Innovative Microbial Immobilization Strategy for Di-n-Butyl Phthalate Biodegradation Using Biochar-Calcium Alginate-Waterborne Polyurethane Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Isolation and Identification of DBP-Degrading Bacteria
2.3. Preparation and Characterization of Biochar
2.3.1. Biochar Production
2.3.2. Basic Properties of the RH and RHB
2.3.3. Chemical Compositions Analysis
2.4. Immobilization Methods
2.5. Analysis of Microbial Immobilized Particles
2.6. Experimental Design
2.7. Analysis of Residual DBP
2.8. Algal Biotoxicity Assessments
2.9. Microbial Community Analysis
2.10. Statistical Analyses
3. Results
3.1. Identification of DBP-Degrading Strain
3.2. Basic Properties of Biochar
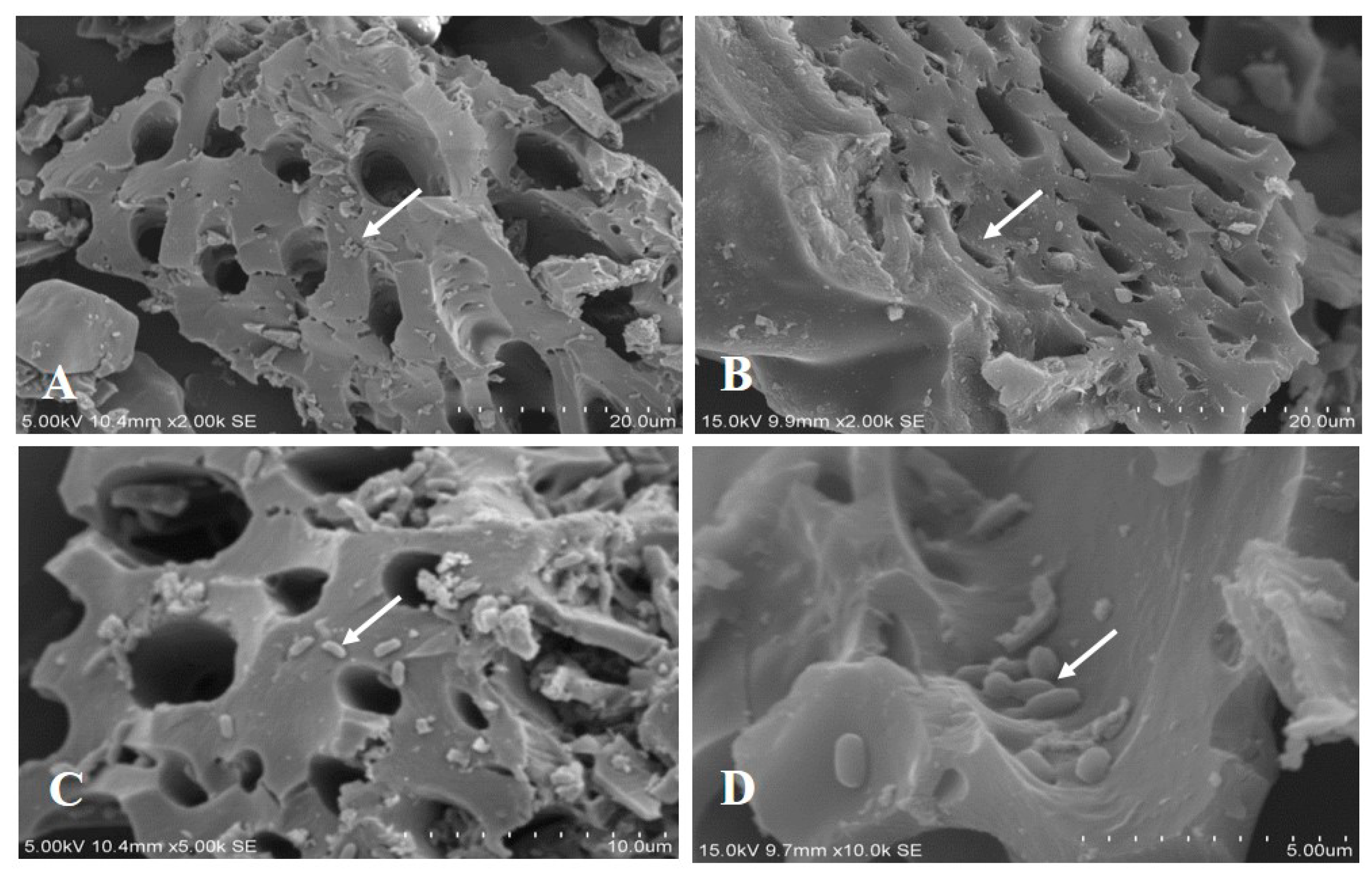
3.3. Characterization of Microbial Immobilized Particles
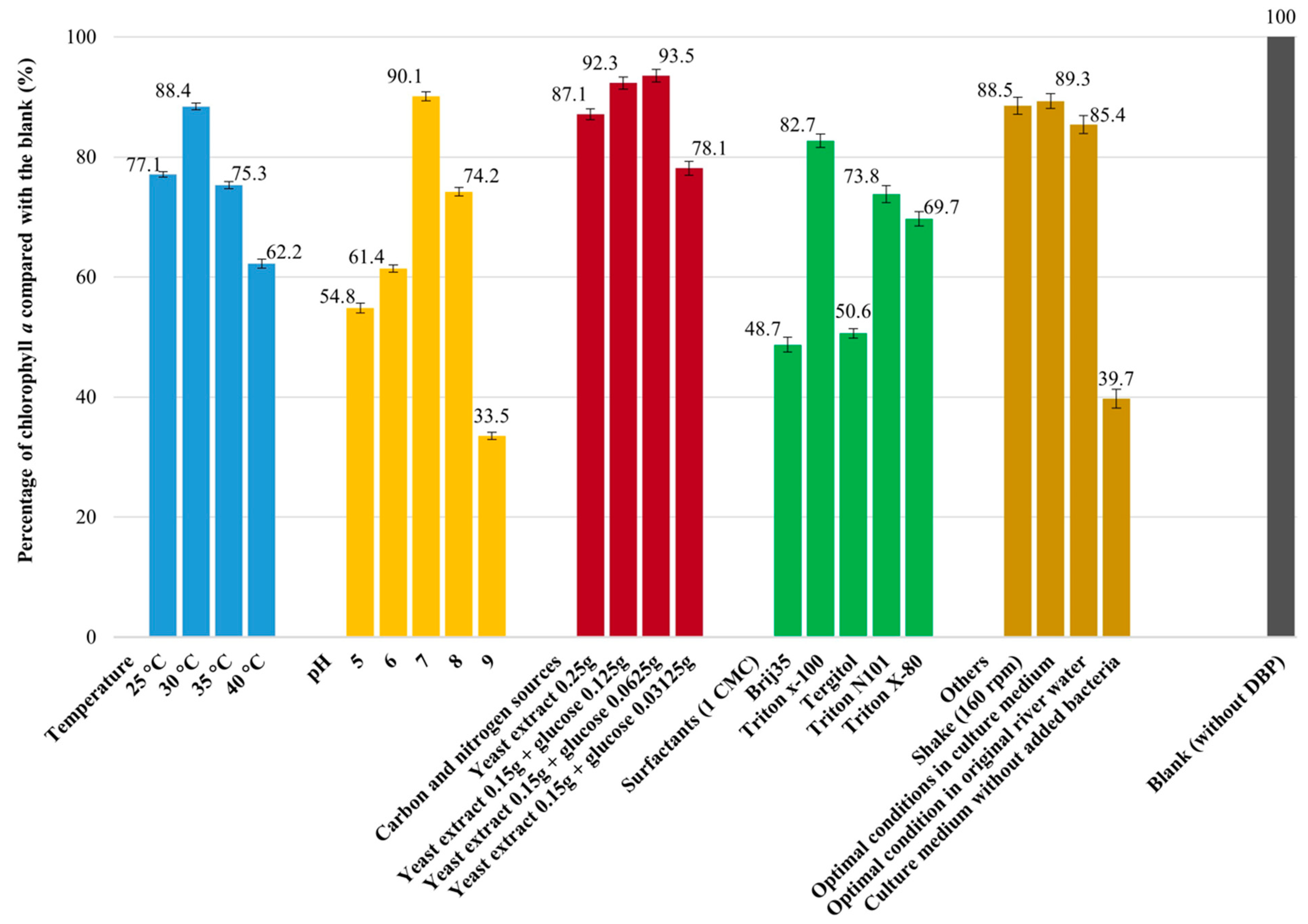
3.4. Optimal Cultural Conditions of DBP Biodegradation
3.5. Algal Biotoxicity of DBP
3.6. Microbial Community Analysis
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Wang, P.; Wang, L.; Sun, G.; Zhao, J.; Zhang, H.; Du, N. The influence of facility agriculture production on phthalate esters distribution in black soils of northeast China. Sci. Total Environ. 2015, 506–507, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Katsikantami, I.; Sifakis, S.; Tzatzarakis, M.N.; Vakonaki, E.; Kalantzi, O.I.; Tsatsakis, A.M.; Rizos, A.K. A global assessment of phthalates burden and related links to health effects. Environ. Int. 2016, 97, 212–236. [Google Scholar] [CrossRef]
- Paluselli, A.; Fauvelle, V.; Galgani, F.; Sempere, R. Phthalate release from plastic fragments and degradation in seawater. Environ. Sci. Technol. 2019, 53, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Perestrelo, R.; Silva, C.L.; Algarra, M.; Câmara, J.S. Evaluation of the occurrence of phthalates in plastic materials used in food packaging. Appl. Sci. 2021, 11, 2130. [Google Scholar] [CrossRef]
- Tumu, K.; Vorst, K.; Curtzwiler, G. Endocrine modulating chemicals in food packaging: A review of phthalates and bisphenols. Compr. Rev. Food. Sci. Food Saf. 2023, 22, 1337–1359. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Akhbarizadeh, R.; Dobaradarana, S.; Schmidtd, T.C.; Nabipourb, I.; Spitze, J. Worldwide bottled water occurrence of emerging contaminants: A review of the recent scientific literature. J. Hazard. Mater. 2020, 392, 122271. [Google Scholar] [CrossRef]
