Agro Active Potential of Bacillus subtilis PE7 against Didymella bryoniae (Auersw.), the Causal Agent of Gummy Stem Blight of Cucumis melo
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture Conditions and Microorganisms
2.2. Dual Culture and Mycelial Growth Inhibition Assay
2.3. Inhibitory Activity of B. subtilis PE7 VOCs on D. bryoniae Growth
2.4. Effect of Chemical Fungicide on B. subtilis PE7 Growth
2.5. In Vitro Examination of D. bryoniae Growth Inhibition by B. subtilis PE7
2.6. Preparation of D. bryoniae Conidial Suspension for Pot Experiments
2.7. In Vivo Pot Experiment
2.8. Quantification of B. subtilis PE7 in Pot Soil
2.9. Nutrient Analysis of Melon Plants
2.10. Data Analysis
3. Results
3.1. Antagonistic Activity of B. subtilis PE7 against D. bryoniae
3.2. Inhibitory Effect of B. subtilis PE7 VOCs on D. bryoniae
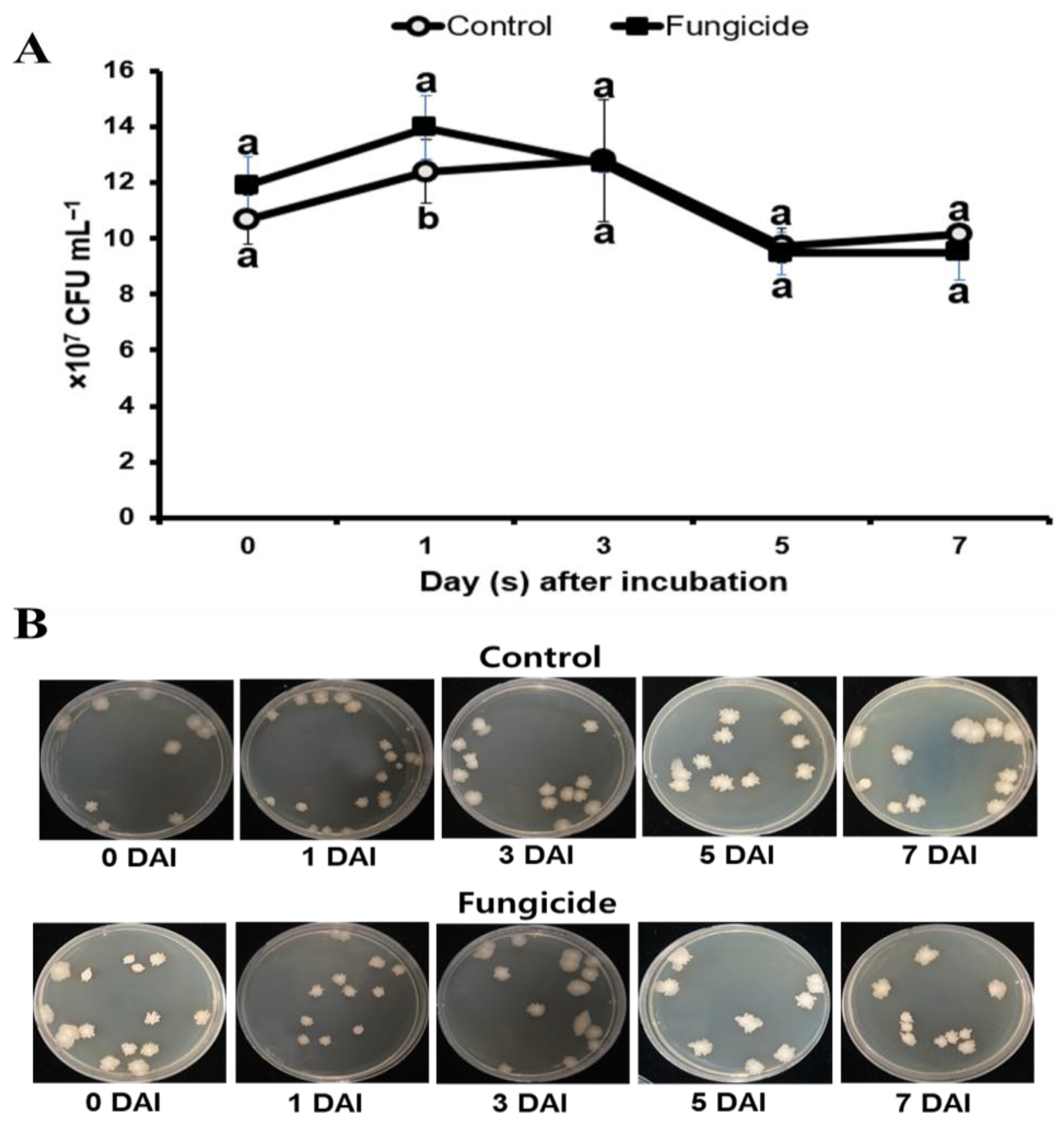
3.3. Influence of Chemical Fungicide on the Survival of B. subtilis PE7
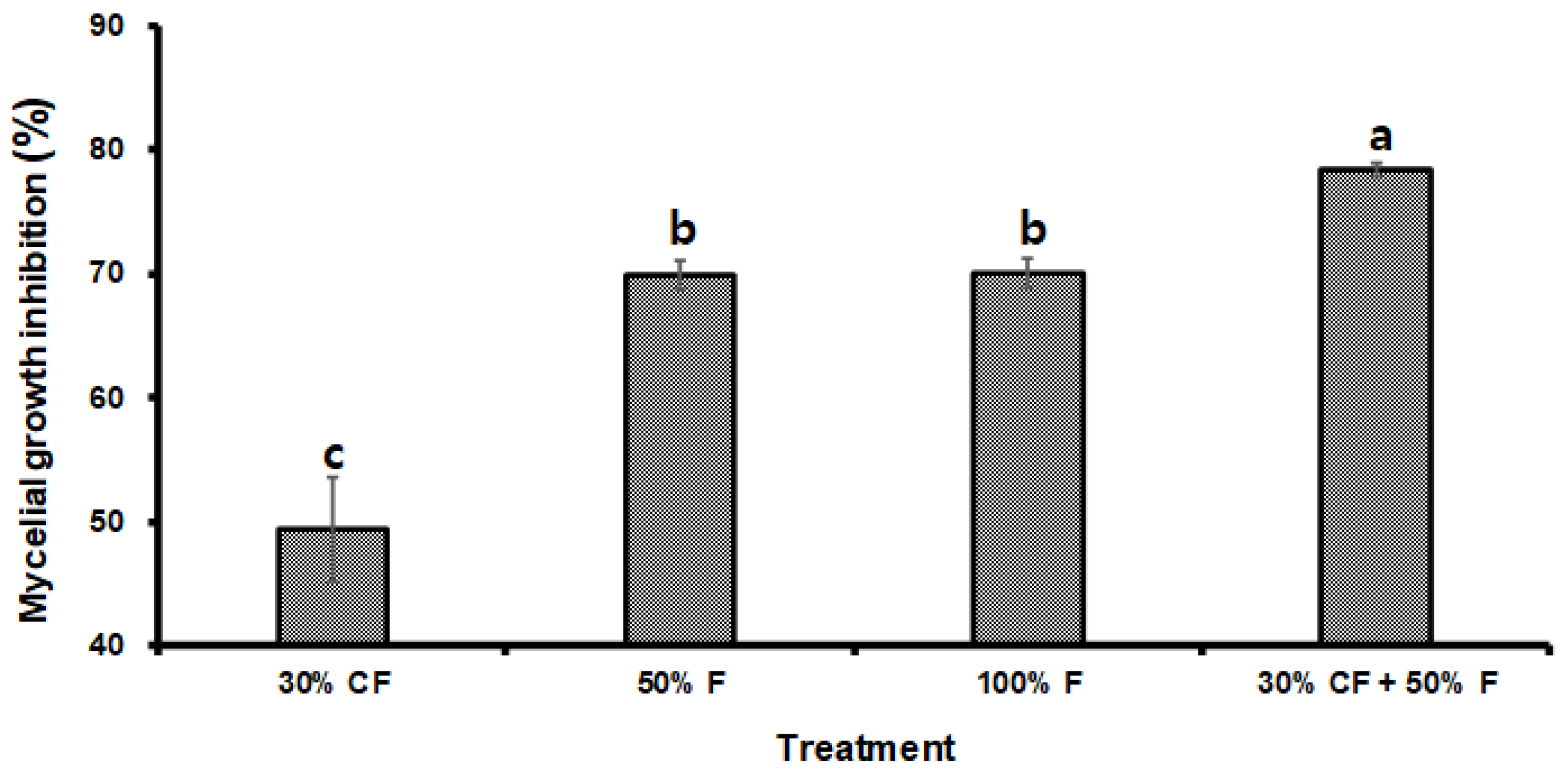
3.4. B. subtilis PE7 Culture Filtrate and Fungicide Co-Exposure for Targeted Suppression of D. bryoniae
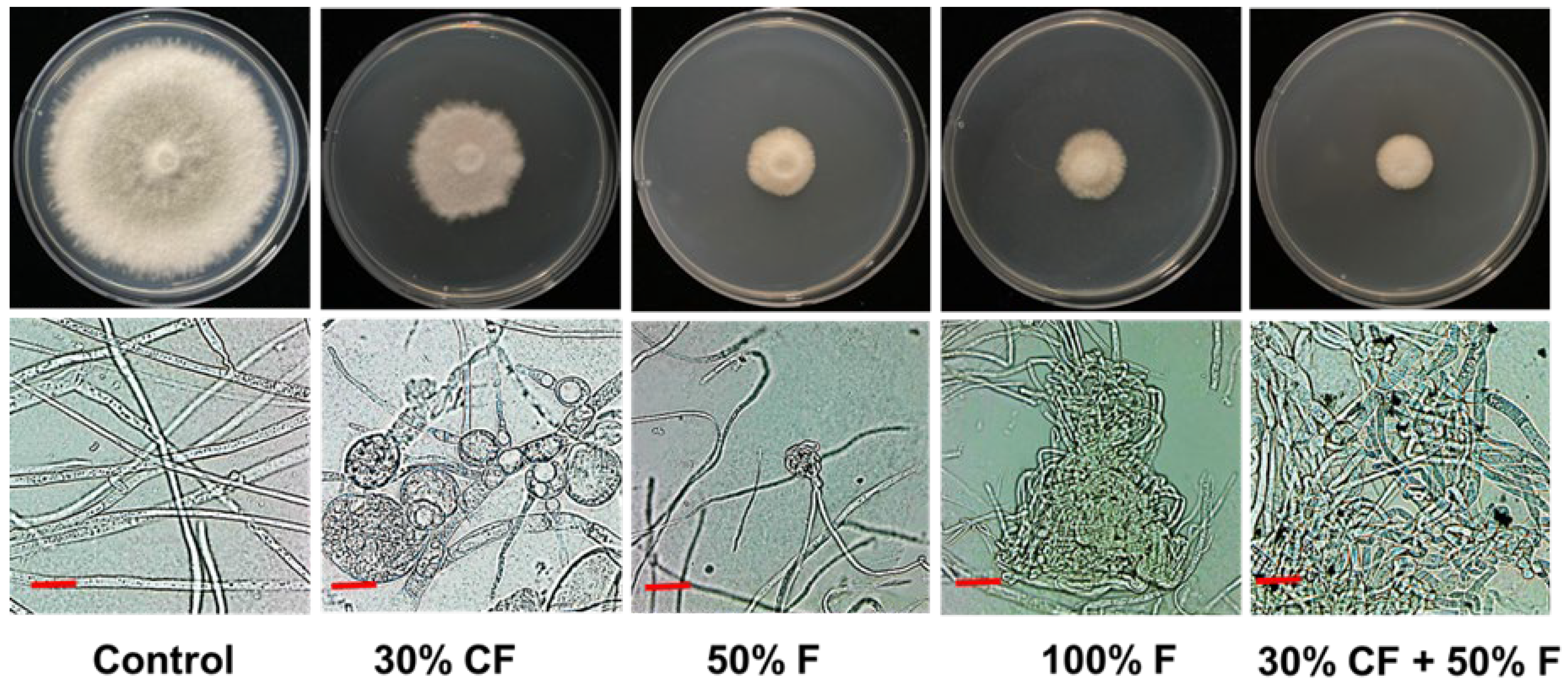
3.5. Effects of B. subtilis PE7 CF and Fungicide on D. bryoniae Hyphal Morphology
3.6. Effect of B. subtilis PE7 Culture on Melon Plant Growth
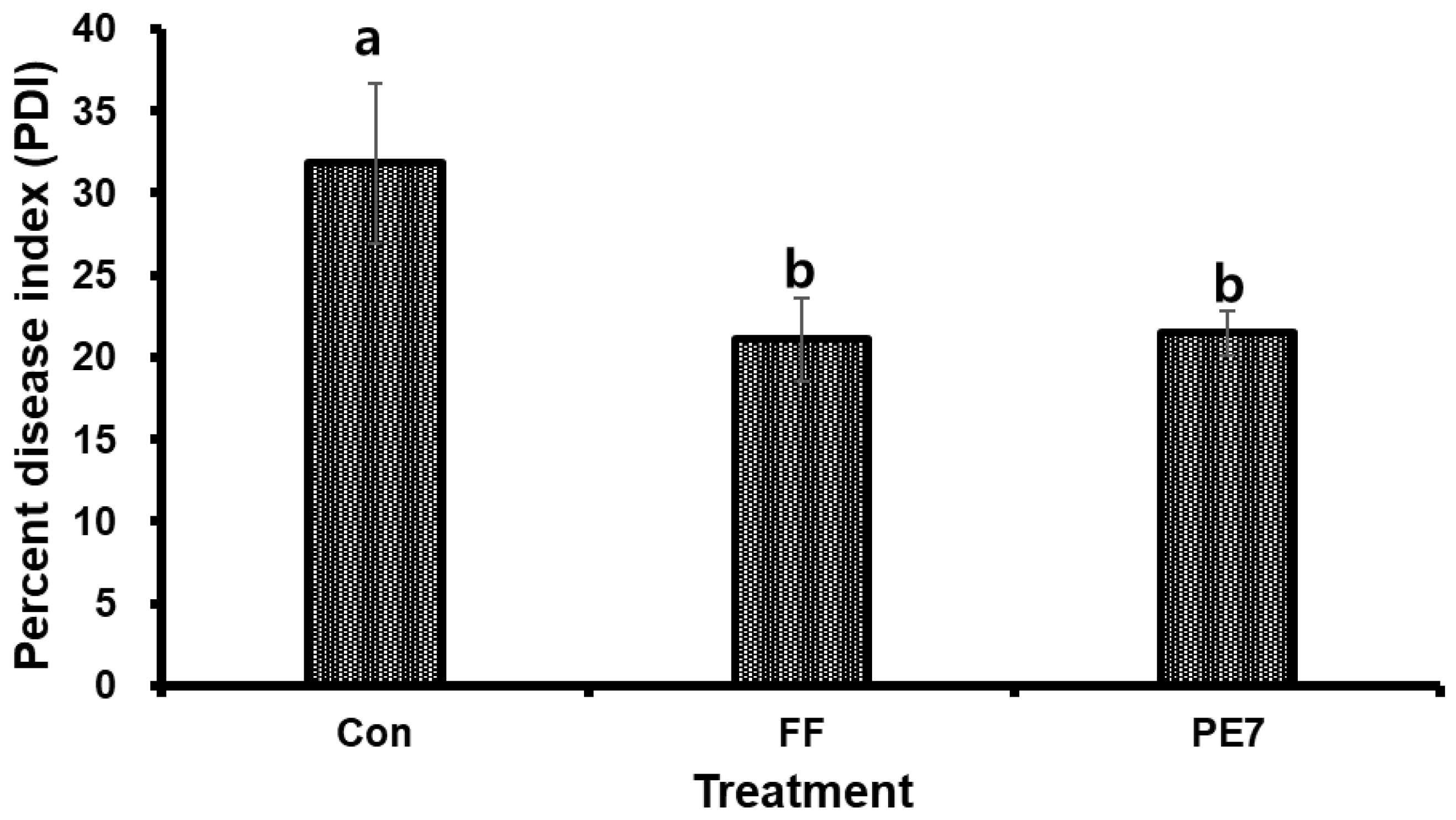
3.7. Effects of B. subtilis PE7 Culture and Fungicide on the Disease Severity of D. bryoniae-Infected Melon Plants
3.8. Nutrient Analysis of Melon Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, Y.; Li, Y.; Zhu, B.; Huang, W.; Chen, J.; Wang, F.; Chen, Y.; Wang, M.; Lai, H.; Zhou, Y. Genome-wide identification of the expansion gene family in netted melon and their transcriptional responses to fruit peel cracking. Front. Plant Sci. 2024, 15, 1332240. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Sun, Y.; He, Y.; Tang, X.; Yang, S.; Huang, J. Comparison of Rhizospheric and Endophytic Bacterial Compositions between Netted and Oriental Melons. Microbiol. Spect. 2023, 11, e04027-22. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, D.; Kwon, T.H.; Lee, B.H.; Lee, S.E. Effective Phyto-sanitary Treatment for Export of Oriental Melons (Cucumis melo var L.) Using Ethyl Formate and Modified Atmosphere Packaging to Control Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). Insects 2023, 14, 442. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Tan, G.F.; Ma, Y.Q.; Meng, P.H.; Zhang, J. Cucurbitaceous Vegetables’ Gummy Stem Blight Research. Agronomy 2022, 12, 1283. [Google Scholar] [CrossRef]
- Keinath, A.P. Reproduction of Didymella bryoniae on nine species of cucurbits under field conditions. Plant Dis. 2014, 98, 1379–1386. [Google Scholar] [CrossRef][Green Version]
- Wu, Z.W.; Cai, X.W.; Mao, X.W.; Zhou, M.G.; Hou, Y.P. Sensitivity and resistance risk analysis of Didymella bryoniae populations to fluopyram. J. Integr. Agric. 2023, 23, 2306–2317. [Google Scholar] [CrossRef]
