Mycophagous Mite, Tyrophagus putrescentiae, Prefers to Feed on Entomopathogenic Fungi, except Metarhizium Generalists
Abstract
1. Introduction
2. Materials and Methods
2.1. Population of Tyrophagus putrescentiae and Maintenance
2.2. Entomopathogenic Fungi Strains and Culture
2.3. Feeding Preference of T. putrescentiae on Entomopathogenic Fungi
2.4. Developmental Duration of T. putrescentiae Fed on Different Entomopathogenic Fungi
2.5. Bioactivity of M. anisopliae and M. robertsii on T. putrescentiae
2.5.1. Bioassay of Metarhizium spp. against Mites
2.5.2. Infection Observation of Metarhizium spp. on Mites
3. Results
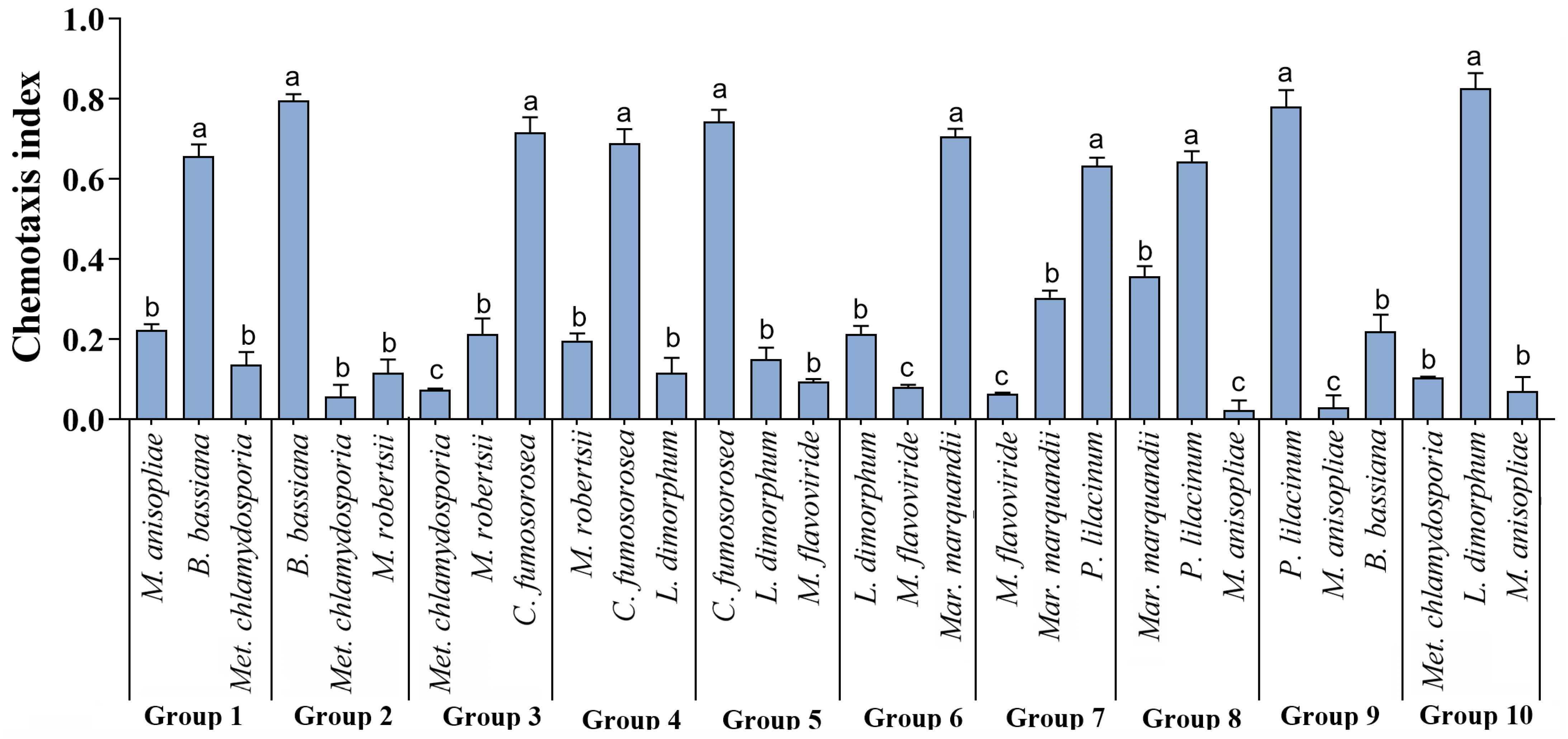
3.1. Feeding Preference of T. putrescentiae
3.2. Development Duration of T. putrescentiae Fed on Different Entomopathogenic Fungi
3.3. The Biological Activity of Metarhizium against T. putrescentiae
3.3.1. Acaricidal Activity
3.3.2. Infection Progress of Metarhizium on T. putrescentiae
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Deliephan, A.; Phillips, T.W.; Subramanyam, B.; Aldrich, C.G.; Maille, J.; Manu, N. Efficacy of Liquid Smoke to Mitigate Infestations of the Storage Mite Tyrophagus putrescentiae, in a Model Semi-Moist Pet Food. Animals 2023, 13, 3188. [Google Scholar] [CrossRef] [PubMed]
- Abbar, S.; Schilling, M.W.; Phillips, T.W. Time-Mortality relationships to control Tyrophagus putrescentiae (Sarcoptiformes: Acaridae) exposed to high and low temperatures. J. Econ. Entomol. 2016, 109, 2215–2220. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.X.; Huang, H.F.; Ge, D.Z.; Yu, L.L.; Xu, C.; Cui, Y.B. Tyrophagus putrescentiae group 4 allergen allergenicity and epitope prediction. Allergol. Immunopathol. 2020, 48, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Erban, T.; Klimov, P.B.; Harant, K.; Talacko, P.; Nesvorna, M.; Hubert, J. Label-free proteomic analysis reveals differentially expressed Wolbachia proteins in Tyrophagus putrescentiae: Mite allergens and markers reflecting population-related proteome differences. J. Proteom. 2021, 249, 104356. [Google Scholar] [CrossRef] [PubMed]
- White, S.A.; Zhang, X.; Campbell, Y.L.; Smith, S.W.; Phillips, T.W.; Freeman, C.; Schilling, M.W. Effectiveness of nets treated with food-grade coatings following various drying methods for controlling mite growth on dry-cured hams. J. Stored Prod. Res. 2023, 100, 102065. [Google Scholar] [CrossRef]