- Rajput, S.; Kumari, A.; Sharma, R.; Rajput, V.D.; Minkina, T.; Arora, S.; Rajinder, K.A.U.R. Seasonal fluctuations in phthalates’ contamination in pond water: A case study. Eurasian. Soil Sci. 2023, 12, 19–27. [Google Scholar] [CrossRef]
- Jafarabadi, A.R.; Dashtbozorg, M.; Raudonytė-Svirbutavičienė, E.; Bakhtiari, A.R. A potential threat to the coral reef environments: Polybrominated diphenyl ethers and phthalate esters in the corals and their ambient environment (Persian Gulf, Iran). Sci. Total Environ. 2021, 775, 145822. [Google Scholar] [CrossRef]
- Ai, S.; Gao, X.; Wang, X.; Li, J.; Fan, B.; Zhao, S.; Liu, Z. Exposure and tiered ecological risk assessment of phthalate esters in the surface water of Poyang Lake, China. Chemosphere 2021, 262, 127864. [Google Scholar] [CrossRef]
- Heo, H.; Choi, M.J.; Park, J.; Nam, T.; Cho, J. Anthropogenic occurrence of phthalate esters in beach seawater in the southeast coast region, South Korea. Water 2019, 12, 122. [Google Scholar] [CrossRef]
- Lee, C.C.; Hsieh, C.Y.; Chen, C.S.; Tien, C.J. Emergent contaminants in sediments and fishes from the Tamsui River (Taiwan): Their spatial-temporal distribution and risk to aquatic ecosystems and human health. Environ. Pollut. 2020, 258, 113733. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.Y.; Liu, C.; Liao, C.S.; Chang, B.V. Occurrence and microbial degradation of phthalate esters in Taiwan river sediments. Chemosphere 2002, 49, 1295–1299. [Google Scholar] [CrossRef] [PubMed]
- Chemical Manufacturers Association. Comments of the Chemical Manufacturers Association Phthalate Esters Panel in Response to Request for Public Input on Seven Phthalate Esters; FR Doc 99–9484; Chemical Manufacturers Association: Washington, DC, USA, 1999. [Google Scholar]
- Ding, M.; Kang, Q.; Zhang, S.; Zhao, F.; Mu, D.; Zhang, H.; Yang, M.; Hu, J. Contribution of phthalates and phthalate monoesters from drinking water to daily intakes for the general population. Chemosphere 2019, 229, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Fu, J.; Zhou, S.; Zhao, P.; Wu, X.; Tang, S.; Zhang, Z. Rapid recognition of di-n-butyl phthalate in food samples with a near infrared fluorescence imprinted sensor based on zeolite imidazolate framework-67. Food. Chem. 2022, 367, 130505. