Lysine Phoshoglycerylation Is Widespread in Bacteria and Overlaps with Acylation
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Culture Conditions
2.2. Sample Preparation for Proteomics
2.3. Mass Spectrometry
2.4. Data Processing and Peptide and Protein Identification
2.5. Re-Analysis of Phosphoproteome Datasets from Other Bacteria
2.6. Additional Methods
3. Results and Discussion
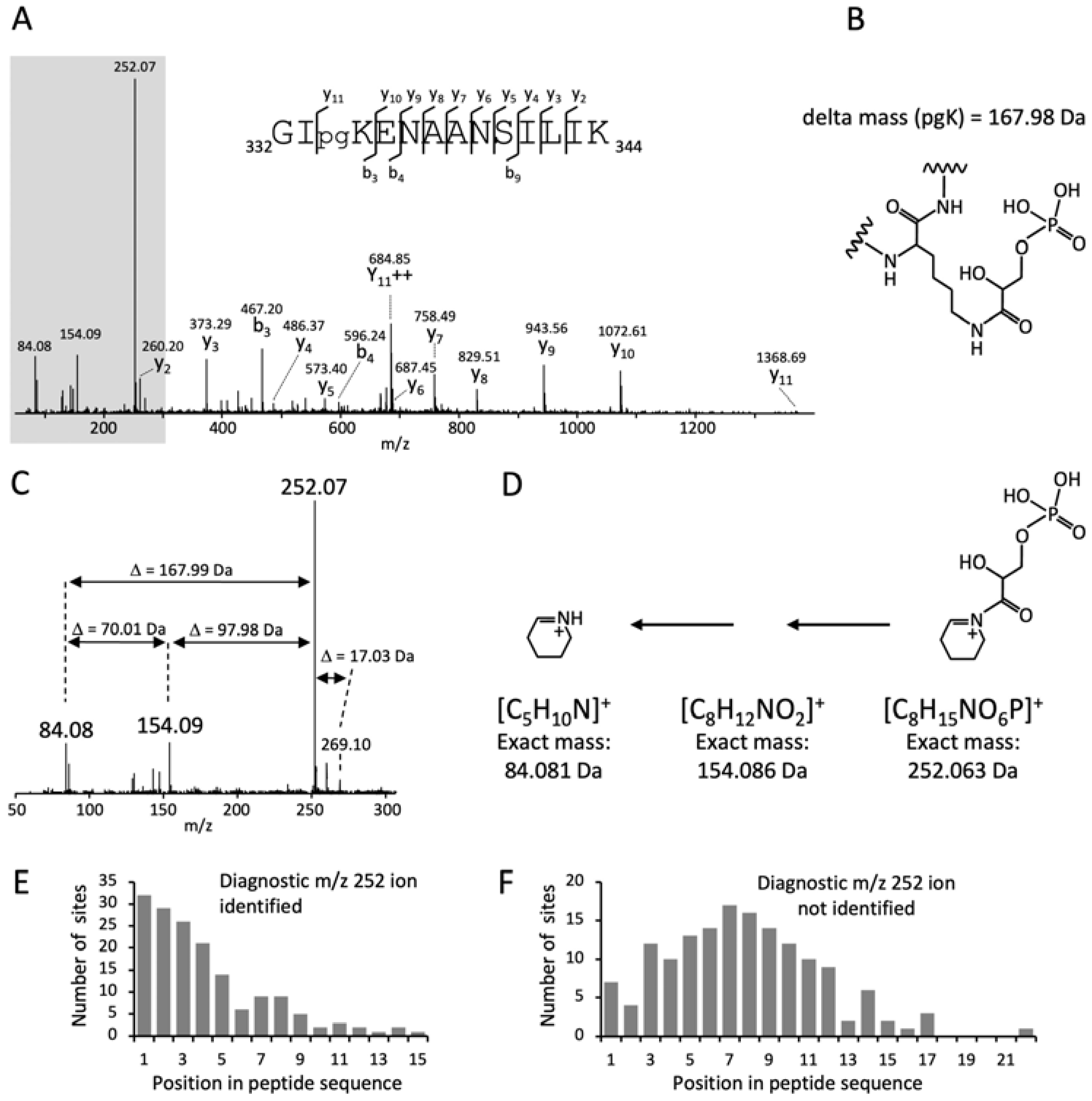
3.1. A Cyclic Immonium Ion of Phosphoglyceryl-Lysine Confirms Phosphoglycerylation
3.2. Identification of Phosphoglycerylation Depends on Protein Abundance
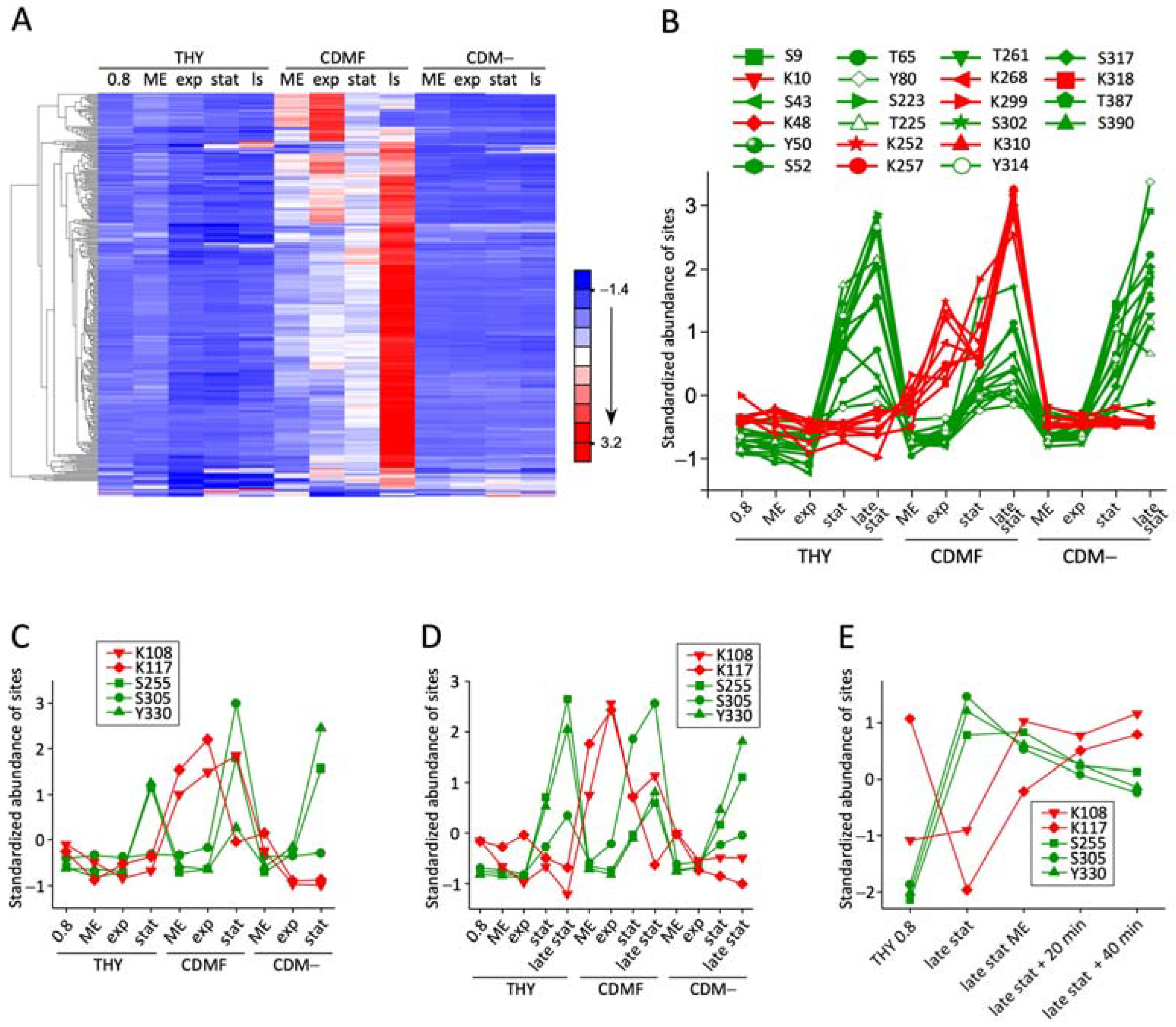
3.3. Phosphoglycerylation Accumulates during Growth with 1% Fructose
3.4. The Phosphoglyceryl Modification Is Mostly Low-Stoichiometric
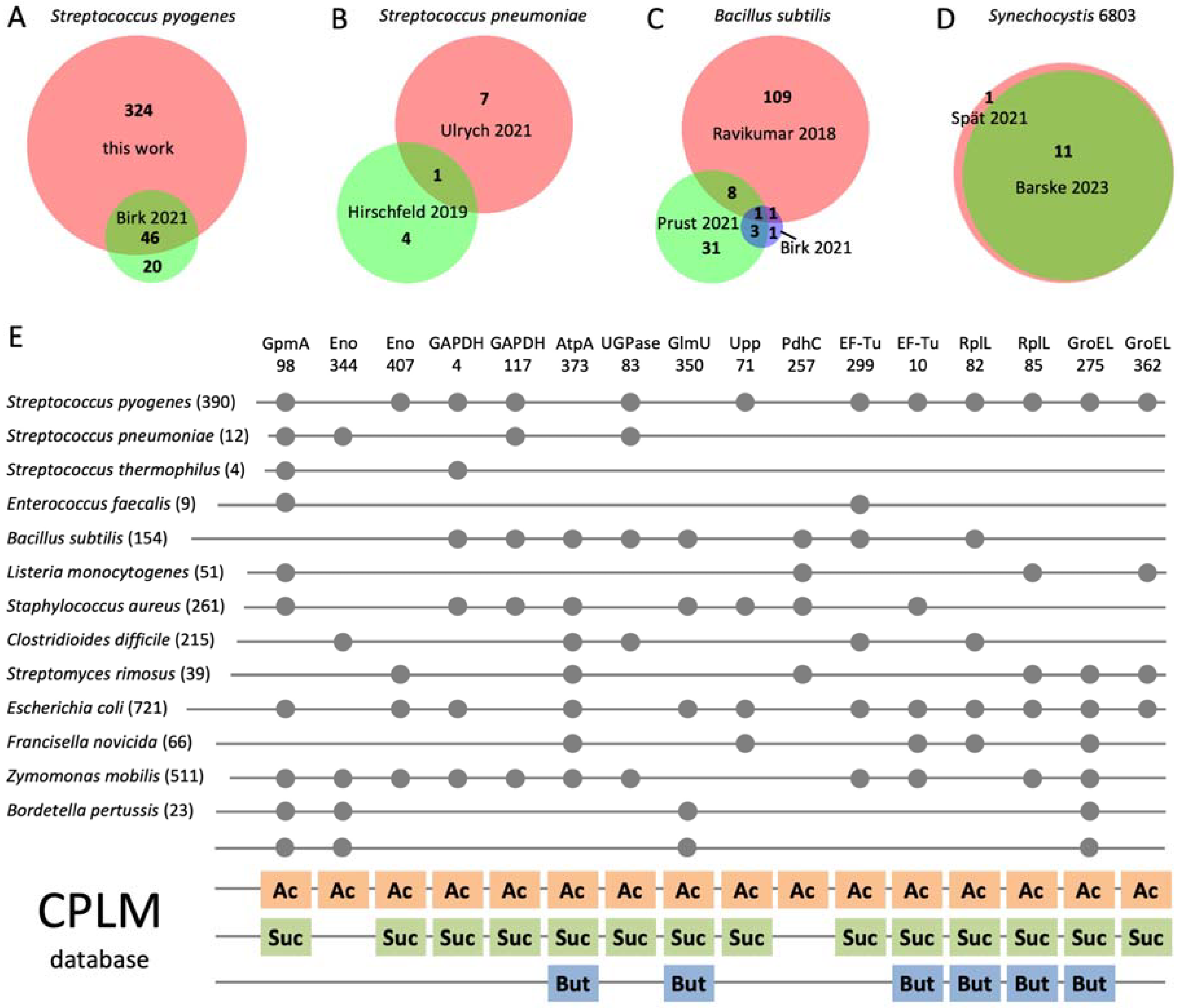
3.5. Phosphoglycerylation Is Conserved in Bacteria and Overlaps with Acylation
3.6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Macek, B.; Forchhammer, K.; Hardouin, J.; Weber-Ban, E.; Grangeasse, C.; Mijakovic, I. Protein post-translational modifications in bacteria. Nat. Reviews. Microbiol. 2019, 17, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.G.; Baumgartner, J.T.; Xie, X.; Jew, K.M.; Basisty, N.; Schilling, B.; Kuhn, M.L.; Wolfe, A.J. Mechanisms, Detection, and Relevance of Protein Acetylation in Prokaryotes. mBio 2019, 10, e02708-18. [Google Scholar] [CrossRef] [PubMed]
- Keenan, E.K.; Zachman, D.K.; Hirschey, M.D. Discovering the landscape of protein modifications. Mol. Cell 2021, 81, 1868–1878. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Guo, L.; Fu, Y.; Huo, M.; Qi, Q.; Zhao, G. Bacterial protein acetylation and its role in cellular physiology and metabolic regulation. Biotechnol. Adv. 2021, 53, 107842. [Google Scholar] [CrossRef] [PubMed]
- Nakayasu, E.S.; Burnet, M.C.; Walukiewicz, H.E.; Wilkins, C.S.; Shukla, A.K.; Brooks, S.; Plutz, M.J.; Lee, B.D.; Schilling, B.; Wolfe, A.J.; et al. Ancient Regulatory Role of Lysine Acetylation in Central Metabolism. mBio 2017, 8, e01894-17. [Google Scholar] [CrossRef] [PubMed]