- Paret, M.L.; Dufault, N.S.; Newark, M.; Freeman, J.H. Management of gummy stem blight (black rot) on cucurbits in Florida. Plant Pathol. Dep. UF/IFAS Ext. 2021, 2018, PP280. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Kibria, M.G.; Talukder, M.M.R.; Akhter, M.S.; Amia, M.F. Evaluation of fungicides for control of gummy stem blight of watermelon caused by Didymella bryoniae. Bangladesh J. Plant Pathol. 2019, 35, 47–52. [Google Scholar]
- Noh, J.; Kim, J.H.; Lim, J.H.; Kim, T.B.; Seong, M.H.; Jung, G.T.; Kim, J.M.; Cheong, S.S.; Oh, N.K.; Lee, W.H. Occurrence of diseases and case of clinical diagnosis on watermelon in South Korea, 2008–2012. Res. Plant Dis. 2014, 20, 8–14. [Google Scholar] [CrossRef][Green Version]
- Pereira, A.S.; dos Santos, G.R.; Sarmento, R.A.; da Silva Galdino, T.V.; de Oliveira Lima, C.H.; Picanço, M.C. Key factors affecting watermelon yield loss in different growing seasons. Sci. Hortic. 2017, 218, 205–212. [Google Scholar] [CrossRef]
- Café-Filho, A.C.; Santos, G.R.; Laranjeira, F.F. Temporal and spatial dynamics of watermelon gummy stem blight epidemics. Eur. J. Plant Pathol. 2010, 128, 473–482. [Google Scholar] [CrossRef]
- Rennberger, G.; Keinath, A.P. Susceptibility of fourteen new cucurbit species to gummy stem blight caused by Stagonosporopsis citrulli under field conditions. Plant Dis. 2018, 102, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.G.; Korir, R.C.; Walter, T.L.; Everts, K.L. Reducing chlorothalonil use in fungicide spray programs for powdery mildew, anthracnose, and gummy stem blight in melons. Plant Dis. 2020, 104, 3213–3220. [Google Scholar] [CrossRef] [PubMed]
- Seblani, R.; Keinath, A.P.; Munkvold, G. Gummy stem blight: One disease, three pathogens. Mol. Plant Pathol. 2023, 24, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Haggag, M.; Wafaa, M.H. Sustainable agriculture management of plant diseases. Online J. Biol. Sci. 2002, 2, 280–284. [Google Scholar]
- Gimode, W.; Bao, K.; Fei, Z.; McGregor, C. QTL associated with gummy stem blight resistance in watermelon. Theor. Appl. Genet. 2020, 134, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Tarafdar, A.; Ghosh, R.; Gopalakrishanan, S. Biological Control as a Tool for Eco-Friendly Management of Plant Pathogens. In Advances in Soil Microbiology: Recent Trends and Future Prospects; Springer: Berlin/Heidelberg, Germany, 2017; pp. 153–188. [Google Scholar]
- Maung, C.E.H.; Choub, V.; Cho, J.Y.; Kim, K.Y. Control of the bacterial soft rot pathogen, Pectobacterium carotovorum by Bacillus velezensis CE 100 in cucumber. Microb. Pathog. 2022, 173, 105807. [Google Scholar] [CrossRef] [PubMed]
- Kumbar, B.; Mahmood, R.; Nagesha, S.N.; Nagaraja, M.S.; Prashant, D.G.; Kerima, O.Z.; Karosiya, A.; Chavan, M. Field application of Bacillus subtilis isolates for controlling late blight disease of potato caused by Phytophthora infestans. Biocatal. Agric. Biotechnol. 2019, 22, 101366. [Google Scholar] [CrossRef]
