Niacinamide Antimicrobial Efficacy and Its Mode of Action via Microbial Cell Cycle Arrest
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms Strains and Growth Conditions
2.2. Antimicrobial Activity (MIC and MBC)
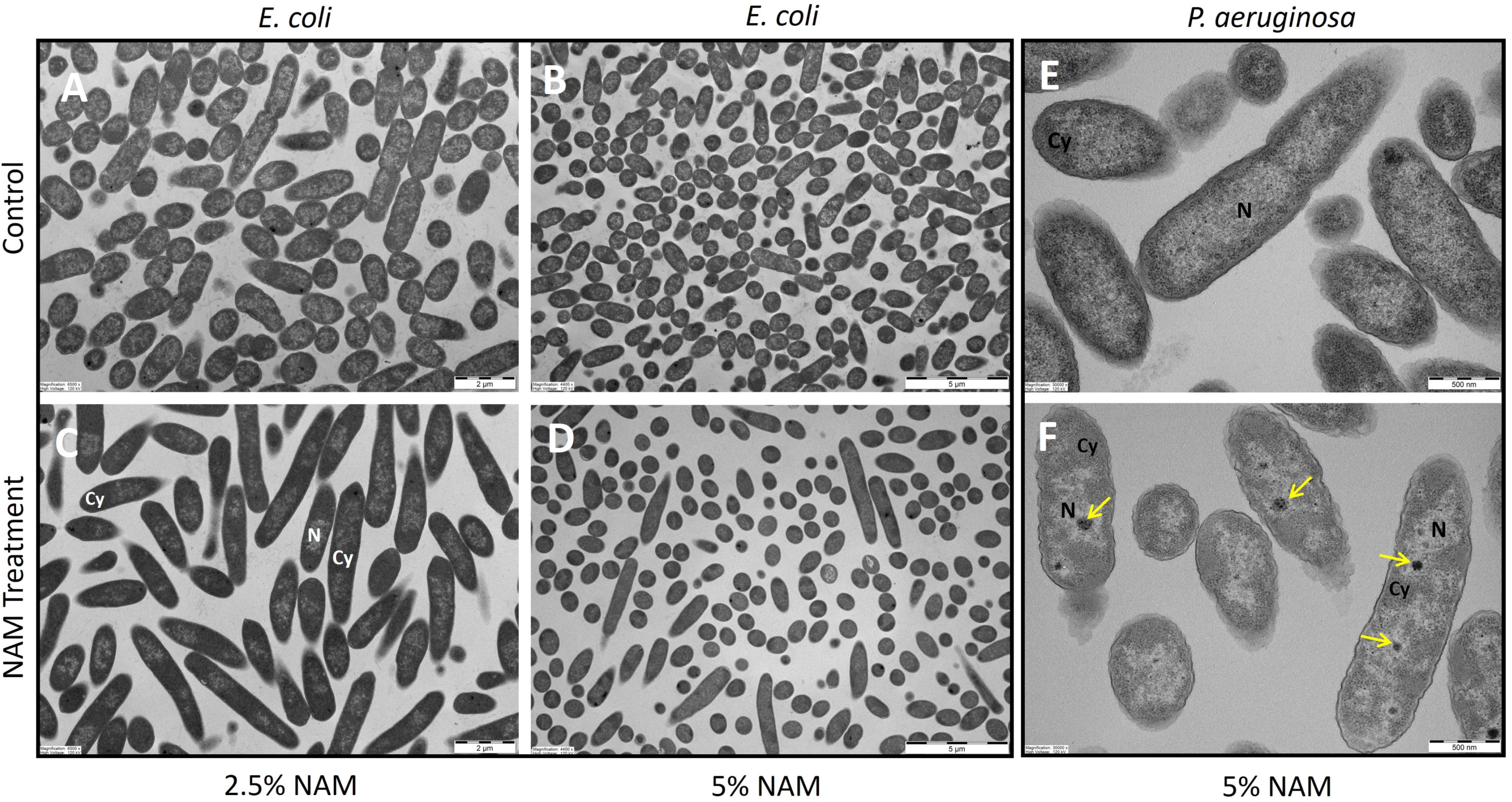
2.3. Transmission Electron Microscopy (TEM)
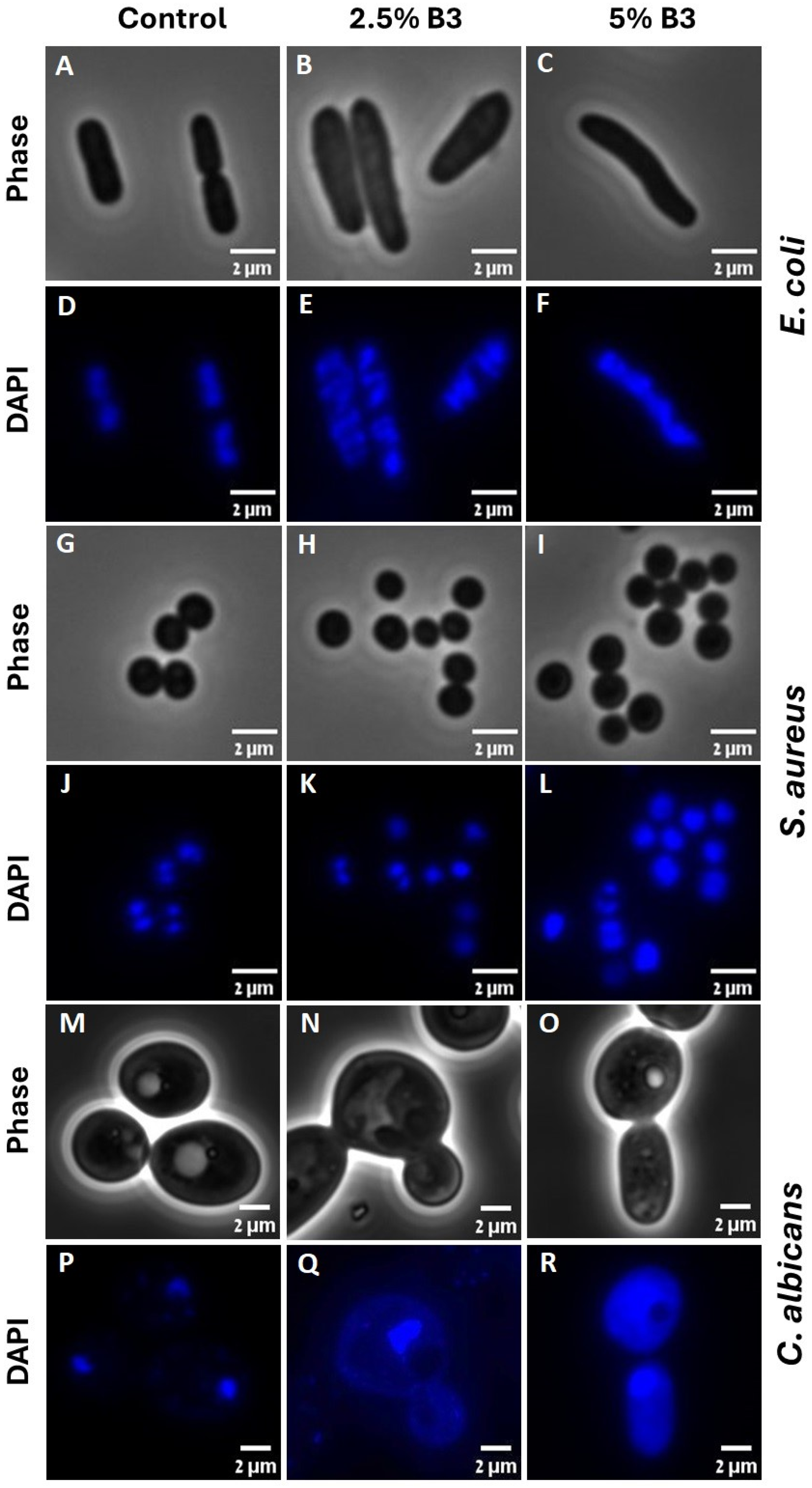
2.4. Phase Microscopy and Image Analysis
2.5. Fluorescence Microscopy and Image Analysis
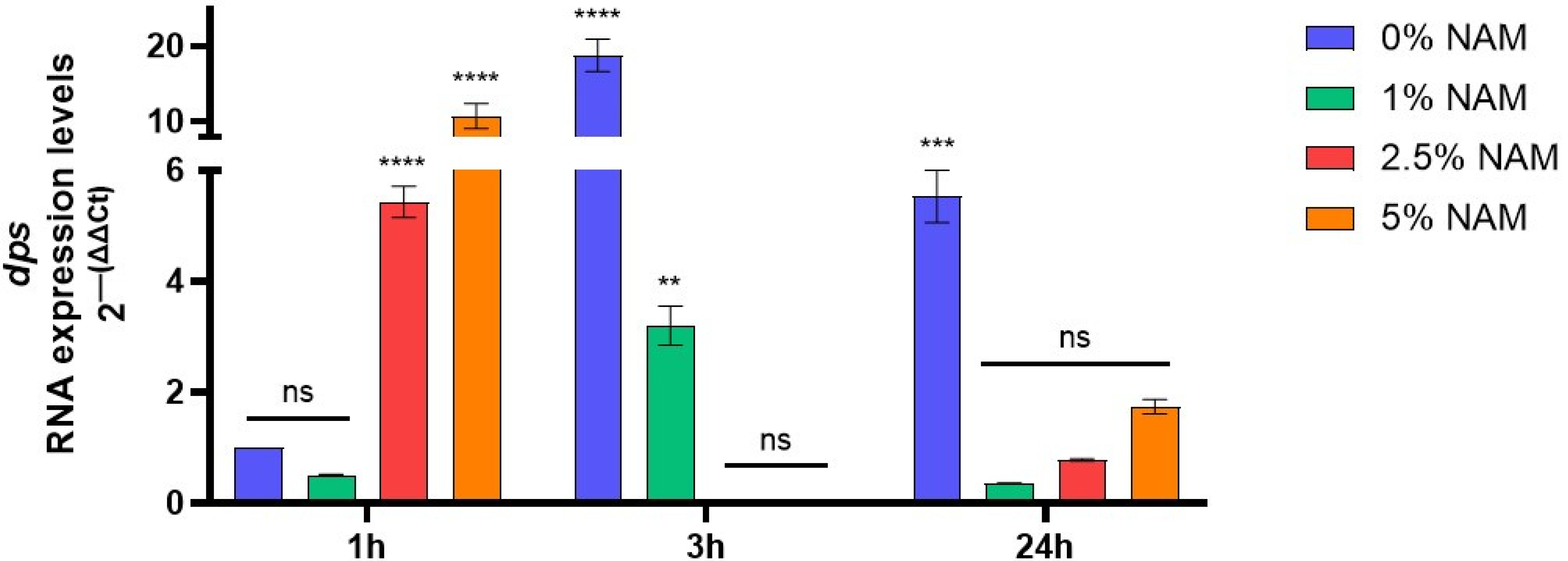
2.6. DNA-Binding Protein (dps) Expression Levels
2.7. Niacinamide Direct Interaction with DNA
2.8. Statistical Analysis
3. Results and Discussion
3.1. Antimicrobial Activity via MIC and MBC
3.2. Morphological Effect of Niacinamide Using TEM
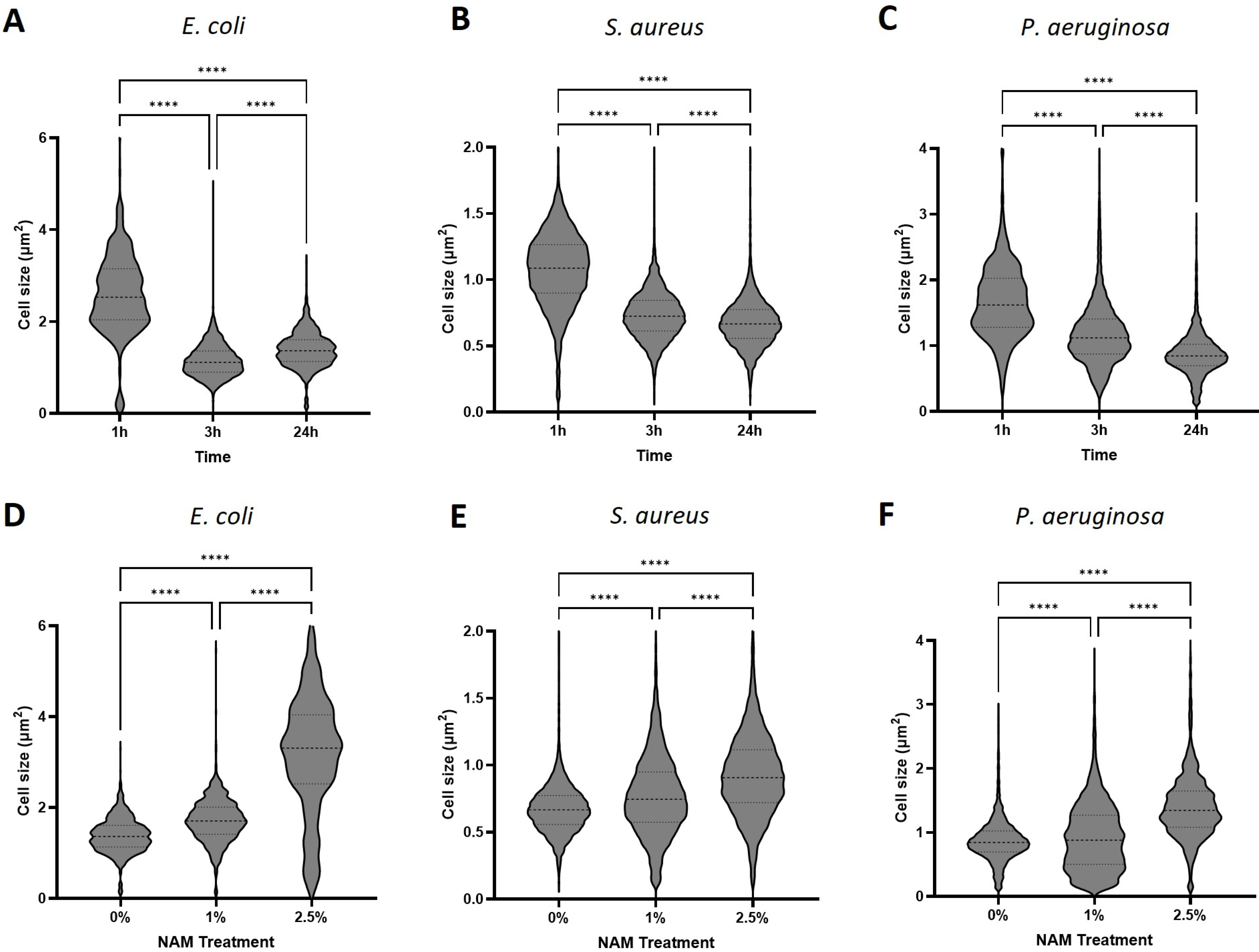
3.3. Phase Microscopy Image Analysis
3.3.1. Cell Volume
3.3.2. Cell Count and Percentage of Dividing Cells
3.4. Fluorescence Microscopy Image Analysis
3.5. dps Gene Expression
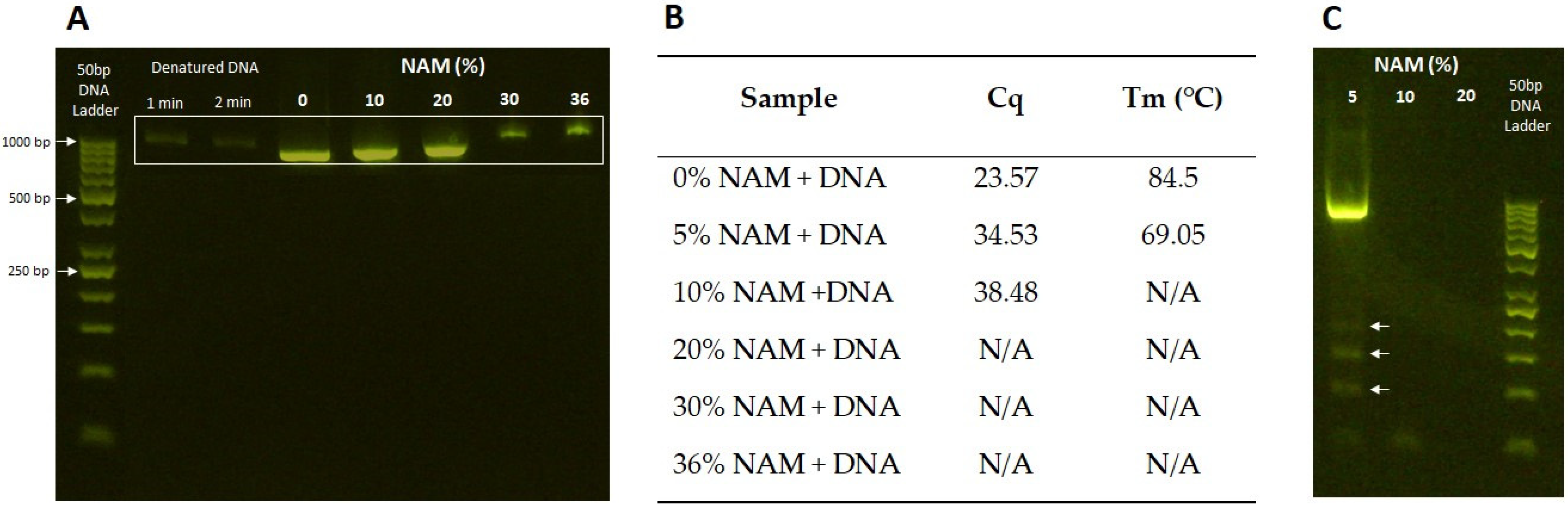
3.6. Interaction with DNA Fragment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Draelos, Z.D.; Ertel, K.; Berge, C. Niacinamide-Containing Facial Moisturizer Improves Skin Barrier and Benefits Subjects with Rosacea. Cutis 2005, 76, 135–141. [Google Scholar] [PubMed]
- Boo, Y.C. Mechanistic Basis and Clinical Evidence for the Applications of Nicotinamide (Niacinamide) to Control Skin Aging and Pigmentation. Antioxidants 2021, 10, 1315. [Google Scholar] [CrossRef] [PubMed]
- Zhen, A.X.; Piao, M.J.; Kang, K.A.; Fernando, P.D.S.M.; Kang, H.K.; Koh, Y.S.; Yi, J.M.; Hyun, J.W. Niacinamide Protects Skin Cells from Oxidative Stress Induced by Particulate Matter. Biomol. Ther. 2019, 27, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Matts, P.; Oblong, J.; Bissett, D. A Review of The Range of Effects of Niacinamide in Human Skin. IFSCC Mag. 2002, 5, 285–289. [Google Scholar]
- Gehring, W. Nicotinic Acid/Niacinamide and the Skin. J. Cosmet. Dermatol. 2004, 3, 88–93. [Google Scholar] [CrossRef]
- Bissett, D.L.; Oblong, J.E.; Berge, C.A. Niacinamide: A B Vitamin That Improves Aging Facial Skin Appearance. Dermatol. Surg. 2005, 31, 860–866. [Google Scholar] [CrossRef]
- Bissett, D.L.; Miyamoto, K.; Sun, P.; Li, J.; Berge, C.A. Topical Niacinamide Reduces Yellowing, Wrinkling, Red Blotchiness, and Hyperpigmented Spots in Aging Facial Skin. Int. J. Cosmet. Sci. 2004, 26, 231–238. [Google Scholar] [CrossRef]
