Rational Design for the Complete Synthesis of Stevioside in Saccharomyces cerevisiae
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Reagents
2.2. DNA Manipulation
2.3. Strain Construction, Transformation, Screening, and Culture
2.4. Metabolite Extraction and Quantification
2.5. Fed-Batch Fermentation of Yeast Strain SST-302III-ST2
3. Results
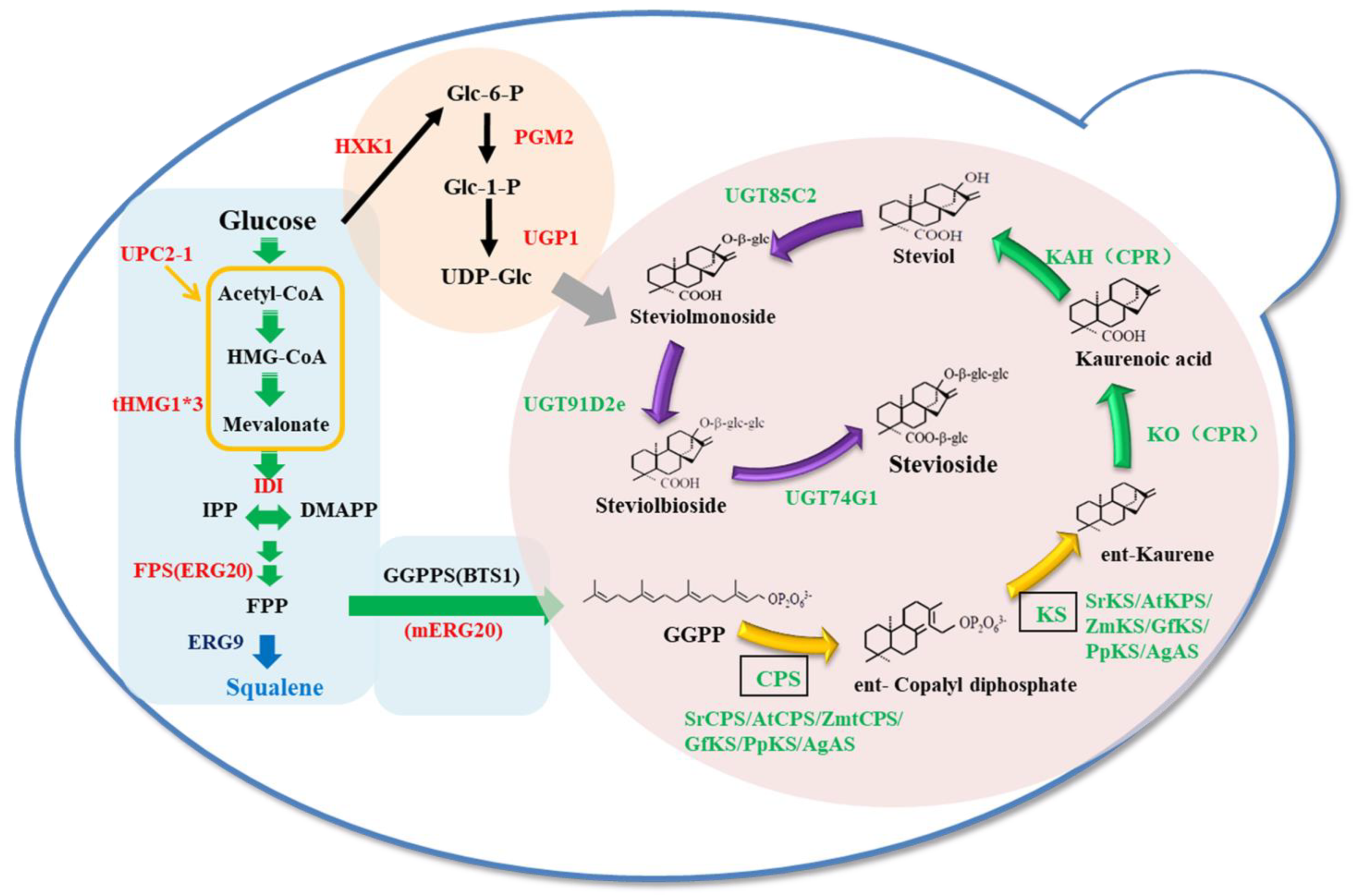
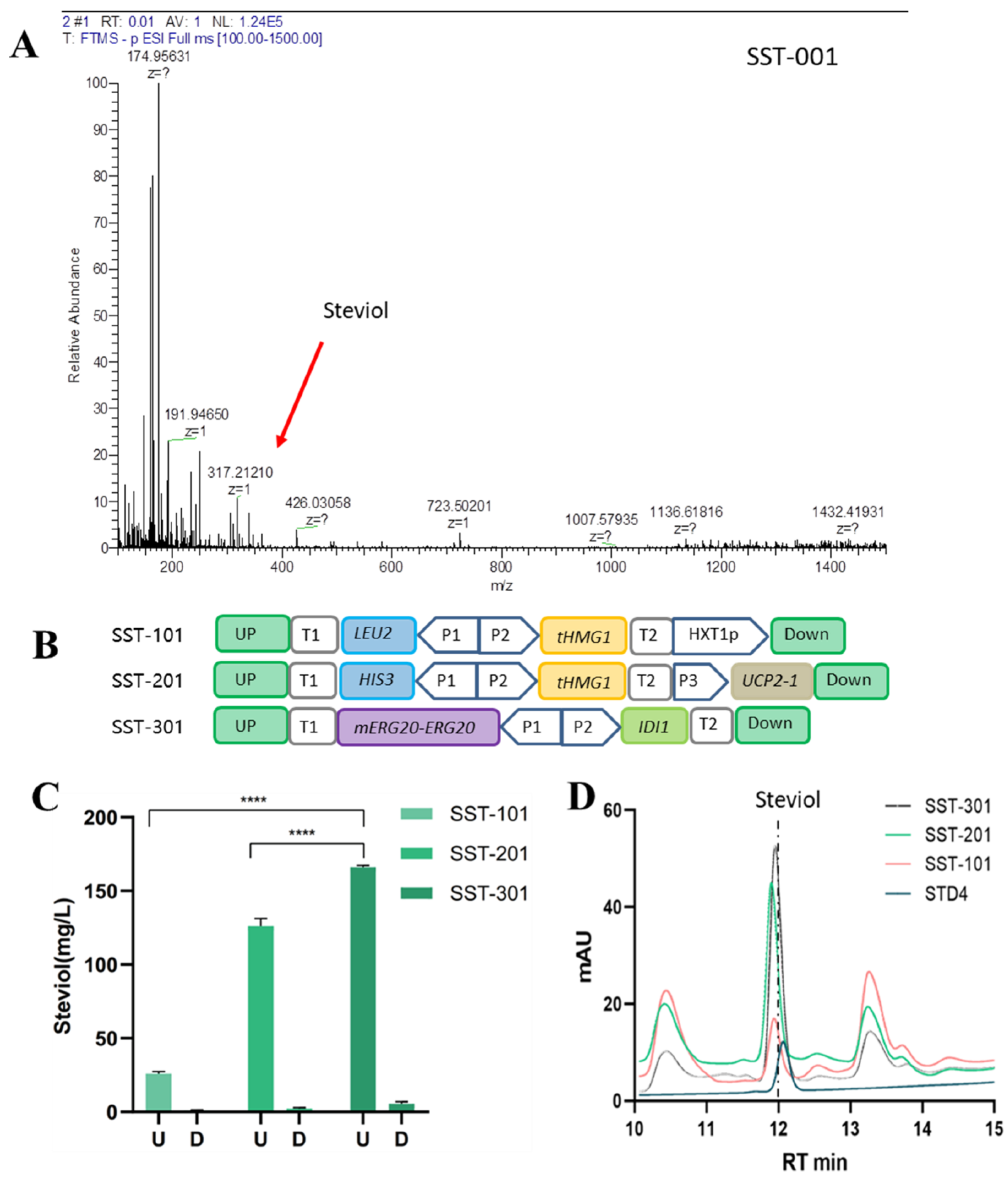
3.1. Construction of S. cerevisiae to Produce Steviol
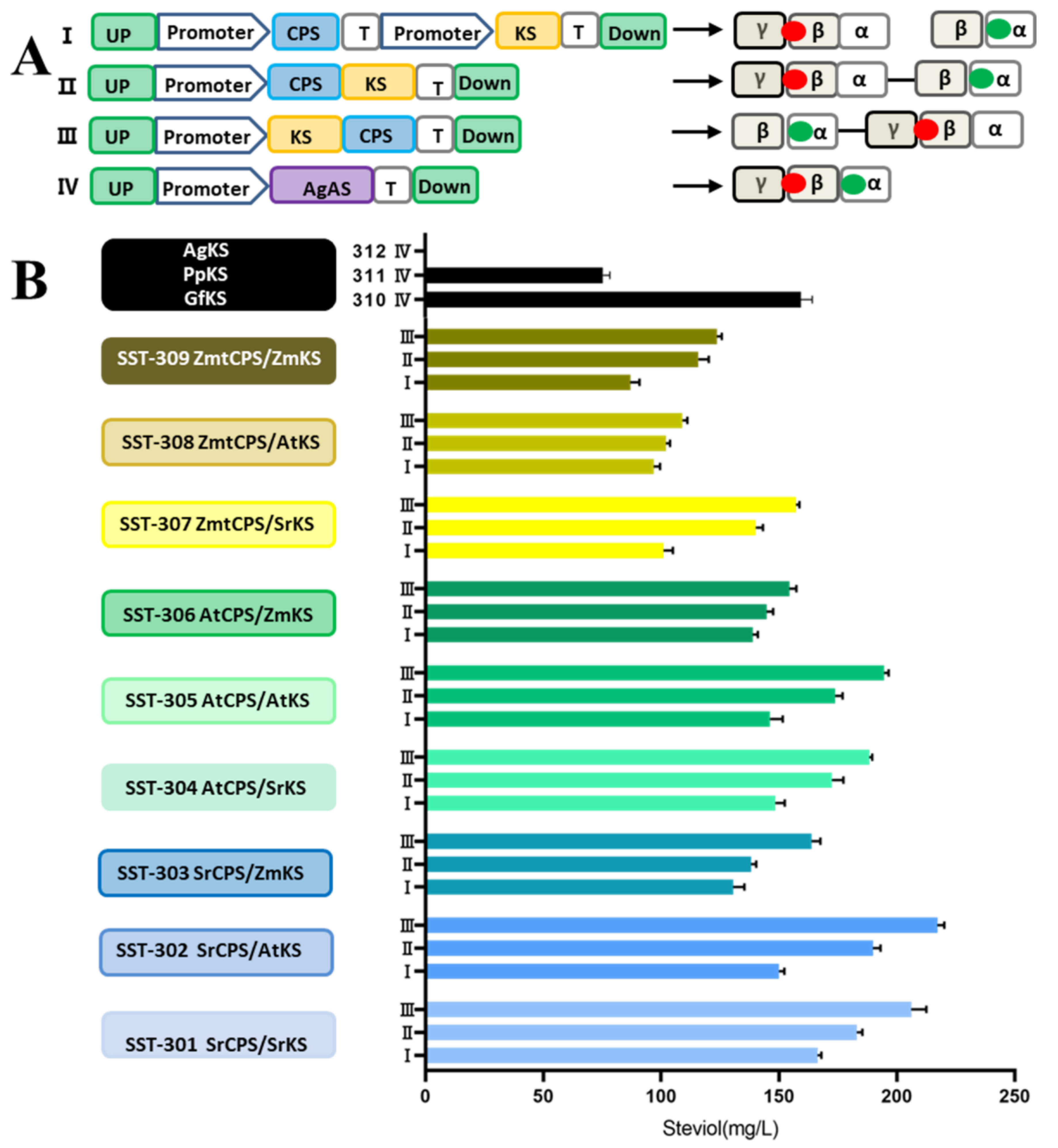
3.2. Studies on Diterpenoid Synthases
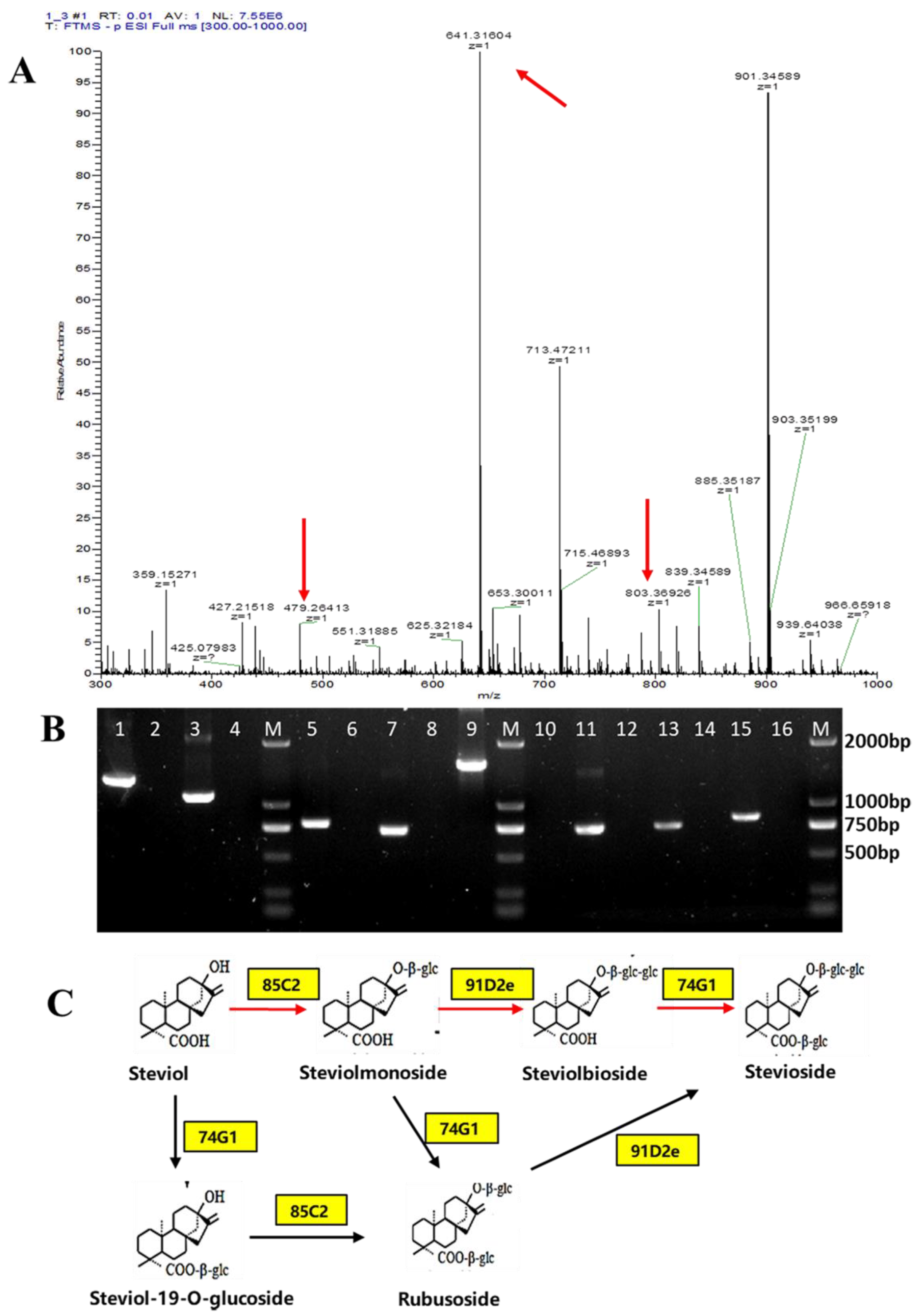
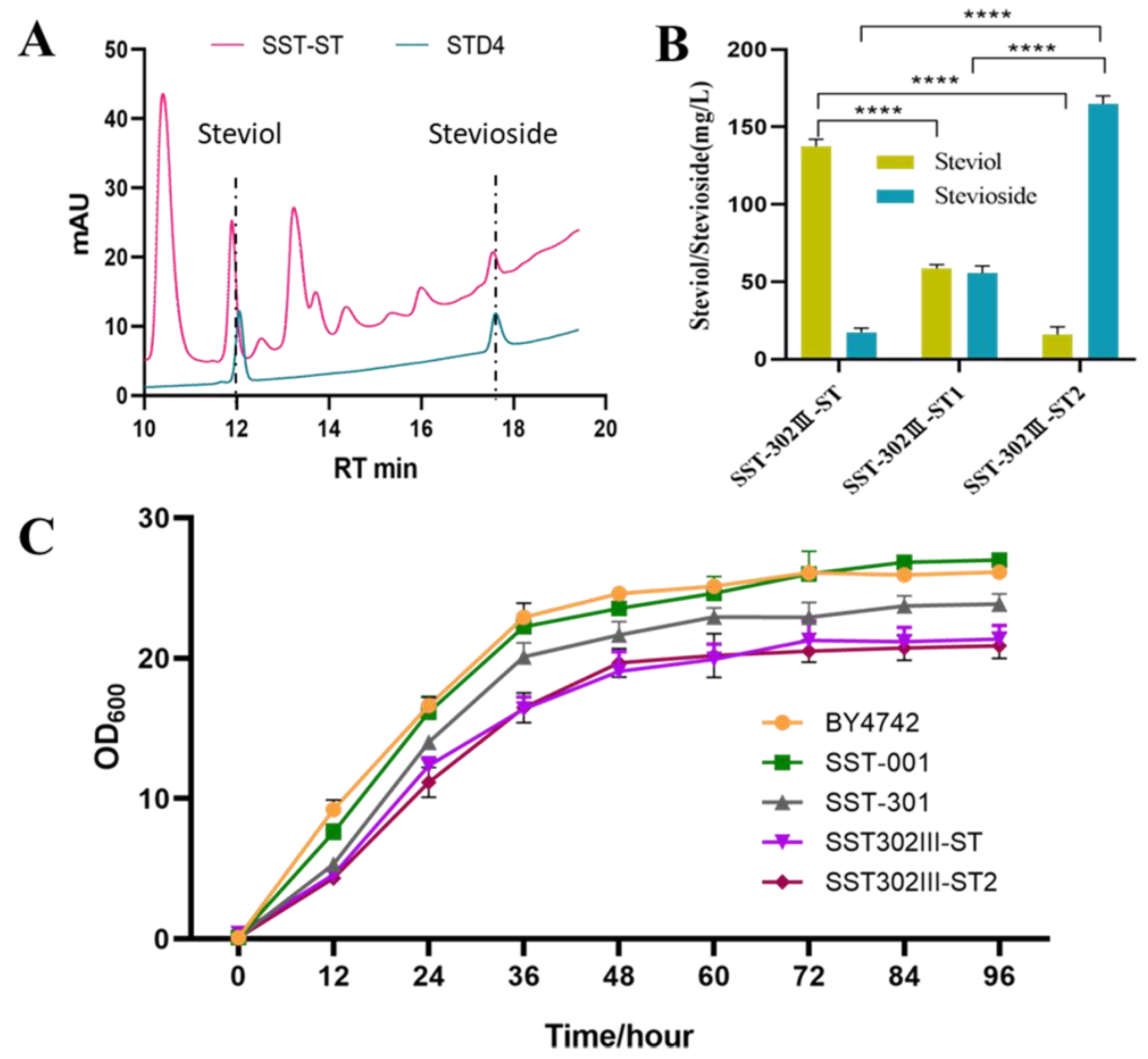
3.3. Complete Biosynthesis of Stevioside in S. cerevisiae
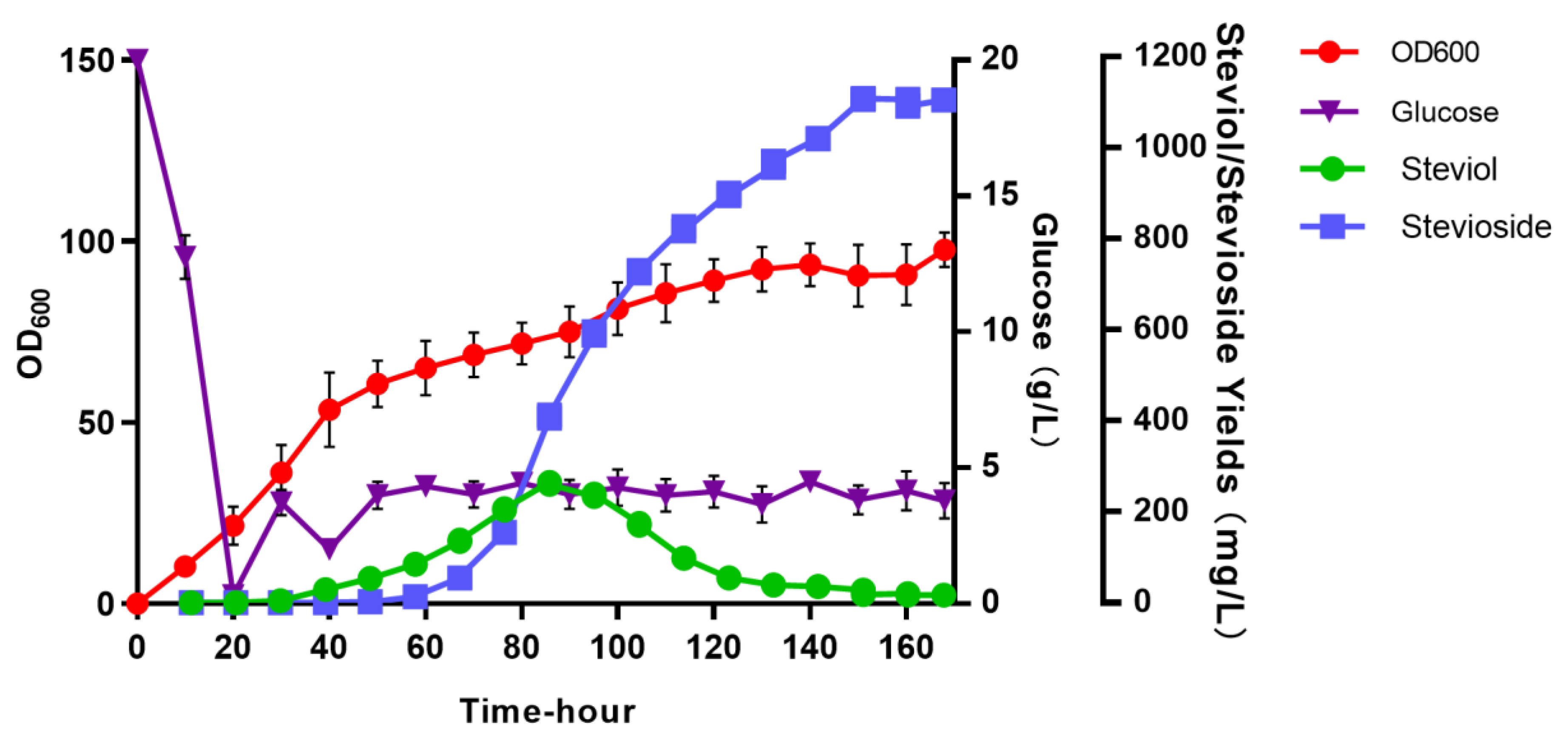
3.4. Batch Feeding Culture in a 10 L Bioreactor
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brandle, J.E.; Telmer, P.G. Steviol glycoside biosynthesis. Phytochemistry 2007, 68, 1855–1863. [Google Scholar] [CrossRef] [PubMed]
- Geuns, J.M. Stevioside. Phytochemistry 2003, 64, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Bondarev, N.I.; Sukhanova, M.A.; Reshetnyak, O.V.; Nosov, A.M. Steviol glycoside content in different organs of Stevia rebaudiana and its dynamics during ontogeny. Biol. Plantarum. 