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, Z.; Ren, Z.; Ding, Y.; Pan, J.; Wang, Y.; Jin, D. Effects of di-n-butyl phthalate on aerobic composting process of agricultural waste: Mainly based on bacterial biomass and community dynamics analysis. Environ. Res. 2022, 212, 113290. [Google Scholar] [CrossRef] [PubMed]
- Kelley, K.E.; Hernández-Díaz, S.; Chaplin, E.L.; Hauser, R.; Mitchell, A.A. Identification of phthalates in medications and dietary supplement formulations in the United States and Canada. Environ. Health Perspect. 2011, 120, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Kaur, R.; Sharma, R.; Kaur, R. Assessment of toxicological effects of di-n-butyl phthalate to a cereal crop (Hordeum vulgare L.). J. Adv. Agric. Technol. 2019, 6, 1. [Google Scholar] [CrossRef]
- Gao, M.; Xu, Y.; Liu, Y.; Wang, S.; Wang, C.; Dong, Y.; Song, Z. Effect of polystyrene on di-butyl phthalate (DBP) bioavailability and DBP-induced phytotoxicity in lettuce. Environ. Pollut. 2021, 268, 115870. [Google Scholar] [CrossRef]
- Kassab, R.B.; Lokman, M.S.; Essawy, E.A. Neurochemical alterations following the exposure to di-n-butyl phthalate in rats. Metab. Brain Dis. 2019, 34, 235–244. [Google Scholar] [CrossRef]
- Liao, C.S.; Hong, Y.H.; Nishikawa, Y.; Kage-Nakadai, E.; Chiou, T.Y.; Wu, C.C. Impacts of Endocrine Disruptor di-n-Butyl Phthalate Ester on Microalga Chlorella vulgaris Verified by Approaches of Proteomics and Gene Ontology. Molecules 2020, 25, 4304. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.; Ahmad, B.; Alarjani, K.M.; saleem Aldosri, N.; Khan, M.S. Biostimulation of Rhodovulum sp., for enhanced degradation of di-n-butyl phthalate under optimum conditions. Chemosphere 2021, 266, 128998. [Google Scholar] [CrossRef]
- Feng, N.X.; Feng, Y.X.; Liang, Q.F.; Chen, X.; Xiang, L.; Zhao, H.M.; Liu, B.L.; Cao, G.; Li, Y.W.; Li, H.; et al. Complete biodegradation of di-n-butyl phthalate (DBP) by a novel Pseudomonas sp. YJB6. Sci. Total Environ. 2021, 761, 143208. [Google Scholar] [CrossRef]