- Maung, C.E.H.; Baek, W.S.; Choi, T.G.; Kim, K.Y. Control of grey mould disease on strawberry using the effective agent, Bacillus amyloliquefaciens Y1. Biocontrol Sci. Technol. 2021, 31, 468–482. [Google Scholar] [CrossRef]
- Vehapi, M.; Inan, B.; Kayacan-Cakmakoglu, S.; Sagdic, O.; Özçimen, D. Optimization of growth conditions for the production of Bacillus subtilis using central composite design and its antagonism against pathogenic fungi. Probiotics Antimicrob. Proteins 2023, 15, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Harish, B.N.; Nagesha, S.N.; Ramesh, B.N.; Shyamalamma, S.; Nagaraj, M.S.; Girish, H.C.; Pradeep, C.; Shiva Kumar, K.S.; Tharun Kumar, K.S.; Pavan, S.N.; et al. Molecular characterization and antifungal activity of lipopeptides produced from Bacillus subtilis against plant fungal pathogen Alternaria alternata. BMC Microbiol. 2023, 23, 179. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Tan, H.; Dai, Y.; Huang, Y.; Yao, H.; Cai, Y.; Yu, G. Application of antimicrobial peptides in plant protection: Making use of the overlooked merits. Front. Plant Sci. 2023, 14, 1139539. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.Q.H.; Thai, T.H.; Tran, T.X.P.; Tran, D.H. Evaluation of Bacillus sp. strains for biological control of gummy stem blight, Didymella bryoniae (Auersw.) in watermelon (Citrullus lanatus). Crop Res. 2023, 58, 238–242. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Xu, W. Bacillus velezensis WB induces systemic resistance in watermelon against Fusarium wilt. Pest Manag. Sci. 2024, 80, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Maung, C.E.H.; Lee, H.G.; Cho, J.Y.; Kim, K.Y. Antifungal compound, methyl hippurate from Bacillus velezensis CE 100 and its inhibitory effect on growth of Botrytis cinerea. World J. Microbiol. Biotechnol. 2021, 37, 159. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.A.; Elmenisy, A.; Abdel-Ghafar, N.Y. Prediction of Tomato Early Blight Disease Under Climate Change Conditions in Egypt. Arab Univ. J. Agric. Sci. 2019, 27, 1985–1995. [Google Scholar] [CrossRef]
- Awan, Z.A.; Shoaib, A.; Schenk, P.M.; Ahmad, A.; Alansi, S.; Paray, B.A. Antifungal potential of volatiles produced by Bacillus subtilis BS-01 against Alternaria solani in Solanum lycopersicum. Front. Plant Sci. 2023, 13, 1089562. [Google Scholar] [CrossRef] [PubMed]
- Trung, N.T.; Thao, N.T.; Le Thanh, N.S.; Dai Nguyen, N.P.; Tuyet, N.T.A.; Cuong, N.T.; Chan, S.S.; Khoo, K.S.; Show, P.L. Antifungal activity of secondary metabolites purified from Bacillus subtilis isolated in Vietnam and evaluated on in vitro and in vivo models. Int. Biodeterior. Biodegrad. 2023, 179, 105558. [Google Scholar]