- Marques, C.; Hadjab, F.; Porcello, A.; Lourenço, K.; Scaletta, C.; Abdel-Sayed, P.; Hirt-Burri, N.; Applegate, L.A.; Laurent, A. Mechanistic Insights into the Multiple Functions of Niacinamide: Therapeutic Implications and Cosmeceutical Applications in Functional Skincare Products. Antioxidants 2024, 13, 425. [Google Scholar] [CrossRef]
- Li, F.; Chong, Z.Z.; Maiese, K. Cell Life Versus Cell Longevity: The Mysteries Surrounding the NAD+ Precursor Nicotinamide. Curr. Med. Chem. 2006, 13, 883–895. [Google Scholar] [CrossRef]
- Surjana, D.; Halliday, G.M.; Damian, D.L. Role of Nicotinamide in DNA Damage, Mutagenesis, and DNA Repair. J. Nucleic Acids 2010, 2010, 157591. [Google Scholar] [CrossRef]
- Jacob, R.; Swendseid, M. Niacin. In Present Knowledge in Nutrition; Ziegler, E.E., Filer, L.J., Eds.; International Life Sciences Press: Washington, DC, USA, 1996; pp. 184–190. [Google Scholar]
- Surjana, D.; Halliday, G.M.; Damian, D.L. Nicotinamide Enhances Repair of Ultraviolet Radiation-Induced DNA Damage in Human Keratinocytes and Ex Vivo Skin. Carcinogenesis 2013, 34, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Li, X.; Gao, H.; Feng, Z.; Li, X.; Zhao, L.; Jia, X.; Zhang, H.; Liu, J. High Doses of Nicotinamide Prevent Oxidative Mitochondrial Dysfunction in a Cellular Model and Improve Motor Deficit in a Drosophila Model of Parkinson’s Disease. J. Neurosci. Res. 2008, 86, 2083–2090. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Lee, K.M.; Im, Y.; Seok, S.H.; Chung, H.; Kim, D.Y.; Han, D.; Lee, C.H.; Hwang, E.H.; Park, S.Y.; et al. Nicotinamide (Niacin) Supplement Increases Lipid Metabolism and ROS-Induced Energy Disruption in Triple-Negative Breast Cancer: Potential for Drug Repositioning as an Anti-Tumor Agent. Mol. Oncol. 2022, 16, 1795–1815. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Ren, Z.; Xu, F.; Zhou, X.; Song, C.; Wang, V.Y.F.; Liu, W.; Lu, L.; Thomson, J.A.; Chen, G. Nicotinamide Promotes Cell Survival and Differentiation as Kinase Inhibitor in Human Pluripotent Stem Cells. Stem Cell Rep. 2018, 11, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Peng, Z.; Yang, L.; Qu, M.; Xiong, X.; Xu, L.; Zhao, X.; Pan, K.; Ouyang, K. Niacin Protects against Butyrate-Induced Apoptosis in Rumen Epithelial Cells. Oxidative Med. Cell. Longev. 2019, 2019, 2179738. [Google Scholar] [CrossRef] [PubMed]