- Kong, X.; Jin, D.; Tai, X.; Yu, H.; Duan, G.; Yan, X.; Pan, J.; Song, J.; Deng, Y. Bioremediation of dibutyl phthalate in a simulated agricultural ecosystem by Gordonia sp. strain QH-11 and the microbial ecological effects in soil. Sci. Total Environ. 2019, 667, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Mphahlele, I.J.; Malinga, S.P.; Dlamini, L.N. Combined biological and photocatalytic degradation of dibutyl phthalate in a simulated wastewater treatment plant. Catalysts 2022, 12, 504. [Google Scholar] [CrossRef]
- Liu, H.; Li, H.; Fang, C.; Mao, H.; Xue, X.; Wang, Q. Removal of Di-n-butyl phthalate from aged leachate under optimal hydraulic condition of leachate treatment process and in the presence of its dominant bacterial strains. Ecotoxicol. Environ. Saf. 2021, 222, 112532. [Google Scholar] [CrossRef]
- Ziembowicz, S.; Kida, M.; Koszelnik, P.; Czarnota, J.; Miąsik, M. Fenton-like degradation of di-n-butyl phthalate in landfill leachate by endogenous catalysts or iron, copper and manganese loaded bottom sediments. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100551. [Google Scholar] [CrossRef]
- Bouabidi, Z.B.; El-Naas, M.H.; Zhang, Z. Immobilization of microbial cells for the biotreatment of wastewater: A review. Environ. Chem. Lett. 2019, 17, 241–257. [Google Scholar] [CrossRef]
- Bera, S.; Mohanty, K. Areca nut (Areca catechu) husks and Luffa (Luffa cylindrica) sponge as microbial immobilization matrices for efficient phenol degradation. J. Water. Process Eng. 2020, 33, 100999. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, L.H.; Zhou, Y.; Ye, B.C. Enhanced biodegradation of di-n-butyl phthalate by Acinetobacter species strain LMB-5 coated with magnetic nanoparticles. Int. Biodeter Biodegr. 2017, 116, 184–190. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Luo, H.; Chen, Q.; Zhu, Z.; Chen, W.; Mo, Y. Bacterial community dynamics and enhanced degradation of di-n-octyl phthalate (DOP) by corncob-sodium alginate immobilized bacteria. Geoderma 2017, 305, 264–274. [Google Scholar] [CrossRef]
- Wang, P.; Gao, J.; Zhao, Y.; Zhang, M.; Zhou, S. Biodegradability of di-(2-ethylhexyl) phthalate by a newly isolated bacterium Achromobacter sp. RX. Sci. Total Environ. 2021, 755, 142476. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, B.; Niu, A.; Cheng, N.; Chen, M.; Zhang, X.; Yu, Z.; Wang, S. Application of biochar immobilized microorganisms for pollutants removal from wastewater: A review. Sci. Total Environ. 2022, 837, 155563. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.L.; Fendorf, S.E.; Toner IV, C.V.; Carski, T.H. Kinetic methods and measurements. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 1275–1307. [Google Scholar]