- Qiao, J.; Zhang, R.; Liu, Y.; Liu, Y. Evaluation of the Biocontrol Efficiency of Bacillus subtilis Wettable Powder on Pepper Root Rot Caused by Fusarium solani. Pathogens 2023, 12, 225. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, D.K.; Johri, B.N. Interactions of Bacillus spp. and plants—With special reference to induced systemic resistance (ISR). Microbiol. Res. 2009, 164, 493–513. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zhao, Z.; Fan, J.; Liang, Y.; Bernier, M.C.; Gao, Y.; Zhao, L.; Opiyo, S.O.; Xia, Y. Bacillus proteolyticus OSUB18 triggers induced systemic resistance against bacterial and fungal pathogens in Arabidopsis. Front. Plant Sci. 2023, 14, 1078100. [Google Scholar] [CrossRef] [PubMed]
- Samaniego-Gámez, B.Y.; Valle-Gough, R.E.; Garruña-Hernández, R.; Reyes-Ramírez, A.; Latournerie-Moreno, L.; Tun-Suárez, J.M.; Villanueva-Alonzo, H.D.J.; Nuñez-Ramírez, F.; Diaz, L.C.; Samaniego-Gámez, S.U.; et al. Induced Systemic Resistance in the Bacillus spp.—Capsicum chinese Jacq.—PepGMV Interaction, Elicited by Defense-Related Gene Expression. Plants 2023, 12, 2069. [Google Scholar] [CrossRef] [PubMed]
- Arnaouteli, S.; Bamford, N.C.; Stanley-Wall, N.R.; Kovács, Á.T. Bacillus subtilis biofilm formation and social interactions. Nat. Rev. Microbiol. 2021, 19, 600–614. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Chauhan, P.; Kumar, N.; Mishra, N.; Pandey, S.; Bajpai, R.; Yadav, J.K.; Sahay, R.; Bahadur, L.; Mishra, A. Microbial biofilm approaches in phytopathogen management. In Microbial Biomolecules; Academic Press: Cambridge, MA, USA, 2023; pp. 77–96. [Google Scholar]
- Malik, M.S.; Rehman, A.; Khan, I.U.; Khan, T.A.; Jamil, M.; Rha, E.S.; Anees, M. Thermo-neutrophilic cellulases and chitinases characterized from a novel putative antifungal biocontrol agent: Bacillus subtilis TD11. PLoS ONE 2023, 18, e0281102. [Google Scholar] [CrossRef] [PubMed]
- Rosazza, T.; Eigentler, L.; Earl, C.; Davidson, F.A.; Stanley-Wall, N.R. Bacillus subtilis extracellular protease production incurs a context-dependent cost. Mol. Microbiol. 2023, 120, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Park, J.; Cho, S.W.; Park, K.H.; Lee, G.P.; Ban, S.J.; Lee, C.R.; Kim, C.S. Isolation and characterization of Bacillus strains for biological control. J. Microbiol. 2003, 41, 196–201. [Google Scholar]
- Liang, N.; Charron, J.B.; Jabaji, S. Comparative transcriptome analysis reveals the biocontrol mechanism of Bacillus velezensis E68 against Fusarium graminearum DAOMC 180378, the causal agent of Fusarium head blight. PLoS ONE 2023, 18, e0277983. [Google Scholar] [CrossRef] [PubMed]
- Grahovac, J.; Pajčin, I.; Vlajkov, V. Bacillus VOCs in the Context of Biological Control. Antibiotics 2023, 12, 581. [Google Scholar] [CrossRef] [PubMed]
- Al-Mutar, D.M.K.; Noman, M.; Alzawar, N.S.A.; Qasim, H.H.; Li, D.; Song, F. The extracellular lipopeptides and volatile organic compounds of Bacillus subtilis DHA41 display broad-spectrum antifungal activity against soil-borne pytopathogenic Fungi. J. Fungi 2023, 9, 797. [Google Scholar] [CrossRef] [PubMed]
- Waghunde, R.R.; Khunt, M.D.; Shelake, R.M.; Hiremani, N.; Patil, V.A.; Kim, J.Y. Endophytes: A potential bioagent for plant disease management. In Microbial Endophytes and Plant Growth; Academic Press: Cambridge, MA, USA, 2023; pp. 19–34. [Google Scholar]