- Popova, L.; Carr, R.A.; Carabetta, V.J. Recent Contributions of Proteomics to Our Understanding of Reversible N(epsilon)-Lysine Acylation in Bacteria. J. Proteome Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Boel, G.; Pichereau, V.; Mijakovic, I.; Maze, A.; Poncet, S.; Gillet, S.; Giard, J.C.; Hartke, A.; Auffray, Y.; Deutscher, J. Is 2-phosphoglycerate-dependent automodification of bacterial enolases implicated in their export? J. Mol. Biol. 2004, 337, 485–496. [Google Scholar] [CrossRef]
- Moellering, R.E.; Cravatt, B.F. Functional lysine modification by an intrinsically reactive primary glycolytic metabolite. Science 2013, 341, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.A.; Sharma, A.; Dehzangi, A.; Tsunoda, T. RAM-PGK: Prediction of Lysine Phosphoglycerylation Based on Residue Adjacency Matrix. Genes 2020, 11, 1524. [Google Scholar] [CrossRef]
- Zhang, W.; Tan, X.; Lin, S.; Gou, Y.; Han, C.; Zhang, C.; Ning, W.; Wang, C.; Xue, Y. CPLM 4.0: An updated database with rich annotations for protein lysine modifications. Nucleic Acids Res. 2022, 50, D451–D459. [Google Scholar] [CrossRef]
- Molto, E.; Pintado, C.; Louzada, R.A.; Bernal-Mizrachi, E.; Andres, A.; Gallardo, N.; Bonzon-Kulichenko, E. Unbiased Phosphoproteome Mining Reveals New Functional Sites of Metabolite-Derived PTMs Involved in MASLD Development. Int. J. Mol. Sci. 2023, 24, 16172. [Google Scholar] [CrossRef] [PubMed]
- Willems, P.; Sterck, L.; Dard, A.; Huang, J.; De Smet, I.; Gevaert, K.; Van Breusegem, F. The Plant PTM Viewer 2.0: In-depth exploration of plant protein modification landscapes. J. Exp. Bot. 2024, erae270. [Google Scholar] [CrossRef]
- Mikkat, S.; Kreutzer, M.; Patenge, N. Dynamic Protein Phosphorylation in Streptococcus pyogenes during Growth, Stationary Phase, and Starvation. Microorganisms 2024, 12, 621. [Google Scholar] [CrossRef]
- Kaufhold, A.; Podbielski, A.; Johnson, D.R.; Kaplan, E.L.; Lutticken, R. M protein gene typing of Streptococcus pyogenes by nonradioactively labeled oligonucleotide probes. J. Clin. Microbiol. 1992, 30, 2391–2397. [Google Scholar] [CrossRef] [PubMed]