- Sumner, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 1201–1229. [Google Scholar]
- Łapczyńska-Kordon, B.; Ślipek, Z.; Słomka-Polonis, K.; Styks, J.; Hebda, T.; Francik, S. Physicochemical properties of biochar produced from goldenrod plants. Materials 2022, 15, 2615. [Google Scholar] [CrossRef] [PubMed]
- Kutchko, B.G.; Kim, A.G. Fly ash characterization by SEM–EDS. Fuel 2006, 85, 2537–2544. [Google Scholar] [CrossRef]
- Liao, C.S.; Nishikawa, Y.; Shih, Y.T. Characterization of di-n-butyl phthalate phytoremediation by Garden lettuce (Lactuca sativa L. var. longifolia) through Kinetics and proteome analysis. Sustainability 2019, 11, 1625. [Google Scholar]
- García-Delgado, C.; Fresno, T.; Rodríguez-Santamaría, J.J.; Díaz, E.; Mohedano, A.F.; Moreno-Jimenez, E. Co-application of activated carbon and compost to contaminated soils: Toxic elements mobility and PAH degradation and availability. Int. J. Environ. Sci. Technol. 2019, 16, 1057–1068. [Google Scholar] [CrossRef]
- Jien, S.H.; Wang, C.S. Effects of biochar on soil properties and erosion potential in a highly weathered soil. Catena 2013, 110, 225–233. [Google Scholar] [CrossRef]
- Jien, S.H. Physical characteristics of biochars and their effects on soil physical properties. In Biochar from Biomass and Waste; Elsevier: Amsterdam, The Netherlands, 2019; pp. 21–35. [Google Scholar]
- Lua, A.C.; Yang, T.; Guo, J. Effects of pyrolysis conditions on the properties of activated carbons prepared from pistachio-nut shells. J. Anal. Appl. Pyrolysis 2004, 72, 279–287. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Wang, J.M.; Feng, Y. The effects of biochar addition on soil physicochemical properties: A review. Catena 2021, 202, 105284. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Weidemann, E.; Buss, W.; Edo, M.; Mašek, O.; Jansson, S. Influence of pyrolysis temperature and production unit on formation of selected PAHs, oxy-PAHs, N-PACs, PCDDs, and PCDFs in biochar—A screening study. Environ. Sci. Pollut. Res. 2018, 25, 3933–3940. [Google Scholar] [CrossRef] [PubMed]