- Ahmed, W.; Zhou, G.; Yang, J.; Munir, S.; Ahmed, A.; Liu, Q.; Zhao, Z.; Ji, G. Bacillus amyloliquefaciens WS-10 as a potential plant growth-promoter and biocontrol agent for bacterial wilt disease of flue-cured tobacco. Egypt. J. Biol. Pest Control 2022, 32, 25. [Google Scholar] [CrossRef]
- Salazar, B.; Ortiz, A.; Keswani, C.; Minkina, T.; Mandzhieva, S.; Pratap Singh, S.; Rekadwad, B.; Borriss, R.; Jain, A.; Singh, H.B.; et al. Bacillus spp. as bio-factories for antifungal secondary metabolites: Innovation beyond whole organism formulations. Microb. Ecol. 2023, 86, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhao, K.; Cai, L.; Zhang, Y.; Fu, Q.; Huang, S. Combination effects of tebuconazole with Bacillus subtilis to control rice false smut and the related synergistic mechanism. Pest Manag. Sci. 2023, 79, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, M.; Hirai, M.; Shoda, M. Integrated biological and chemical control of damping-off caused by Rhizoctonia solani using Bacillus subtilis RB14-C and flutolanil. J. Biosci. Bioeng. 2001, 91, 173–177. [Google Scholar] [CrossRef]
- Ons, L.; Bylemans, D.; Thevissen, K.; Cammue, B.P. Combining biocontrol agents with chemical fungicides for integrated plant fungal disease control. Microorganisms 2020, 8, 1930. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hagberg, I.; Novitsky, L.; Hadj-Moussa, H.; Avis, T.J. Interaction of antimicrobial cyclic lipopeptides from Bacillus subtilis influences their effect on spore germination and membrane permeability in fungal plant pathogens. Fungal Biol. 2014, 118, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liang, Y.; Wu, M.; Chen, Z.; Lin, J.; Yang, L. Natural products from Bacillus subtilis with antimicrobial properties. Chin. J. Chem. Eng. 2015, 23, 744–754. [Google Scholar] [CrossRef]
- Noh, J.S.; Hwang, S.H.; Maung, C.E.H.; Cho, J.Y.; Kim, K.Y. Enhanced control efficacy of Bacillus subtilis NM4 via integration of chlorothalonil on potato early blight caused by Alternaria solani. Microb. Pathog. 2024, 190, 106604. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Li, C.H.; Ali, Q.; Zhao, W.; Chi, Y.K.; Shafiq, M.; Ali, F.; Yu, X.Y.; Yu, Q.; Zhao, J.T.; et al. Bacterial and Fungal Biocontrol Agents for Plant Disease Protection: Journey from Lab to Field, Current Status, Challenges, and Global Perspectives. Molecules 2023, 28, 6735. [Google Scholar] [CrossRef] [PubMed]
- Musheer, N.; Jamil, A.; Choudhary, A. Solo and combined applications of fungicides and bio-agents to reduce severity of Fusarium oxysporum and induce antioxidant metabolites in Ocimum tenuiflorum L. J. Plant Pathol. 2023, 105, 237–251. [Google Scholar] [CrossRef]
- Gu, Z.W.; Yin, J.H.; Wu, H.; Liang, Y.Q.; Wu, W.H.; Lu, Y.; Li, R.; Tan, S.B.; He, C.P.; Yi, K.X. Synergistic mechanism of Bacillus subtilis Czk1 combined with propiconazole and tebuconazole mixtures against Pyrrhoderma noxium. Chem. Biol. Technol. Agric. 2023, 10, 113. [Google Scholar] [CrossRef]