2002, 47, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Kaul, K.; Bajpai-Gupta, S.; Kaul, V.K.; Kumar, S. A comprehensive analysis of fifteen genes of steviol glycosides biosynthesis pathway in Stevia rebaudiana (Bertoni). Gene 2012, 492, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Richman, A.S.; Gijzen, M.; Starratt, A.N.; Yang, Z.; Brandle, J.E. Diterpene synthesis in Stevia rebaudiana: Recruitment and up-regulation of key enzymes from the gibberellin biosynthetic pathway. Plant J. 1999, 19, 411–421. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Wang, Y.; Chen, L.; Yan, M.; Chen, K.; Xu, L.; Ouyang, P. Production of Rebaudioside A from Stevioside Catalyzed by the Engineered Saccharomyces cerevisiae. Appl. Biochem. Biotechnol. 2016, 178, 1586–1598. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Jin, J.; Zheng, J.; Wong, L.; Chua, N.H.; Jang, I.C. Comparative Transcriptomics Unravel Biochemical Specialization of Leaf Tissues of Stevia for Diterpenoid Production. Plant Physiol. 2015, 169, 2462–2480. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hou, K.; Qin, P.; Liu, H.; Yi, B.; Yang, W.; Wu, W. RNA-Seq for gene identification and transcript profiling of three Stevia rebaudiana genotypes. BMC Genom. 2014, 15, 571. [Google Scholar] [CrossRef]
- Ashok, K.K.Y.; Singh, S.; Dhyani, D.; Ahuja, P.S. A review on the improvement of stevia [Stevia rebaudiana (Bertoni)]. Can. J. Plant Sci. 2011, 91, 1–27. [Google Scholar] [CrossRef]