- Buss, W.; Mašek, O.; Graham, M.; Wüst, D. Inherent organic compounds in biochar–their content, composition and potential toxic effects. J. Environ. Manag. 2015, 156, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Kumari, M.; Cameotra, S.S. Biodegradation of the allelopathic chemical m-tyrosine by Bacillus aquimaris SSC5 involves the homogentisate central pathway. PLoS ONE 2013, 8, e75928. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Screening of Bacillus strains isolated from mangrove ecosystems in Peninsular Malaysia for microplastic degradation. Environ. Pollut. 2017, 231, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Kim, I.G.; Kang, K.H.; Oh, T.K.; Park, Y.H. Bacillus marisflavi sp. nov. and Bacillus aquimaris sp. nov., isolated from sea water of a tidal flat of the Yellow Sea in Korea. Int. J. Syst. Evol. Micr. 2003, 53, 1297–1303. [Google Scholar] [CrossRef]
- Namane, A.; Amrouche, F.; Arrar, J.; Ali, O.; Hellal, A. Bacterial behaviour in the biodegradation of phenol by indigenous bacteria immobilized in Ca-alginate beads. Environ. Technol. 2018, 41, 1829–1836. [Google Scholar] [CrossRef]
- Raj, A.S.; Anup, G.; Sivalingam, R.; Chandran, P. Biodegradation of chlorpyrifos by charcoal–alginate immobilized bacterial consortium isolated from pesticide-polluted Kuzhikandam Creek, Kerala, India. Bioremediat. J. 2024, 1–21. [Google Scholar] [CrossRef]
- Dong, H.; Wang, W.; Song, Z.; Dong, H.; Wang, J.; Sun, S.; Zhang, Z.; Ke, M.; Zhang, Z.; Wu, W.M.; et al. A high-efficiency denitrification bioreactor for the treatment of acrylonitrile wastewater using waterborne polyurethane immobilized activated sludge. Bioresour. Technol. 2017, 239, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Lü, T.T.; Yu, D.S.; Chen, G.H.; Wang, X.X.; Huang, S.; Liu, C.C.; Tang, P. NH4+-N adsorption behavior of nitrifying sludge immobilized in waterborne polyurethane (WPU) pellets. Biochem. Eng. J. 2019, 143, 196–201. [Google Scholar] [CrossRef]
- Liao, C.S.; Chen, L.C.; Chen, B.S.; Lin, S.H. Bioremediation of endocrine disruptor di-n-butyl phthalate ester by Deinococcus radiodurans and Pseudomonas stutzeri. Chemosphere 2010, 78, 342–346. [Google Scholar] [CrossRef] [PubMed]



| RH | RHB-300 | RHB-450 | RHB-600 | |
|---|---|---|---|---|
| pH (1:10 w/v) | 5.92 | 7.65 | 7.99 | 8.03 |
| Total carbon (%) | 40.5 | 31.2 | 28.2 | 26.8 |
| Total nitrogen (%) | 0.57 | 0.41 | 0.32 | 0.28 |
| C/N ratio | 71.1 | 76.1 | 88.1 | 95.7 |