- de Novais, C.B.; Giovannetti, M.; de Faria, S.M.; Sbrana, C. Two herbicides, two fungicides and spore-associated bacteria affect Funneliformis mosseae extraradical mycelium structural traits and viability. Mycorrhiza 2019, 29, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Hirozawa, M.T.; Ono, M.A.; Suguiura, I.M.D.S.; Bordini, J.G.; Ono, E.Y.S. Lactic acid bacteria and Bacillus spp. as fungal biological control agents. J. Appl. Microbiol. 2023, 134, 083. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmad, M.; Hussain, A.; Jamil, M. Integrated use of phosphate-solubilizing Bacillus subtilis strain IA6 and zinc-solubilizing Bacillus sp. strain IA16: A promising approach for improving cotton growth. Folia Microbiol. 2021, 66, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Sayago, P.; Juncosa, F.; Albarracín Orio, A.G.; Luna, D.F.; Molina, G.; Lafi, J.; Ducasse, D.A. Bacillus subtilis ALBA01 alleviates onion pink root by antagonizing the pathogen Setophoma terrestris and allowing physiological status maintenance. Eur. J. Plant Pathol. 2020, 157, 509–519. [Google Scholar] [CrossRef]
- Jabborova, D.; Enakiev, Y.; Sulaymanov, K.; Kadirova, D.; Ali, A.; Annapurna, K. Plant growth promoting bacteria Bacillus subtilis promote growth and physiological parameters of Zingiber officinale Roscoe. Plant Sci. Today 2021, 8, 66–71. [Google Scholar] [CrossRef]
- Shafi, J.; Tian, H.; Ji, M. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef]
- Chen, F.; Wang, M.; Zheng, Y.; Luo, J.; Yang, X.; Wang, X. Quantitative changes of plant defense enzymes and phytohormone in biocontrol of cucumber Fusarium wilt by Bacillus subtilis B579. World J. Microbiol. Biotechnol. 2010, 26, 675–684. [Google Scholar] [CrossRef]
- Han, S.E.; Cho, J.Y.; Kim, K.Y.; Maung, C.E.H. Role of an antagonistic bacterium, Bacillus subtilis PE7, in growth promotion of netted melon (Cucumis melo L. var. reticulatus Naud.). Can. J. Microbiol. 2023, 70, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Han, S.E.; Kim, K.S.; Maung, C.E.H.; Kim, K.Y. Growth enhancement of tomato by a plant growth promoting bacterium, Bacillus subtilis PE7. Korean J. Soil Sci. Fertil. 2023, 56, 398–406. [Google Scholar] [CrossRef]
- Jensen, C.N.G.; Pang, J.K.Y.; Hahn, C.M.; Gottardi, M.; Husted, S.; Moelbak, L.; Kovács, Á.T.; Fimognari, L.; Schulz, A. Differential influence of Bacillus subtilis strains on Arabidopsis root architecture through common and distinct plant hormonal pathways. Plant Sci. 2024, 339, 111936. [Google Scholar] [CrossRef] [PubMed]
- Lastochkina, O.; Garshina, D.; Allagulova, C.; Fedorova, K.; Koryakov, I.; Vladimirova, A. Application of endophytic Bacillus subtilis and salicylic acid to improve wheat growth and tolerance under combined drought and Fusarium root rot stresses. Agronomy 2020, 10, 1343. [Google Scholar] [CrossRef]