- Wang, J. Discussing the mechanism of sweetness, sweetness and bitter aftertaste of stevia glycosides. Food Ind. Technol. 2010, 31, 417–420. [Google Scholar] [CrossRef]
- Ceunen, S.; Geuns, J.M. Steviol glycosides: Chemical diversity, metabolism, and function. J. Nat. Prod. 2013, 76, 1201–1228. [Google Scholar] [CrossRef]
- Olsson, K.; Carlsen, S.; Semmler, A.; Simón, E.; Mikkelsen, M.D.; Møller, B.L. Microbial production of next-generation stevia sweeteners. Microb. Cell Fact. 2016, 15, 207. [Google Scholar] [CrossRef]
- Carothers, J.M.; Goler, J.A.; Keasling, J.D. Chemical synthesis using synthetic biology. Curr. Opin. Biotechnol. 2009, 20, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Ro, D.K.; Paradise, E.M.; Ouellet, M.; Fisher, K.J.; Newman, K.L.; Ndungu, J.M.; Ho, K.A.; Eachus, R.A.; Ham, T.S.; Kirby, J.; et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 2006, 440, 940–943. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Nielsen, J. Synthetic Biology of Yeast. Biochemistry 2019, 58, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, S.; Xiong, Z.; Wang, Y. Pathway mining-based integration of critical enzyme parts for de novo biosynthesis of steviolglycosides sweetener in Escherichia coli. Cell Res. 2016, 26, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, X.; Zhang, C.; Zhou, X.; Xu, X.; Han, L.; Lv, X.; Liu, Y.; Liu, S.; Li, J.; et al. De novo biosynthesis of rubusoside and rebaudiosides in engineered yeasts. Nat. Commun. 2022, 13, 3040. [Google Scholar] [CrossRef]