- van de Rijn, I.; Kessler, R.E. Growth characteristics of group A streptococci in a new chemically defined medium. Infect. Immun. 1980, 27, 444–448. [Google Scholar] [CrossRef]
- Klahn, S.; Mikkat, S.; Riediger, M.; Georg, J.; Hess, W.R.; Hagemann, M. Integrative analysis of the salt stress response in cyanobacteria. Biol. Direct 2021, 16, 26. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; Garcia-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef]
- Okuda, S.; Watanabe, Y.; Moriya, Y.; Kawano, S.; Yamamoto, T.; Matsumoto, M.; Takami, T.; Kobayashi, D.; Araki, N.; Yoshizawa, A.C.; et al. jPOSTrepo: An international standard data repository for proteomes. Nucleic Acids Res. 2017, 45, D1107–D1111. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.C.; Stucky, M.; Wakefield, C.; Melott, J.M.; Akbani, R.; Weinstein, J.N.; Broom, B.M. Interactive Clustered Heat Map Builder: An easy web-based tool for creating sophisticated clustered heat maps. F1000Research 2020, 8, 1750. [Google Scholar] [CrossRef]
- Hulsen, T.; de Vlieg, J.; Alkema, W. BioVenn—A web application for the comparison and visualization of biological lists using area-proportional Venn diagrams. BMC Genom. 2008, 9, 488. [Google Scholar] [CrossRef]
- Sievers, F.; Higgins, D.G. The Clustal Omega Multiple Alignment Package. Methods Mol. Biol. 2021, 2231, 3–16. [Google Scholar] [CrossRef]
- Creasy, D.M.; Cottrell, J.S. Error tolerant searching of uninterpreted tandem mass spectrometry data. Proteomics 2002, 2, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Tholey, A.; Reed, J.; Lehmann, W.D. Electrospray tandem mass spectrometric studies of phosphopeptides and phosphopeptide analogues. J. Mass Spectrom. 1999, 34, 117–123. [Google Scholar] [CrossRef]
- Couttas, T.A.; Raftery, M.J.; Bernardini, G.; Wilkins, M.R. Immonium ion scanning for the discovery of post-translational modifications and its application to histones. J. Proteome Res. 2008, 7, 2632–2641. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.W.; Schlosser, A.; Wei, J.; Lehmann, W.D. Collision-induced reporter fragmentations for identification of covalently modified peptides. Anal. Bioanal. Chem. 2007, 389, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Fenaille, F.; Tabet, J.C.; Guy, P.A. Study of peptides containing modified lysine residues by tandem mass spectrometry: Precursor ion scanning of hexanal-modified peptides. Rapid Commun. Mass Spectrom. 2004, 18, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, T.; Harrison, A.G. Ion chemistry of protonated lysine derivatives. J. Mass Spectrom. 1996, 31, 1237–1243. [Google Scholar] [CrossRef]
- Muroski, J.M.; Fu, J.Y.; Nguyen, H.H.; Ogorzalek Loo, R.R.; Loo, J.A. Leveraging Immonium Ions for Targeting Acyl-Lysine Modifications in Proteomic Datasets. Proteomics 2021, 21, e2000111. [Google Scholar] [CrossRef] [PubMed]
- Muroski, J.M.; Fu, J.Y.; Nguyen, H.H.; Wofford, N.Q.; Mouttaki, H.; James, K.L.; McInerney, M.J.; Gunsalus, R.P.; Loo, J.A.; Ogorzalek Loo, R.R. The Acyl-Proteome of Syntrophus aciditrophicus Reveals Metabolic Relationships in Benzoate Degradation. Mol. Cell. Proteom. 2022, 21, 100215. [Google Scholar] [CrossRef]
- Trelle, M.B.; Jensen, O.N. Utility of immonium ions for assignment of epsilon-N-acetyllysine-containing peptides by tandem mass spectrometry. Anal. Chem. 2008, 80, 3422–3430. [Google Scholar] [CrossRef]
- Wan, N.; Wang, N.; Yu, S.; Zhang, H.; Tang, S.; Wang, D.; Lu, W.; Li, H.; Delafield, D.G.; Kong, Y.; et al. Cyclic immonium ion of lactyllysine reveals widespread lactylation in the human proteome. Nat. Methods 2022, 19, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Zolg, D.P.; Wilhelm, M.; Schmidt, T.; Medard, G.; Zerweck, J.; Knaute, T.; Wenschuh, H.; Reimer, U.; Schnatbaum, K.; Kuster, B. ProteomeTools: Systematic Characterization of 21 Post-translational Protein Modifications by Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS) Using Synthetic Peptides. Mol. Cell. Proteom. 2018, 17, 1850–1863. [Google Scholar] [CrossRef] [PubMed]
- Hunt, D.F.; Yates, J.R., 3rd; Shabanowitz, J.; Winston, S.; Hauer, C.R. Protein sequencing by tandem mass spectrometry. Proc. Natl. Acad. Sci. USA 1986, 83, 6233–6237. [Google Scholar] [CrossRef] [PubMed]