| Water content (%) | 15.9 | 9.6 | 8.4 | 7.3 |
| CEC (cmol(+) kg−1) | 46.1 | 24.2 | 26.2 | 35.5 |
| Element | RH | RHB-300 | RHB-450 | RHB-600 | ||||
|---|---|---|---|---|---|---|---|---|
| Weight | Atomic | Weight | Atomic | Weight | Atomic | Weight | Atomic | |
| C | 77.97 | 83.68 | 63.97 | 70.52 | 77.64 | 83.28 | 76.58 | 82.73 |
| O | 17.88 | 14.41 | 35.08 | 29.03 | 18.76 | 15.11 | 18.59 | 15.08 |
| Si | 4.08 | 1.87 | 0.95 | 0.45 | 3.27 | 1.50 | 4.57 | 2.11 |
| K | 0.08 | 0.03 | 0.00 | 0.00 | 0.24 | 0.08 | 0.18 | 0.06 |
| Ca | 0.00 | 0.00 | 0.00 | 0.00 | 0.09 | 0.03 | 0.07 | 0.02 |
| Mn | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Totals | 100.00 | 100.00 | 100.00 | 100.00 | ||||
| Alginate-Ca (%) | Water Content (%) | Weight of Single Particle (mg) |
|---|---|---|
| 0.5 | 18.96 | 31.31 ± 0.85 |
| 2 | 26.45 | 55.94 ± 1.34 |
| 3 | 35.66 | 68.65 ± 1.88 |
| Alginate-Ca (%) | Initial Weight (g) | Weight after 48 h Shaking (g) | Weight Loss Ratio (%) | Weight after 96 h Shaking (g) | Weight Loss Ratio (%) |
|---|---|---|---|---|---|
| 0.5 | 5 | 4.39 ± 0.22 | 12.23 | 3.85 ± 0.12 | 23.17 |
| 2 | 5 | 4.47 ± 0.21 | 10.52 | 4.08 ± 0.14 | 18.42 |
| 3 | 5 | 4.59 ± 0.22 | 8.23 | 4.24 ± 0.15 | 15.21 |
| Treatment | Free Cells | Alginate-Ca | Alginate-Ca/WPU | ||||||
|---|---|---|---|---|---|---|---|---|---|
| k1 (day−1) | t1/2 (days) | r2 | k1 (day−1) | t1/2 (days) | r2 | k1 (day−1) | t1/2 (days) | r2 | |
| Temperature | |||||||||
| 25 °C | 0.16 | 4.42 | 0.91 | 0.19 | 3.57 | 0.88 | 0.28 | 2.39 | 0.85 |
| 30 °C | 0.18 | 3.89 | 0.88 | 0.22 | 3.12 | 0.84 | 0.38 | 1.82 | 0.92 |
| 35 °C | 0.14 | 5.11 | 0.88 | 0.16 | 4.31 | 0.87 | 0.27 | 2.61 | 0.77 |
| 40 °C | 0.15 | 4.61 | 0.85 | 0.13 | 4.95 | 0.82 | 0.22 | 3.09 | 0.72 |
| pH | |||||||||
| 5 | 0.11 | 6.32 | 0.84 | 0.13 | 5.39 | 0.91 | 0.19 | 3.61 | 0.85 |
| 6 | 0.13 | 5.23 | 0.94 | 0.18 | 3.69 | 0.87 | 0.22 | 3.16 | 0.84 |
| 7 | 0.18 | 3.88 | 0.88 | 0.22 | 3.13 | 0.84 | 0.38 | 1.81 | 0.91 |
| 8 | 0.15 | 4.59 | 0.84 | 0.15 | 4.34 | 0.86 | 0.26 | 2.55 | 0.77 |
| 9 | 0.11 | 6.41 | 0.89 | 0.08 | 8.11 | 0.89 | 0.12 | 5.62 | 0.85 |
| Carbon and nitrogen sources | |||||||||
| Yeast extract 0.25 g | 0.31 | 2.29 | 0.89 | 0.45 | 1.54 | 0.87 | 0.38 | 1.81 | 0.86 |
| Yeast extract 0.15 g + glucose 0.125 g | 0.28 | 2.49 | 0.92 | 0.39 | 1.77 | 0.89 | 0.47 | 1.46 | 0.83 |