- Mathapathi, M.S.; Mallemalla, P.; Vora, S.; Iyer, V.; Tiwari, J.K.; Chakrabortty, A.; Majumdar, A. Niacinamide Leave-on Formulation Provides Long-Lasting Protection against Bacteria in Vivo. Exp. Dermatol. 2016, 26, 827–829. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Gong, T.; Ma, Q.; Jing, M.; Zheng, T.; Yan, J.; Chen, J.; Pan, Y.; Sun, Q.; Zhou, X.; et al. Nicotinamide Could Reduce Growth and Cariogenic Virulence of Streptococcus mutans. J. Oral Microbiol. 2022, 14, 2056291. [Google Scholar] [CrossRef] [PubMed]
- Losasso, V.; Agarwal, K.; Waskar, M.; Majumdar, A.; Crain, J.; Winn, M.; Hoptroff, M. Small Molecules Enhance the Potency of Natural Antimicrobial Peptides. Biophys. J. 2022, 121, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Bettenworth, D.; Nowacki, T.M.; Ross, M.; Kyme, P.; Schwammbach, D.; Kerstiens, L.; Thoennissen, G.B.; Bokemeyer, C.; Hengst, K.; Berdel, W.E.; et al. Nicotinamide Treatment Ameliorates the Course of Experimental Colitis Mediated by Enhanced Neutrophil-Specific Antibacterial Clearance. Mol. Nutr. Food Res. 2014, 58, 1474–1490. [Google Scholar] [CrossRef]
- Savarino, A.; Martini, C.; Orofino, G.C.; Cantamessa, C.; Castelli, L.; Pich, P.G.; Sinicco, A.; Pugliese, A. Apoptotic DNA Fragmentation and Its in Vitro Prevention by Nicotinamide, in Lymphocytes from HIV-1-Seropositive Patients and in HIV-1-Infected MT-4 Cells. Cell Biochem. Funct. 1997, 15, 171–179. [Google Scholar] [CrossRef]
- Murray, M.F. Nicotinamide: An Oral Antimicrobial Agent with Activity against Both Mycobacterium Tuberculosis and Human Immunodeficiency Virus. Clin. Infect. Dis. 2003, 36, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, K.P.; Sirsi, M.; Vaidyanathan, C.S. Nicotinamide-Adenine Dinucleotide-Glycohydrolase Activity in Experimental Tuberculosis. Biochem. J. 1965, 94, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Boshoff, H.I.M.; Xu, X.; Tahlan, K.; Dowd, C.S.; Pethe, K.; Camacho, L.R.; Park, T.H.; Yun, C.S.; Schnappinger, D.; Ehrt, S.; et al. Biosynthesis and Recycling of Nicotinamide Cofactors in Mycobacterium Tuberculosis: An Essential Role for NAD in Nonreplicating bacilli. J. Biol. Chem. 2008, 283, 19329–19341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.S.; Sang, W.W.; Wang, Y.O.; Liu, W. Nicotinamide Phosphoribosyltransferase/Sirtuin 1 Pathway Is Involved in Human Immunodeficiency Virus Type 1 Tat-Mediated Long Terminal Repeat Transactivation. J. Cell. Biochem. 