- Zhou, D.; Ren, Y.; Zhou, Y.; Tao, X.; Liao, Y.; Yuan, C.; Lu, M.; Cui, Y. Expression, purification, and activity of novel allergen Tyr p 31 from Tyrophagus putrescentiae. Int. J. Biol. Macromol. 2024, 258, 128856. [Google Scholar] [CrossRef] [PubMed]
- Smrz, J.; Soukalova, H.; Catska, V.; Hubert, J. Feeding Patterns of Tyrophagus putrescentiae (Sarcoptiformes: Acaridae) Indicate That Mycophagy Is Not a Single and Homogeneous Category of Nutritional Biology. J. Insect Sci. 2016, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G.L.; Esswein, I.Z.; Heidrich, D.; Dresch, F.; Maciel, M.J.; Pagani, D.M.; Valente, P.; Scroferneker, M.L.; Johann, L.; Ferla, N.J.; et al. Population growth of the stored product pest Tyrophagus putrescentiae (Acari: Acaridae) on environmentally and medically important fungi. Exp. Appl. Acarol. 2019, 78, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Hubert, J.; Nesvorna, M.; Kopecky, J. The effect of Tyrophagus putrescentiae on Fusarium poae transmission and fungal community in stored barley in a laboratory experiment. Insect Sci. 2014, 21, 65–73. [Google Scholar] [CrossRef]
- Abou El-Atta, D.A.E.-M.; Osman, M.A. Development and reproductive potential of Tyrophagus putrescentiae (Acari: Acaridae) on plant-parasitic nematodes and artificial diets. Exp. Appl. Acarol. 2016, 68, 477–483. [Google Scholar] [CrossRef]
- Rybanska, D.; Hubert, J.; Markovic, M.; Erban, T. Dry Dog Food Integrity and Mite Strain Influence the Density-Dependent Growth of the Stored-Product Mite Tyrophagus putrescentiae (Acari: Acaridida). J. Econ. Entomol. 2016, 109, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Erban, T.; Rybanska, D.; Hubert, J. Population Growth of the Generalist Mite Tyrophagus putrescentiae (Acari: Acaridida) Following Adaptation to High- or Low-Fat and High- or Low-Protein Diets and the Effect of Dietary Switch. Environ. Entomol. 2015, 44, 1599–1604. [Google Scholar] [CrossRef]
- Sharififard, M.; Mossadegh, M.S.; Vazirianzadeh, B.; Latifi, S.M. Biocontrol of the Brown-Banded Cockroach, Supella longipalpa F. (Blattaria: Blattellidae), with Entomopathogenic Fungus, Metharhizium anisopliae. J. Arthropod-Borne Dis. 2016, 10, 335–346. [Google Scholar] [PubMed]