- Mao, Y.; Chen, Z.; Ren, Y.; Sun, Y.; Wang, Y. Whole-Cell Biocatalyst for Rubusoside Production in Saccharomyces cerevisiae. J. Agric. Food Chem. 2021, 69, 13155–13163. [Google Scholar] [CrossRef]
- Ye, L.D.; Lv, X.M.; Yu, H.W. Assembly of biosynthetic pathways in Saccharomyces cerevisiae using a marker recyclable integrative plasmid toolbox. Front. Chem. Sci. Eng. 2017, 11, 126–132. [Google Scholar] [CrossRef]
- Reider Apel, A.; d’Espaux, L.; Wehrs, M.; Sachs, D.; Li, R.A.; Tong, G.J.; Garber, M.; Nnadi, O.; Zhuang, W.; Hillson, N.J.; et al. A Cas9-based toolkit to program gene expression in Saccharomyces cerevisiae. Nucleic Acids Res. 2017, 45, 496–508. [Google Scholar] [CrossRef]
- Nan, W.; Zhao, F.; Zhang, C.; Ju, H.; Lu, W. Promotion of compound K production in Saccharomyces cerevisiae by glycerol. Microb. Cell Fact. 2020, 19, 41. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.; Liu, M.; Qu, J.; Yao, M.; Li, B.; Ding, M.; Liu, H.; Xiao, W.; Yuan, Y. Primary and Secondary Metabolic Effects of a Key Gene Deletion (ΔYPL062W) in Metabolically Engineered Terpenoid-Producing Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2019, 85, e01990-18. [Google Scholar] [CrossRef] [PubMed]