2010, 110, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D.; Matsubara, A.; Smiles, K. The Effect of 2% Niacinamide on Facial Sebum Production. J. Cosmet. Laser Ther. 2006, 8, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-H.; Liu, D.; Chen, Y.-C.; Liao, M.-H.; Lee, W.-R.; Shen, S.-C. Activation of Deoxyribonuclease I by Nicotinamide as a New Strategy to Attenuate Tetracycline-Resistant Biofilms of Cutibacterium acnes. Pharmaceutics 2021, 13, 819. [Google Scholar] [CrossRef] [PubMed]
- Grange, P.A.; Raingeaud, J.; Calvez, V.; Dupin, N. Nicotinamide Inhibits Propionibacterium Acnes-Induced IL-8 Production in Keratinocytes through the NF-ΚB and MAPK Pathways. J. Dermatol. Sci. 2009, 56, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Khodaeiani, E.; Fouladi, R.F.; Amirnia, M.; Saeidi, M.; Karimi, E.R. Topical 4% Nicotinamide vs. 1% Clindamycin in Moderate Inflammatory Acne Vulgaris. Int. J. Dermatol. 2013, 52, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.R.; Liao, Z.B.; Tan, F.; Zhu, Z.Y.; Jiang, Y.; Cao, Y.Y. Effect of Nicotinamide against Candida albicans. Front. Microbiol. 2019, 10, 595. [Google Scholar] [CrossRef]
- Ciebiada-Adamiec, A.; Małafiej, E.; Ciebiada, I. Inhibitory Effect of Nicotinamide on Enzymatic Activity of Selected Fungal Strains Causing Skin Infection. Mycoses 2010, 53, 204–207. [Google Scholar] [CrossRef]
- Wurtele, H.; Tsao, S.; Lépine, G.; Mullick, A.; Tremblay, J.; Drogaris, P.; Lee, E.H.; Thibault, P.; Verreault, A.; Raymond, M. Modulation of Histone H3 Lysine 56 Acetylation as an Antifungal Therapeutic Strategy. Nat. Med. 2010, 16, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Carmona, J.J. Sirtuin-Mediated Mechanisms of Homeostasis and Aging in Metazoans. Ph.D. Thesis, Harvard University, Cambridge, MA, USA, 2010. [Google Scholar]
- Sauve, A.A.; Schramm, V.L. Sir2 Regulation by Nicotinamide Results from Switching between Base Exchange and Deacetylation Chemistry. Biochemistry 2003, 42, 9249–9256. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Q.; Zeng, L.; Huang, X. Protein Acetylation/Deacetylation: A Potential Strategy for Fungal Infection Control. Front. Microbiol. 2020, 11, 574736. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Jara, J.; Ortega, Á.; Lozano Terol, G.; Sola Martínez, R.A.; Cánovas Díaz, M.; de Diego Puente, T. Bacterial Sirtuins Overview: An Open Niche to Explore. Front. Microbiol. 2021, 12, 744416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sprung, R.; Pei, J.; Tan, X.; Kim, S.; Zhu, H.; Liu, C.F.; Grishin, N.V.; Zhao, Y. Lysine Acetylation Is a Highly Abundant and Evolutionarily Conserved Modification in Escherichia coli. Mol. Cell. Proteom. 2009, 8, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Thao, S.; Chen, C.S.; Zhu, H.; Escalante-Semerena, J.C. Nε-Lysine Acetylation of a Bacterial Transcription Factor Inhibits Its DNA-Binding Activity. PLoS ONE 2010, 5, e15123. [Google Scholar] [CrossRef]
- Gallego-Jara, J.; Conesa, A.É.; Puente, T.d.D.; Terol, G.L.; Díaz, M.C. Characterization of CobB Kinetics and Inhibition by Nicotinamide. PLoS ONE 2017, 12, e0189689. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.G.; Xie, X.; Basisty, N.; Byrnes, J.; McSweeney, S.; Schilling, B.; Wolfe, A.J. Post-Translational Protein Acetylation: An Elegant Mechanism for Bacteria to Dynamically Regulate Metabolic Functions. Front. Microbiol. 2019, 10, 1604. [Google Scholar] [CrossRef] [PubMed]
- Hocher, A.; Laursen, S.P.; Radford, P.; Tyson, J.; Lambert, C.; Stevens, K.M.; Picardeau, M.; Elizabeth Sockett, R.; Luger, K. Histone-Organized Chromatin in Bacteria. bioRxiv 2023. [Google Scholar] [CrossRef]
- Orban, K.; Finkel, S.E. Dps Is a Universally Conserved Dual-Action DNA-Binding and Ferritin Protein. J. Bacteriol. 2022, 204, e0003622. [Google Scholar] [CrossRef]
- Ziklo, N.; Bibi, M.; Salama, P. The Antimicrobial Mode of Action of Maltol and Its Synergistic Efficacy with Selected Cationic Surfactants. Cosmetics 2021, 8, 86–104. [Google Scholar] [CrossRef]
- AlSaleh, A.; Shahid, M.; Farid, E.; Kamal, N.; Bindayna, K. Synergistic Antimicrobial Effect of Ascorbic Acid and Nicotinamide with Rifampicin and Vancomycin against SCC Mec Type IV Methicillin-Resistant Staphylococcus aureus (MRSA). Access Microbiol. 2023, 5, 000475.v4. [Google Scholar] [CrossRef] [PubMed]
- Weiser, R.; Green, A.E.; Bull, M.J.; Cunningham-Oakes, E.; Jolley, K.A.; Maiden, M.C.J.; Hall, A.J.; Winstanley, C.; Weightman, A.J.; Donoghue, D.; et al. Not All Pseudomonas Aeruginosa Are Equal: Strains from Industrial Sources Possess Uniquely Large Multireplicon Genomes. Microb. Genom. 2019, 5, e000276. [Google Scholar] [CrossRef] [PubMed]