- Li, H.; Liu, J.; Hou, Z.; Luo, X.; Lin, J.; Jiang, N.; Hou, L.; Ma, L.; Li, C.; Qu, S. Activation of mycelial defense mechanisms in the oyster mushroom Pleurotus ostreatus induced by Tyrophagus putrescentiae. Food Res. Int. 2022, 160, 111708. [Google Scholar] [CrossRef]
- Hubert, J.; Nesvorna, M.; Sopko, B.; Green, S.J. Diet modulation of the microbiome of the pest storage mite Tyrophagus putrescentiae. FEMS Microbiol. Ecol. 2023, 99, fiad011. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S. Insect Pathogenic Fungi: Genomics, Molecular Interactions, and Genetic Improvements. Annu. Rev. Entomol. 2017, 62, 73–90. [Google Scholar] [CrossRef]
- Li, J.; Xia, Y. Host–Pathogen Interactions between Metarhizium spp. and Locusts. J. Fungi 2022, 8, 602. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, J. The Registration Situation and Use of Mycopesticides in the World. J. Fungi 2023, 9, 940. [Google Scholar] [CrossRef]
- Sharma, I.; Bhardwaj, S. Management of the European red mite, Panonychus ulmi (Acari: Tetranychidae) in apple orchards of North Western Himalayan region of India. Int. J. Acarol. 2023, 49, 416–421. [Google Scholar] [CrossRef]
- Basso, V.; Dillon, A.J.P.; Toldi, M.; Kramer, C.G.; Bonato, C.V. Beauveria bassiana submerged spores for control of two-spotted spider mite (Tetranychus urticae Koch (Acari: Tetranychidae)): Production, stability, and virulence. Arch. Microbiol. 2024, 206. [Google Scholar] [CrossRef]
- Peng, Z.Y.; Huang, S.T.; Chen, J.T.; Li, N.; Wei, Y.; Nawaz, A.; Deng, S.Q. An update of a green pesticide: Metarhizium anisopliae. All Life 2022, 15, 1141–1159. [Google Scholar] [CrossRef]
- Greenfield, B.P.J.; Lord, A.M.; Dudley, E.; Butt, T.M. Conidia of the insect pathogenic fungus, Metarhizium anisopliae, fail to adhere to mosquito larval cuticle. R. Soc. Open Sci. 2014, 1, 140193. [Google Scholar] [CrossRef]
- Moonjely, S.; Bidochka, M.J. Generalist and specialist Metarhizium insect pathogens retain ancestral ability to colonize plant roots. Fungal Ecol. 2019, 41, 209–217. [Google Scholar] [CrossRef]