- Amiri, P.; Shahpiri, A.; Asadollahi, M.A.; Momenbeik, F.; Partow, S. Metabolic engineering of Saccharomyces cerevisiae for linalool production. Biotechnol. Lett. 2016, 38, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Scalcinati, G.; Knuf, C.; Partow, S.; Chen, Y.; Maury, J.; Schalk, M.; Daviet, L.; Nielsen, J.; Siewers, V. Dynamic control of gene expression in Saccharomyces cerevisiae engineered for the production of plant sesquitepene α-santalene in a fed-batch mode. Metab. Eng. 2012, 14, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Zhou, J.; Tong, Y.; Su, P.; Li, X.; Liu, Y.; Liu, N.; Wu, X.; Zhang, Y.; Wang, J.; et al. Engineering chimeric diterpene synthases and isoprenoid biosynthetic pathways enables high-level production of miltiradiene in yeast. Metab. Eng. 2020, 60, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.J.; Gao, W.; Rong, Q.; Jin, G.; Chu, H.; Liu, W.; Yang, W.; Zhu, Z.; Li, G.; Zhu, G.; et al. Modular pathway engineering of diterpenoid synthases and the mevalonic acid pathway for miltiradiene production. J. Am. Chem. Soc. 2012, 134, 3234–3241. [Google Scholar] [CrossRef] [PubMed]
- Hedden, P.; Phillips, A.L. Gibberellin metabolism: New insights revealed by the genes. Trends Plant Sci. 2000, 5, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Brown, R.; Köllner, T.G.; Fu, J.; Chen, X.; Wong, G.K.; Gershenzon, J.; Peters, R.J.; Chen, F. Origin and early evolution of the plant terpene synthase family. Proc. Natl. Acad. Sci. USA 2022, 119, e2100361119. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.C.; Woo, H.M. Biosynthesis of the Calorie-Free Sweetener Precursor ent-Kaurenoic Acid from CO2 Using Engineered Cyanobacteria. ACS Synth. Biol. 2020, 9, 2979–2985. [Google Scholar] [CrossRef]
- Li, W.; Cui, L.; Mai, J.; Shi, T.Q.; Lin, L.; Zhang, Z.G.; Ledesma-Amaro, R.; Dong, W.; Ji, X.J. Advances in Metabolic Engineering Paving the Way for the Efficient Biosynthesis of Terpenes in Yeasts. J. Agric. Food Chem. 2022, 70, 9246–9261. [Google Scholar] [CrossRef]
- Zhou, K.; Gao, Y.; Hoy, J.A.; Mann, F.M.; Honzatko, R.B.; Peters, R.J. Insights into diterpene cyclization from structure of bifunctional abietadiene synthase from Abies grandis. J. Biol. Chem. 2012, 287, 6840–6850. [Google Scholar] [CrossRef] [PubMed]
- Richman, A.; Swanson, A.; Humphrey, T.; Chapman, R.; McGarvey, B.; Pocs, R.; Brandle, J. Functional genomics uncovers three glucosyltransferases involved in the synthesis of the major sweet glucosides of Stevia rebaudiana. Plant J. 2005, 41, 56–67. [Google Scholar] [CrossRef]
- Kishore, G.M.L.; Motion, M.L.; Hicks, P.M.L.; Houghton-Larsen, J.L.; Hansen, J.L.; Halkjear Hansen, E.L.; Tavares, S.L.; Blom, C.L. Recombinant Production of Steviol. Glycosides. Patent WO2013022989A2, 14 February 2013. [Google Scholar]