- Racki, L.R.; Tocheva, E.I.; Dieterle, M.G.; Sullivan, M.C.; Jensen, G.J.; Newman, D.K. Polyphosphate Granule Biogenesis Is Temporally and Functionally Tied to Cell Cycle Exit during Starvation in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2017, 114, E2440–E2449. [Google Scholar] [CrossRef] [PubMed]
- Nyström, T. Stationary-Phase Physiology. Annu. Rev. Microbiol. 2004, 58, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Lange, R.; Hengge-Aronis, R. Growth Phase-Regulated Expression of BolA and Morphology of Stationary-Phase Escherichia coli Cells Are Controlled by the Novel Sigma Factor σ(S). J. Bacteriol. 1991, 173, 4474–4481. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.P.; Clements, M.O.; Foster, S.J. Characterization of the Starvation-Survival Response of Staphylococcus aureus. J. Bacteriol. 1998, 180, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Shiratsuchi, A.; Shimamoto, N.; Nitta, M.; Tuan, T.Q.; Firdausi, A.; Gawasawa, M.; Yamamoto, K.; Ishihama, A.; Nakanishi, Y. Role for Σ38 in Prolonged Survival of Escherichia coli in Drosophila Melanogaster. J. Immunol. 2014, 192, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, J.; Srivastava, P. Molecular Basis of Stationary Phase Survival and Applications. Front. Microbiol. 2017, 8, 2000. [Google Scholar] [CrossRef]
- Huisman, G.W.; Siegele, D.A.; Zambrano, M.M.; Kolter, R. Morphological and Physiological Changes During Stationary Phase. In Escherichia coli and Salmonella: Cellular and Molecular Biology; Neidhardt, F.C., Ed.; ASM Press: Washington, DC, USA, 1996; Volume 2, pp. 1672–1682. [Google Scholar]
- Charoenwong, D.; Andrews, S.; Mackey, B. Role of RpoS in the Development of Cell Envelope Resilience and Pressure Resistance in Stationary-Phase Escherichia coli. Appl. Environ. Microbiol. 2011, 77, 5220–5229. [Google Scholar] [CrossRef]
- Jenkins, D.E.; Schultz, J.E.; Matin, A.; Latter, G.I. Starvation-Induced Cross Protection against Heat or H2O2 Challenge in Escherichia coli. J. Bacteriol. 1988, 170, 3910–3914. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.E.; Chaisson, S.A.; Matin, A. Starvation-Induced Cross Protection against Osmotic Challenge in Escherichia coli. J. Bacteriol. 1990, 172, 2779–2781. [Google Scholar] [CrossRef] [PubMed]