- Chen, W.; Xie, W.; Cai, W.; Thaochan, N.; Hu, Q. Entomopathogenic Fungi Biodiversity in the Soil of Three Provinces Located in Southwest China and First Approach to Evaluate Their Biocontrol Potential. J. Fungi 2021, 7, 984. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, H.; Pang, S.; Yin, X.; Cao, B.; Huang, J.; Xu, X.; Weng, Q.; Hu, Q. Destruxin A inhibits the hemocytin-mediated hemolymph immunity of host insects to facilitate Metarhizium infection. Cell Rep. 2024, 43, 113686. [Google Scholar] [CrossRef]
- Qu, S.-X.; Ma, L.; Li, H.-P.; Song, J.-D.; Hong, X.-Y. Chemosensory proteins involved in host recognition in the stored-food mite Tyrophagus putrescentiae. Pest Manag. Sci. 2016, 72, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.X.; Li, H.P.; Ma, L.; Song, J.D.; Hou, L.J.; Lin, J.S. Temperature-Dependent Development and Reproductive Traits of Tyrophagus putrescentiae (Sarcoptiformes: Acaridae) Reared on Different Edible Mushrooms. Environ. Entomol. 2015, 44, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Geedi, R.; Canas, L.; Reding, M.E.; Ranger, C.M. Attraction of Myzus persicae (Hemiptera: Aphididae) to Volatiles Emitted From the Entomopathogenic Fungus Beauveria bassiana. Envrion. Entomol. 2023, 52, 31–38. [Google Scholar] [CrossRef]
- Brütsch, T.; Felden, A.; Reber, A.; Chapuisat, M. Ant queens (Hymenoptera: Formicidae) are attracted to fungal pathogens during the initial stage of colony founding. Myrmecol. News 2014, 20, 71–76. [Google Scholar]
- Hummadi, E.H.; Dearden, A.; Generalovic, T.; Clunie, B.; Harrott, A.; Cetin, Y.; Demirbek, M.; Khoja, S.; Eastwood, D.; Dudley, E.; et al. Volatile organic compounds of Metarhizium brunneum influence the efficacy of entomopathogenic nematodes in insect control. Biol. Control 2021, 155, 104527. [Google Scholar] [CrossRef]
- Canfield, M.S.; Wrenn, W.J. Tyrophagus putrescentiae mites grown in dog food cultures and the effect mould growth has on mite survival and reproduction. Vet. Dermatol. 2010, 21, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.Q.; Liu, J.J.; Li, H.P.; Luo, X.; Ma, L.; Qu, S.X. Biological parameters and host preference of Tyrophagus putrescentiae (Schrank) on different Pleurotus ostreatus cultivars. Syst. Appl. Acarol. 2022, 27, 1–8. [Google Scholar] [CrossRef]