- Schilling, B.; Basisty, N.; Christensen, D.G.; Sorensen, D.; Orr, J.S.; Wolfe, A.J.; Rao, C.V. Global Lysine Acetylation in Escherichia coli Results from Growth Conditions That Favor Acetate Fermentation. J. Bacteriol. 2019, 201, e00768-18. [Google Scholar] [CrossRef] [PubMed]
- Birk, M.S.; Charpentier, E.; Frese, C.K. Automated Phosphopeptide Enrichment for Gram-Positive Bacteria. J. Proteome Res. 2021, 20, 4886–4892. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, C.; Gomez-Mejia, A.; Bartel, J.; Hentschker, C.; Rohde, M.; Maass, S.; Hammerschmidt, S.; Becher, D. Proteomic Investigation Uncovers Potential Targets and Target Sites of Pneumococcal Serine-Threonine Kinase StkP and Phosphatase PhpP. Front. Microbiol. 2019, 10, 3101. [Google Scholar] [CrossRef] [PubMed]
- Ulrych, A.; Fabrik, I.; Kupcik, R.; Vajrychova, M.; Doubravova, L.; Branny, P. Cell Wall Stress Stimulates the Activity of the Protein Kinase StkP of Streptococcus pneumoniae, Leading to Multiple Phosphorylation. J. Mol. Biol. 2021, 433, 167319. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, V.; Nalpas, N.C.; Anselm, V.; Krug, K.; Lenuzzi, M.; Sestak, M.S.; Domazet-Loso, T.; Mijakovic, I.; Macek, B. In-depth analysis of Bacillus subtilis proteome identifies new ORFs and traces the evolutionary history of modified proteins. Sci. Rep. 2018, 8, 17246. [Google Scholar] [CrossRef] [PubMed]
- Prust, N.; van der Laarse, S.; van den Toorn, H.W.P.; van Sorge, N.M.; Lemeer, S. In-Depth Characterization of the Staphylococcus aureus Phosphoproteome Reveals New Targets of Stk1. Mol. Cell. Proteom. 2021, 20, 100034. [Google Scholar] [CrossRef]
- Spat, P.; Barske, T.; Macek, B.; Hagemann, M. Alterations in the CO2 availability induce alterations in the phosphoproteome of the cyanobacterium Synechocystis sp. PCC 6803. New Phytol. 2021, 231, 1123–1137. [Google Scholar] [CrossRef]