- Howell, M.; Daniel, J.J.; Brown, P.J.B. Live Cell Fluorescence Microscopy to Observe Essential Processes during Microbial Cell Growth. J. Vis. Exp. 2017, 24, 56497. [Google Scholar] [CrossRef]
- Sharpe, M.E.; Hauser, P.M.; Sharpe, R.G.; Errington, J. Bacillus Subtilis Cell Cycle as Studied by Fluorescence Microscopy: Constancy of Cell Length at Initiation of DNA Replication and Evidence for Active Nucleoid Partitioning. J. Bacteriol. 1998, 180, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, T.; Kukolj, E.; Brachner, A.; Beltzung, E.; Bruno, M.; Kostrhon, S.; Opravil, S.; Hudecz, O.; Mechtler, K.; Warren, G.; et al. SIRT2 Regulates Nuclear Envelope Reassembly via ANKLE2 Deacetylation. J. Cell Sci. 2016, 129, 4607–4621. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Park, Y.W.; Lee, M.Y.; Jeong, K.H.; Lee, J.Y. Structural Analysis and Insight into Effector Binding of the Niacin-Responsive Repressor NiaR from Bacillus halodurans. Sci. Rep. 2020, 10, 21039. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, J.B. Niacin Status Impacts Chromatin Structure. J. Nutr. 2009, 139, 2397–2401. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rouleau, M.; Aubin, R.A.; Poirier, G.G. Pol (ADP-Ribosyl)Ated Chromatin Domains: Access Granted. J. Cell Sci. 2004, 117, 815–825. [Google Scholar] [CrossRef] [PubMed]
- De Murcia, G.; Poirier, D.G.G.; Murcia, D.E. Review: Modulation of Chromatin Structure by Poly(ADP-Ribosyl)Ation. Biochem. Cell Biol. 1988, 66, 626–635. [Google Scholar] [CrossRef]
- Pletnev, P.; Osterman, I.; Sergiev, P.; Bogdanov, A.; Dontsova, O. Survival Guide: Escherichia coli in the Stationary Phase. Acta Naturae 2015, 7, 22–33. [Google Scholar] [CrossRef]
- Dorman, C.J. Function of Nucleoid-Associated Proteins in Chromosome Structuring and Transcriptional Regulation. J. Mol. Microbiol. Biotechnol. 2014, 24, 316–331. [Google Scholar] [CrossRef]
- Nair, S.; Finkel, S.E. Dps Protects Cells against Multiple Stresses during Stationary Phase. J. Bacteriol. 2004, 186, 4192–4198. [Google Scholar] [CrossRef]
- Srivastava, S.; Somasagara, R.R.; Hegde, M.; Nishana, M.; Tadi, S.K.; Srivastava, M.; Choudhary, B.; Raghavan, S.C. Quercetin, a Natural Flavonoid Interacts with DNA, Arrests Cell Cycle and Causes Tumor Regression by Activating Mitochondrial Pathway of Apoptosis. Sci. Rep. 2016, 6, 24049. [Google Scholar] [CrossRef]