- Hubert, J.; Nesvorna, M.; Pekar, S.; Green, S.J.; Klimov, P.B. Cardinium inhibits Wolbachia in its mite host, Tyrophagus putrescentiae, and affects host fitness. FEMS Microbiol. Ecol. 2021, 97, fiab123. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.W.; Yin, X.Y.; Pang, S.Y.; Jiang, Y.L.; Weng, Q.F.; Hu, Q.B.; Wang, J.J. Mechanism of destruxin a inhibits juvenile hormone binding protein transporting juvenile hormone to affect insect growth. Pestic. Biochem. Phys. 2023, 197, 105654. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.W.; Hu, Q.B.; Weng, Q.F.; Wang, J.J. Hemocytin, the special aggregation factor connecting insect hemolymph immunity, a potential target of insecticidal immunosuppresant. Pestic. Biochem. Phys. 2024, 198, 105704. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, R.D.B.; dos Santos-Mallet, J.R.; Lowenberger, C.; Ventura, A.; Golo, P.S.; Bittencourt, V.; Angelo, I.D. A Novel Model of Pathogenesis of Metarhizium anisopliae Propagules through the Midguts of Aedes aegypti Larvae. Insects 2023, 14, 328. [Google Scholar] [CrossRef]
- Barelli, L.; Behie, S.W.; Hu, S.S.; Bidochka, M.J. Profiling Destruxin Synthesis by Specialist and Generalist Metarhizium Insect Pathogens during Coculture with Plants. Appl. Environ. Microbiol. 2022, 88, e0247421. [Google Scholar] [CrossRef]
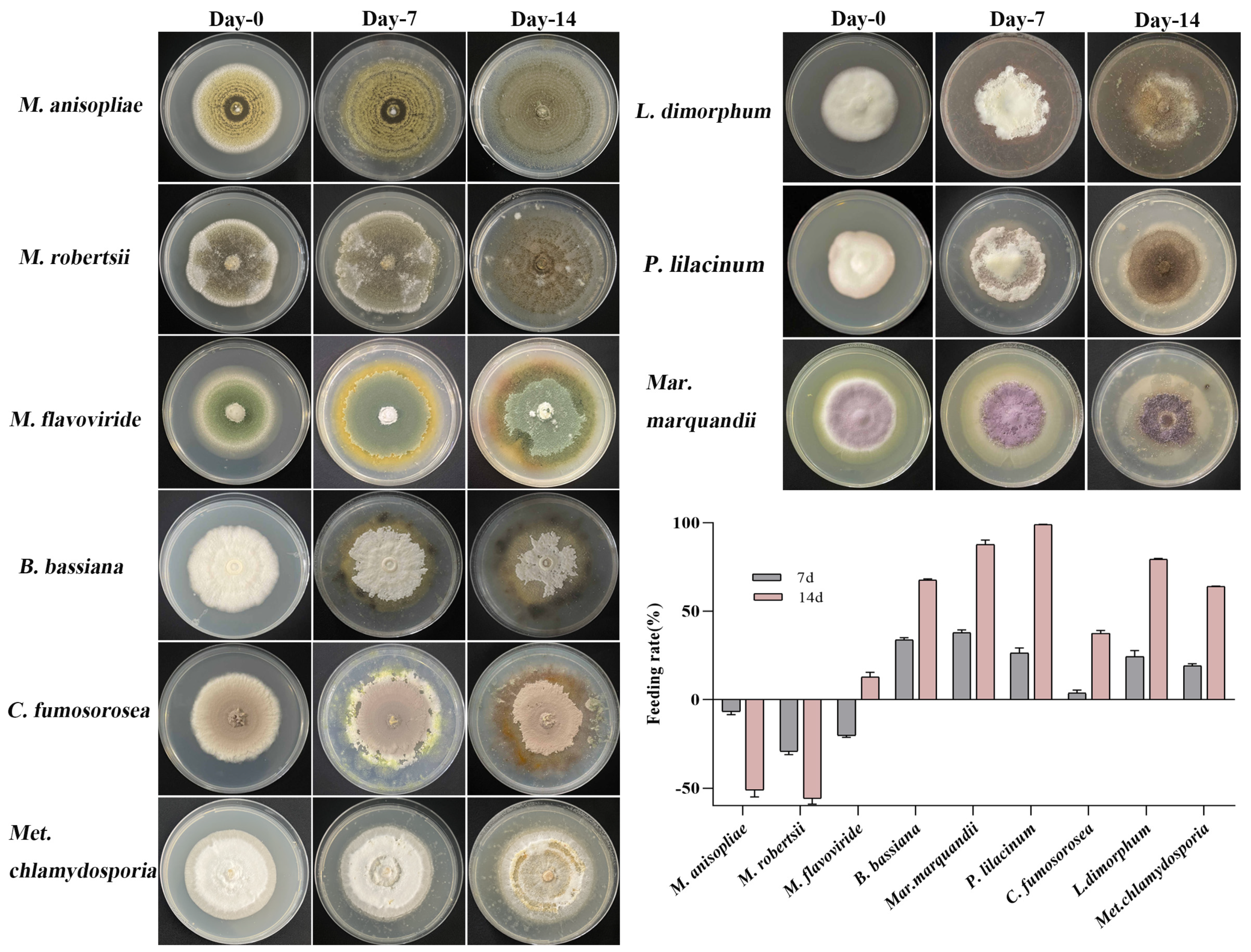





| Strain No. | Species |
|---|---|
| MaGX3701 | Metarhizium anisopliae |
| MrGX0603 | Metarhizium robertsii |
| MfGX33Y01 | Metarhizium flavoviride |
| BbGX6502 | Beauveria bassiana |
| PiGX19S01 | Purpureocillium lilacinum |
| MmGX4202 | Marquandii marquandii |
| CfGX4206 | Cordyceps fumosorosea |
| LdGX4702 | Lecanicillium dimorphum |
| McGX08A01 | Metacordyceps chlamydosporia |
| Quadrant | I | II | III |
|---|---|---|---|
| Group 1 | M. anisopliae | B. bassiana | Met. chlamydosporia |
| Group 2 | B. bassiana | Met. chlamydosporia | M. robertsii |
| Group 3 | Met. chlamydosporia | M. robertsii | C. fumosorosea |
| Group 4 | M. robertsii | C. fumosorosea | L. dimorphum |
| Group 5 | C. fumosorosea | L. dimorphum | M. flavoviride |
| Group 6 | L. dimorphum | M. flavoviride | Mar. marquandii |
| Group 7 | M. flavoviride | Mar. marquandii | P. lilacinum |
| Group 8 | Mar. marquandii | P. lilacinum | M. anisopliae |
| Group 9 | P. lilacinum | M. anisopliae | B. bassiana |
| Group 10 | Met. chlamydosporia | L. dimorphum | M. anisopliae |
| EPF | Mite Developmental Duration (d) | |||||||
|---|---|---|---|---|---|---|---|---|