- Barske, T.; Spat, P.; Schubert, H.; Walke, P.; Macek, B.; Hagemann, M. The Role of Serine/Threonine-Specific Protein Kinases in Cyanobacteria—SpkB Is Involved in Acclimation to Fluctuating Conditions in Synechocystis sp. PCC 6803. Mol. Cell. Proteom. 2023, 22, 100656. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.; Haller, L.; Blein-Nicolas, M.; Zivy, M.; Canette, A.; Verbrugghe, M.; Mezange, C.; Boulay, M.; Gardan, R.; Samson, S.; et al. Identification of Hanks-Type Kinase PknB-Specific Targets in the Streptococcus thermophilus Phosphoproteome. Front. Microbiol. 2019, 10, 1329. [Google Scholar] [CrossRef] [PubMed]
- Iannetta, A.A.; Minton, N.E.; Uitenbroek, A.A.; Little, J.L.; Stanton, C.R.; Kristich, C.J.; Hicks, L.M. IreK-Mediated, Cell Wall-Protective Phosphorylation in Enterococcus faecalis. J. Proteome Res. 2021, 20, 5131–5144. [Google Scholar] [CrossRef] [PubMed]
- Smits, W.K.; Mohammed, Y.; de Ru, A.H.; Cordo, V.; Friggen, A.H.; van Veelen, P.A.; Hensbergen, P.J. Clostridioides difficile Phosphoproteomics Shows an Expansion of Phosphorylated Proteins in Stationary Growth Phase. mSphere 2022, 7, e0091121. [Google Scholar] [CrossRef]
- Saric, E.; Quinn, G.A.; Nalpas, N.; Paradzik, T.; Kazazic, S.; Filic, Z.; Semanjski, M.; Herron, P.; Hunter, I.; Macek, B.; et al. Phosphoproteome Dynamics of Streptomyces rimosus during Submerged Growth and Antibiotic Production. mSystems 2022, 7, e0019922. [Google Scholar] [CrossRef] [PubMed]
- Potel, C.M.; Lin, M.H.; Heck, A.J.R.; Lemeer, S. Widespread bacterial protein histidine phosphorylation revealed by mass spectrometry-based proteomics. Nat. Methods 2018, 15, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Ziveri, J.; Chhuon, C.; Jamet, A.; Rytter, H.; Prigent, G.; Tros, F.; Barel, M.; Coureuil, M.; Lays, C.; Henry, T.; et al. Critical Role of a Sheath Phosphorylation Site On the Assembly and Function of an Atypical Type VI Secretion System. Mol. Cell. Proteom. 2019, 18, 2418–2432. [Google Scholar] [CrossRef] [PubMed]
- Tatli, M.; Hebert, A.S.; Coon, J.J.; Amador-Noguez, D. Genome Wide Phosphoproteome Analysis of Zymomonas mobilis Under Anaerobic, Aerobic, and N(2)-Fixing Conditions. Front. Microbiol. 2019, 10, 1986. [Google Scholar] [CrossRef] [PubMed]