- Chen, G.H.; Li, C.; Feng, X.D. Advances in UDP-Glc biosynthesis. J. Biol. 2023, 40, 95–100. [Google Scholar] [CrossRef]
- Li, X.; Yu, W.W.; Lv, X.Q. Metabolic engineering of Saccharomyces cerevisiae to synthesize β-glucan efficiently. Food Ferment. Ind. 2022, 48, 1–7. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yang, G.Y.; Chen, X.; Liu, Q.; Zhang, X.; Deng, Z.; Feng, Y. Biosynthesis of plant-derived ginsenoside Rh2 in yeast via repurposing a key promiscuous microbial enzyme. Metab. Eng. 2017, 42, 25–32. [Google Scholar] [CrossRef]
- Redden, H.; Morse, N.; Alper, H.S. The synthetic biology toolbox for tuning gene expression in yeast. FEMS Yeast Res. 2015, 15, 1–10. [Google Scholar] [CrossRef]
- Wu, G.; Yan, Q.; Jones, J.A.; Tang, Y.J.; Fong, S.S.; Koffas, M.A.G. Metabolic Burden: Cornerstones in Synthetic Biology and Metabolic Engineering Applications. Trends Biotechnol. 2016, 34, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Vogt, T. Glycosyltransferases in secondary plant metabolism: Tranquilizers and stimulant controllers. Planta 2001, 213, 164–174. [Google Scholar] [CrossRef]
- Gachon, C.M.; Langlois-Meurinne, M.; Saindrenan, P. Plant secondary metabolism glycosyltransferases: The emerging functional analysis. Trends Plant Sci. 2005, 10, 542–549. [Google Scholar] [CrossRef]