| Egg | Larvae | Quiescence | First Instar Nymph | Quiescence | Second Instar Nymph | Quiescence | Total | |
| M. flavoviride | 3.71 ± 0.05 a | 1.94 ± 0.07 a | 0.65 ± 0.04 cd | 1.69 ± 0.03 b | 0.58 ± 0.04 c | 1.75 ± 0.06 ab | 0.75 ± 0.05 c | 11.06 ± 0.07 b |
| B. bassiana | 3.65 ± 0.07 a | 1.54 ± 0.06 c | 0.54 ± 0.03 d | 1.33 ± 0.05 d | 0.63 ± 0.04 bc | 1.58 ± 0.04 bc | 0.86 ± 0.05 abc | 10.13 ± 0.10 cd |
| P. lilacinum | 3.50 ± 0.09 ab | 1.80 ± 0.08 b | 0.73 ± 0.06 bc | 1.52 ± 0.08 bc | 0.74 ± 0.05 ab | 1.72 ± 0.05 ab | 0.79 ± 0.05 bc | 10.08 ± 0.17 cd |
| Mar. marquandii | 3.14 ± 0.05 c | 1.64 ± 0.04 bc | 0.61 ± 0.03 cd | 1.08 ± 0.04 e | 0.58 ± 0.04 c | 1.73 ± 0.04 ab | 0.79 ± 0.05 bc | 9.57 ± 0.08 d |
| C. fumosorosea | 3.62 ± 0.05 a | 1.34 ± 0.04 d | 0.64 ± 0.04 cd | 1.32 ± 0.06 d | 0.80 ± 0.05 a | 1.83 ± 0.07 a | 0.86 ± 0.05 abc | 10.42 ± 0.14 c |
| L. dimorphum | 3.34 ± 0.07 b | 1.65 ± 0.05 bc | 0.83 ± 0.06 ab | 1.35 ± 0.06 cd | 0.70 ± 0.05 abc | 1.52 ± 0.05 c | 0.93 ± 0.03 ab | 10.34 ± 0.09 c |
| Met. chlamydosporia | 3.57 ± 0.08 a | 1.99 ± 0.07 a | 0.91 ± 0.03 a | 1.95 ± 0.09 a | 0.81 ± 0.04 a | 1.73 ± 0.04 ab | 1.00 ± 0.07 a | 11.97 ± 0.13 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, C.; Chen, Q.; Hu, X.; Zeng, Y.; Zhang, K.; Hu, Q.; Weng, Q. Mycophagous Mite, Tyrophagus putrescentiae, Prefers to Feed on Entomopathogenic Fungi, except Metarhizium Generalists. Microorganisms 2024, 12, 1042. https://doi.org/10.3390/microorganisms12061042
Ou C, Chen Q, Hu X, Zeng Y, Zhang K, Hu Q, Weng Q. Mycophagous Mite, Tyrophagus putrescentiae, Prefers to Feed on Entomopathogenic Fungi, except Metarhizium Generalists. Microorganisms. 2024; 12(6):1042. https://doi.org/10.3390/microorganisms12061042
Chicago/Turabian StyleOu, Cuiyi, Qichun Chen, Xiangyu Hu, Yuhao Zeng, Ke Zhang, Qiongbo Hu, and Qunfang Weng. 2024. "Mycophagous Mite, Tyrophagus putrescentiae, Prefers to Feed on Entomopathogenic Fungi, except Metarhizium Generalists" Microorganisms 12, no. 6: 1042. https://doi.org/10.3390/microorganisms12061042
APA StyleOu, C., Chen, Q., Hu, X., Zeng, Y., Zhang, K., Hu, Q., & Weng, Q. (2024). Mycophagous Mite, Tyrophagus putrescentiae, Prefers to Feed on Entomopathogenic Fungi, except Metarhizium Generalists. Microorganisms, 12(6), 1042. https://doi.org/10.3390/microorganisms12061042