- Luu, L.D.W.; Zhong, L.; Kaur, S.; Raftery, M.J.; Lan, R. Comparative Phosphoproteomics of Classical Bordetellae Elucidates the Potential Role of Serine, Threonine and Tyrosine Phosphorylation in Bordetella Biology and Virulence. Front. Cell. Infect. Microbiol. 2021, 11, 660280. [Google Scholar] [CrossRef]
- Pearson, C.L.; Loshon, C.A.; Pedersen, L.B.; Setlow, B.; Setlow, P. Analysis of the function of a putative 2,3-diphosphoglyceric acid-dependent phosphoglycerate mutase from Bacillus subtilis. J. Bacteriol. 2000, 182, 4121–4123. [Google Scholar] [CrossRef][Green Version]
- Weinert, B.T.; Scholz, C.; Wagner, S.A.; Iesmantavicius, V.; Su, D.; Daniel, J.A.; Choudhary, C. Lysine succinylation is a frequently occurring modification in prokaryotes and eukaryotes and extensively overlaps with acetylation. Cell Rep. 2013, 4, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, H.; Guo, Z.; Zou, S.; Long, F.; Wu, J.; Li, P.; Zhao, G.P.; Zhao, W. Global Insights Into Lysine Acylomes Reveal Crosstalk between Lysine Acetylation and Succinylation in Streptomyces coelicolor Metabolic Pathways. Mol. Cell. Proteom. 2021, 20, 100148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, T.; Wang, L.; Huang, Y.; Fan, R.; Ma, K.; Kan, Y.; Tan, M.; Xu, J.Y. Global landscape of lysine acylomes in Bacillus subtilis. J. Proteom. 2023, 271, 104767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, J.; Dong, Q.; Zhu, J.; Peng, R.; He, C.; Li, Y.; Lin, R.; Jiang, P.; Zheng, M.; et al. Lysine Acylation Modification Landscape of Brucella abortus Proteome and its Virulent Proteins. Front. Cell Dev. Biol. 2022, 10, 839822. [Google Scholar] [CrossRef] [PubMed]
- Mo, R.; Yang, M.; Chen, Z.; Cheng, Z.; Yi, X.; Li, C.; He, C.; Xiong, Q.; Chen, H.; Wang, Q.; et al. Acetylome analysis reveals the involvement of lysine acetylation in photosynthesis and carbon metabolism in the model cyanobacterium Synechocystis sp. PCC 6803. J. Proteome Res. 2015, 14, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Nisar, A.; Gongye, X.; Huang, Y.; Khan, S.; Chen, M.; Wu, B.; He, M. Genome-Wide Analyses of Proteome and Acetylome in Zymomonas mobilis Under N2-Fixing Condition. Front. Microbiol. 