- Xu, J.; Wang, G.A.; Gao, L.; Wu, L.; Lei, Q.; Deng, H.; Li, F. Enabling Programmable Dynamic DNA Chemistry Using Small-Molecule DNA Binders. Nat. Commun. 2023, 14, 4248. [Google Scholar] [CrossRef]
- Lipfert, J.; Klijnhout, S.; Dekker, N.H. Torsional Sensing of Small-Molecule Binding Using Magnetic Tweezers. Nucleic Acids Res. 2010, 38, 7122–7132. [Google Scholar] [CrossRef]
- Kolbeck, P.J.; Vanderlinden, W.; Nicolaus, T.; Gebhardt, C.; Cordes, T.; Lipfert, J. Intercalative DNA Binding Governs Fluorescence Enhancement of SYBR Gold. bioRxiv 2020. [Google Scholar] [CrossRef]
- Wang, J.C. The Degree of Unwinding of the DNA Helix by Ethidium I. Titration of Twisted PM2 DNA Molecules in Alkaline Cesium Chloride Density Gradients. J. Mol. Biol. 1974, 89, 783–801. [Google Scholar] [CrossRef]
- Perieteanu, M.C.; McGee, L.M.C.; Shaw, C.D.; MacMillan, D.S.; Khalaf, A.I.; Gillingwater, K.; Beveridge, R.; Carter, K.C.; Suckling, C.J.; Scott, F.J. Selective Anti-Leishmanial Strathclyde Minor Groove Binders Using an N-Oxide Tail-Group Modification. Int. J. Mol. Sci. 2022, 23, 11912. [Google Scholar] [CrossRef]


| Niacinamide (ppm) | ||
|---|---|---|
| Microorganism | MIC100 | MBC100 |
| E. coli | 30,000 | 60,000 |
| S. aureus | 25,000 | >60,000 |
| P. aeruginosa | 15,000 | 60,000 |
| C. albicans | 30,000 | 60,000 |
| A. brasiliensis | 40,000 | >60,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziklo, N.; Bibi, M.; Sinai, L.; Salama, P. Niacinamide Antimicrobial Efficacy and Its Mode of Action via Microbial Cell Cycle Arrest. Microorganisms 2024, 12, 1581. https://doi.org/10.3390/microorganisms12081581
Ziklo N, Bibi M, Sinai L, Salama P. Niacinamide Antimicrobial Efficacy and Its Mode of Action via Microbial Cell Cycle Arrest. Microorganisms. 2024; 12(8):1581. https://doi.org/10.3390/microorganisms12081581
Chicago/Turabian StyleZiklo, Noa, Maayan Bibi, Lior Sinai, and Paul Salama. 2024. "Niacinamide Antimicrobial Efficacy and Its Mode of Action via Microbial Cell Cycle Arrest" Microorganisms 12, no. 8: 1581. https://doi.org/10.3390/microorganisms12081581
APA StyleZiklo, N., Bibi, M., Sinai, L., & Salama, P. (2024). Niacinamide Antimicrobial Efficacy and Its Mode of Action via Microbial Cell Cycle Arrest. Microorganisms, 12(8), 1581. https://doi.org/10.3390/microorganisms12081581