- Bowles, D.; Isayenkova, J.; Lim, E.K.; Poppenberger, B. Glycosyltransferases: Managers of small molecules. Curr. Opin. Plant Biol. 2005, 8, 254–263. [Google Scholar] [CrossRef]
- Lian, J.; Mishra, S.; Zhao, H. Recent advances in metabolic engineering of Saccharomyces cerevisiae: New tools and their applications. Metab. Eng. 2018, 50, 85–108. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Liu, Y.; Ma, X.; Ma, C.; Jiang, Y.; Su, J. Rational Design for the Complete Synthesis of Stevioside in Saccharomyces cerevisiae. Microorganisms 2024, 12, 1125. https://doi.org/10.3390/microorganisms12061125
Huang W, Liu Y, Ma X, Ma C, Jiang Y, Su J. Rational Design for the Complete Synthesis of Stevioside in Saccharomyces cerevisiae. Microorganisms. 2024; 12(6):1125. https://doi.org/10.3390/microorganisms12061125
Chicago/Turabian StyleHuang, Wei, Yongheng Liu, Xiaomei Ma, Cilang Ma, Yuting Jiang, and Jianyu Su. 2024. "Rational Design for the Complete Synthesis of Stevioside in Saccharomyces cerevisiae" Microorganisms 12, no. 6: 1125. https://doi.org/10.3390/microorganisms12061125
APA StyleHuang, W., Liu, Y., Ma, X., Ma, C., Jiang, Y., & Su, J. (2024). Rational Design for the Complete Synthesis of Stevioside in Saccharomyces cerevisiae. Microorganisms, 12(6), 1125. https://doi.org/10.3390/microorganisms12061125