2021, 12, 740555. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Y.; Xu, Z.; Liu, X.; Tan, M.; Ye, B.C. Protein Acetylation and Butyrylation Regulate the Phenotype and Metabolic Shifts of the Endospore-forming Clostridium acetobutylicum. Mol. Cell. Proteom. 2018, 17, 1156–1169. [Google Scholar] [CrossRef]
- Brunk, E.; Chang, R.L.; Xia, J.; Hefzi, H.; Yurkovich, J.T.; Kim, D.; Buckmiller, E.; Wang, H.H.; Cho, B.K.; Yang, C.; et al. Characterizing posttranslational modifications in prokaryotic metabolism using a multiscale workflow. Proc. Natl. Acad. Sci. USA 2018, 115, 11096–11101. [Google Scholar] [CrossRef]
- Fatema, N.; Li, X.; Gan, Q.; Fan, C. Characterizing lysine acetylation of glucokinase. Protein Sci. 2024, 33, e4845. [Google Scholar] [CrossRef]
- Schastnaya, E.; Doubleday, P.F.; Maurer, L.; Sauer, U. Non-enzymatic acetylation inhibits glycolytic enzymes in Escherichia coli. Cell Rep. 2023, 42, 111950. [Google Scholar] [CrossRef]
- Venkat, S.; Chen, H.; Stahman, A.; Hudson, D.; McGuire, P.; Gan, Q.; Fan, C. Characterizing Lysine Acetylation of Isocitrate Dehydrogenase in Escherichia coli. J. Mol. Biol. 2018, 430, 1901–1911. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikkat, S.; Kreutzer, M.; Patenge, N. Lysine Phoshoglycerylation Is Widespread in Bacteria and Overlaps with Acylation. Microorganisms 2024, 12, 1556. https://doi.org/10.3390/microorganisms12081556
Mikkat S, Kreutzer M, Patenge N. Lysine Phoshoglycerylation Is Widespread in Bacteria and Overlaps with Acylation. Microorganisms. 2024; 12(8):1556. https://doi.org/10.3390/microorganisms12081556
Chicago/Turabian StyleMikkat, Stefan, Michael Kreutzer, and Nadja Patenge. 2024. "Lysine Phoshoglycerylation Is Widespread in Bacteria and Overlaps with Acylation" Microorganisms 12, no. 8: 1556. https://doi.org/10.3390/microorganisms12081556
APA StyleMikkat, S., Kreutzer, M., & Patenge, N. (2024). Lysine Phoshoglycerylation Is Widespread in Bacteria and Overlaps with Acylation. Microorganisms, 12(8), 1556. https://doi.org/10.3390/microorganisms12081556