- Kumar, S.; Anjali, A.R.; Masurkar, P.; Singh, U.B.; Tripathi, R.; Bhupenchandra, I.; Minkina, T.; Keswani, C. Bacillus subtilis-Mediated induction of disease resistance and promotion of plant growth of vegetable crops. In Applications of Bacillus and Bacillus Derived Genera in Agriculture, Biotechnology and Beyond; Springer Nature: Singapore, 2024; pp. 165–211. [Google Scholar]
- Pomerleau, M.; Charron-Lamoureux, V.; Leonard, L.; Grenier, F.; Rodrigue, S.; Beauregard, P.B. Adaptive laboratory evolution reveals biofilm regulating genes as key players in B. subtilis root colonization. mSystems 2024, 9, e0084323. [Google Scholar] [CrossRef] [PubMed]
- Blake, C.; Christensen, M.N.; Kovács, Á.T. Molecular aspects of plant growth promotion and protection by Bacillus subtilis. Mol. Plant-Microbe Interact. 2021, 34, 15–25. [Google Scholar] [CrossRef]
- Chang, P.E.; Wu, Y.H.; Tai, C.Y.; Lin, I.H.; Wang, W.D.; Tseng, T.S.; Chuang, H.W. Examining the transcriptomic and biochemical signatures of Bacillus subtilis strains: Impacts on plant growth and abiotic stress tolerance. Int. J. Mol. Sci. 2023, 24, 13720. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, Y.; Zhang, N.; Xun, W.; Feng, H.; Miao, Y.; Shao, J.; Shen, Q.; Zhang, R. Chemical communication in plant–microbe beneficial interactions: A toolbox for precise management of beneficial microbes. Curr. Opin. Microbiol. 2023, 72, 102269. [Google Scholar] [CrossRef] [PubMed]
- Posada, L.F.; Álvarez, J.C.; Romero-Tabarez, M.; de-Bashan, L.; Villegas-Escobar, V. Enhanced molecular visualization of root colonization and growth promotion by Bacillus subtilis EA-CB0575 in different growth systems. Microbiol. Res. 2018, 217, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Jin, J.; Li, Q.; Zhao, C.; Nan, W.; Wang, X.; Ma, R.; Bi, Y. An intact cytokinin-signaling pathway is required for Bacillus sp. LZR216-promoted plant growth and root system architecture alteration in Arabidopsis thaliana seedlings. Plant Growth Regul. 2018, 84, 507–518. [Google Scholar] [CrossRef]
- Roy, B.; Maitra, D.; Biswas, A.; Chowdhury, N.; Ganguly, S.; Bera, M.; Dutta, S.; Golder, S.; Roy, S.; Ghosh, J.; et al. Efficacy of high-altitude biofilm-forming novel Bacillus subtilis species as plant growth-promoting Rhizobacteria on Zea mays L. Appl. Biochem. Biotechnol. 2023, 196, 643–666. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.I.; Gomaa, E.Z. Effect of plant growth promoting Bacillus subtilis and Pseudomonas fluorescence on growth and pigment composition of radish plants (Raphanus sativus) under NaCl stress. Photosynthetica 2012, 50, 263–272. [Google Scholar] [CrossRef]
- Kang, S.M.; Radhakrishnan, R.; Lee, K.E.; You, Y.H.; Ko, J.H.; Kim, J.H.; Lee, I.J. Mechanism of plant growth promotion elicited by Bacillus sp. LKE15 in oriental melon. Acta Agric. Scand. Sect. B Soil Plant Sci. 2015, 65, 637–647. [Google Scholar]