| Yeast extract 0.15 g + glucose 0.0625 g | 0.34 | 2.03 | 0.85 | 0.45 | 1.52 | 0.86 | 0.54 | 1.28 | 0.79 |
| Yeast extract 0.15 g + glucose 0.03125 g | 0.15 | 3.77 | 0.92 | 0.32 | 2.11 | 0.81 | 0.31 | 2.29 | 0.86 |
| Surfactants (1 CMC) | |||||||||
| Brij 35 | 0.12 | 5.58 | 0.83 | 0.14 | 4.76 | 0.88 | 0.15 | 4.41 | 0.92 |
| Triton X-100 | 0.23 | 2.99 | 0.95 | 0.25 | 2.81 | 0.94 | 0.32 | 2.16 | 0.93 |
| Tergitol | 0.16 | 4.22 | 0.81 | 0.20 | 3.42 | 0.75 | 0.16 | 4.30 | 0.87 |
| Triton N101 | 0.16 | 4.46 | 0.86 | 0.16 | 4.19 | 0.85 | 0.27 | 2.59 | 0.78 |
| Triton X-80 | 0.22 | 3.22 | 0.78 | 0.16 | 4.21 | 0.95 | 0.25 | 2.81 | 0.92 |
| Others | |||||||||
| Shake (160 rpm) | 0.21 | 3.59 | 0.85 | 0.23 | 3.08 | 0.89 | 0.39 | 1.78 | 0.89 |
| Optimal conditions in culture medium | 0.27 | 2.53 | 0.98 | 2.04 | 3.12 | 0.97 | 0.43 | 1.61 | 0.94 |
| Optimal condition in original river water | 0.13 | 5.21 | 0.88 | 0.18 | 3.79 | 0.86 | 0.45 | 1.65 | 0.93 |
| Culture medium without added bacteria | 0.01 | 84.83 | 0.81 | 0.01 | 61.81 | 0.79 | 0.01 | 50.93 | 0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.-D.; Jien, S.-H.; Yang, C.-W.; Lin, Y.-H.; Liao, C.-S. Innovative Microbial Immobilization Strategy for Di-n-Butyl Phthalate Biodegradation Using Biochar-Calcium Alginate-Waterborne Polyurethane Composites. Microorganisms 2024, 12, 1265. https://doi.org/10.3390/microorganisms12071265
Cao X-D, Jien S-H, Yang C-W, Lin Y-H, Liao C-S. Innovative Microbial Immobilization Strategy for Di-n-Butyl Phthalate Biodegradation Using Biochar-Calcium Alginate-Waterborne Polyurethane Composites. Microorganisms. 2024; 12(7):1265. https://doi.org/10.3390/microorganisms12071265
Chicago/Turabian StyleCao, Xuan-Di, Shih-Hao Jien, Chu-Wen Yang, Yi-Hsuan Lin, and Chien-Sen Liao. 2024. "Innovative Microbial Immobilization Strategy for Di-n-Butyl Phthalate Biodegradation Using Biochar-Calcium Alginate-Waterborne Polyurethane Composites" Microorganisms 12, no. 7: 1265. https://doi.org/10.3390/microorganisms12071265
APA StyleCao, X.-D., Jien, S.-H., Yang, C.-W., Lin, Y.-H., & Liao, C.-S. (2024). Innovative Microbial Immobilization Strategy for Di-n-Butyl Phthalate Biodegradation Using Biochar-Calcium Alginate-Waterborne Polyurethane Composites. Microorganisms, 12(7), 1265. https://doi.org/10.3390/microorganisms12071265