- Zhang, J.; Ahmed, W.; Dai, Z.; Zhou, X.; He, Z.; Wei, L.; Ji, G. Microbial consortia: An engineering tool to suppress clubroot of Chinese cabbage by changing the rhizosphere bacterial community composition. Biology 2022, 11, 918. [Google Scholar] [CrossRef]




| Length (cm) | Fresh Weight (g) | Dry Weight (g) | Leaf Number | Leaf Area (cm2) | Strain PE7 in Pot Soil | ||||
|---|---|---|---|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | Shoot | Root | ||||
| Con | 86.02 ± 1.22 b | 21.40 ± 1.76 a | 73.52 ± 3.78 b | 13.77 ± 2.56 b | 8.14 ± 0.52 b | 1.17 ± 0.21 b | 22.67 ± 0.52 b | 1111.83 ± 35.76 c | - |
| FF | 82.72 ± 5.65 b | 23.72 ± 0.79 a | 77.06 ± 9.06 b | 15.31 ± 3.83 ab | 7.90 ± 0.69 b | 1.16 ± 0.26 b | 24.33 ± 1.97 a | 1269.07 ± 12.36 b | - |
| PE7 | 108.87 ± 5.17 a | 24.98 ± 1.71 a | 94.22 ± 4.23 a | 19.62 ± 4.21 a | 9.41 ± 0.25 a | 1.54 ± 0.40 a | 24.83 ± 0.98 a | 1388.66 ± 96.14 a | 1.73 ± 0.34 × 107 CFU mL−1 |
| Treatment | Macronutrient (mg plant−1) | |||||
| N | P | K | Mg | Ca | ||
| Con | 132.84 ± 5.37 b | 11.57 ± 0.57 a | 416.66 ± 8.98 b | 83.98 ± 8.52 a | 284.83 ± 12.86 b | |
| FF | 131.94 ± 5.47 b | 11.46 ± 1.17 a | 416.66 ± 8.98 b | 86.75 ± 7.66 a | 340.66 ± 14.57 a | |
| PE7 | 199.23 ± 7.92 a | 11.13 ± 0.92 a | 488.27 ± 20.50 a | 98.76 ± 10.63 a | 328.86 ± 16.50 a | |
| Treatment | Micronutrient (mg plant−1) | |||||
| Mn | B | Cu | Fe | Mo | Zn | |
| Con | 3.56 ± 0.20 b | 171.60 ± 1.73 b | 962.57 ± 275.3 a | 31.38 ± 11.27 a | 7.25 ± 0.51 a | 0.24 ± 0.03 c |
| FF | 3.78 ± 0.11 b | 162.85 ± 2.41 b | 1275.7 ± 227.5 a | 29.01 ± 5.63 a | 6.43 ± 0.51 a | 0.34 ± 0.01 b |
| PE7 | 7.56 ± 0.59 a | 344.34 ± 24.53 a | 1450.4 ± 322.1 a | 30.82 ± 13.03 a | 4.07 ± 0.98 b | 0.50 ± 0.06 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.K.; Han, S.E.; Vasantha-Srinivasan, P.; Jung, W.J.; Maung, C.E.H.; Kim, K.Y. Agro Active Potential of Bacillus subtilis PE7 against Didymella bryoniae (Auersw.), the Causal Agent of Gummy Stem Blight of Cucumis melo. Microorganisms 2024, 12, 1691. https://doi.org/10.3390/microorganisms12081691
Jeong SK, Han SE, Vasantha-Srinivasan P, Jung WJ, Maung CEH, Kim KY. Agro Active Potential of Bacillus subtilis PE7 against Didymella bryoniae (Auersw.), the Causal Agent of Gummy Stem Blight of Cucumis melo. Microorganisms. 2024; 12(8):1691. https://doi.org/10.3390/microorganisms12081691
Chicago/Turabian StyleJeong, Seo Kyoung, Seong Eun Han, Prabhakaran Vasantha-Srinivasan, Woo Jin Jung, Chaw Ei Htwe Maung, and Kil Yong Kim. 2024. "Agro Active Potential of Bacillus subtilis PE7 against Didymella bryoniae (Auersw.), the Causal Agent of Gummy Stem Blight of Cucumis melo" Microorganisms 12, no. 8: 1691. https://doi.org/10.3390/microorganisms12081691
APA StyleJeong, S. K., Han, S. E., Vasantha-Srinivasan, P., Jung, W. J., Maung, C. E. H., & Kim, K. Y. (2024). Agro Active Potential of Bacillus subtilis PE7 against Didymella bryoniae (Auersw.), the Causal Agent of Gummy Stem Blight of Cucumis melo. Microorganisms, 12(8), 1691. https://doi.org/10.3390/microorganisms12081691







