The Heterogeneous Habitat of Taiga Forests Changes the Soil Microbial Functional Diversity
Abstract
1. Introduction
2. Materials and Methods
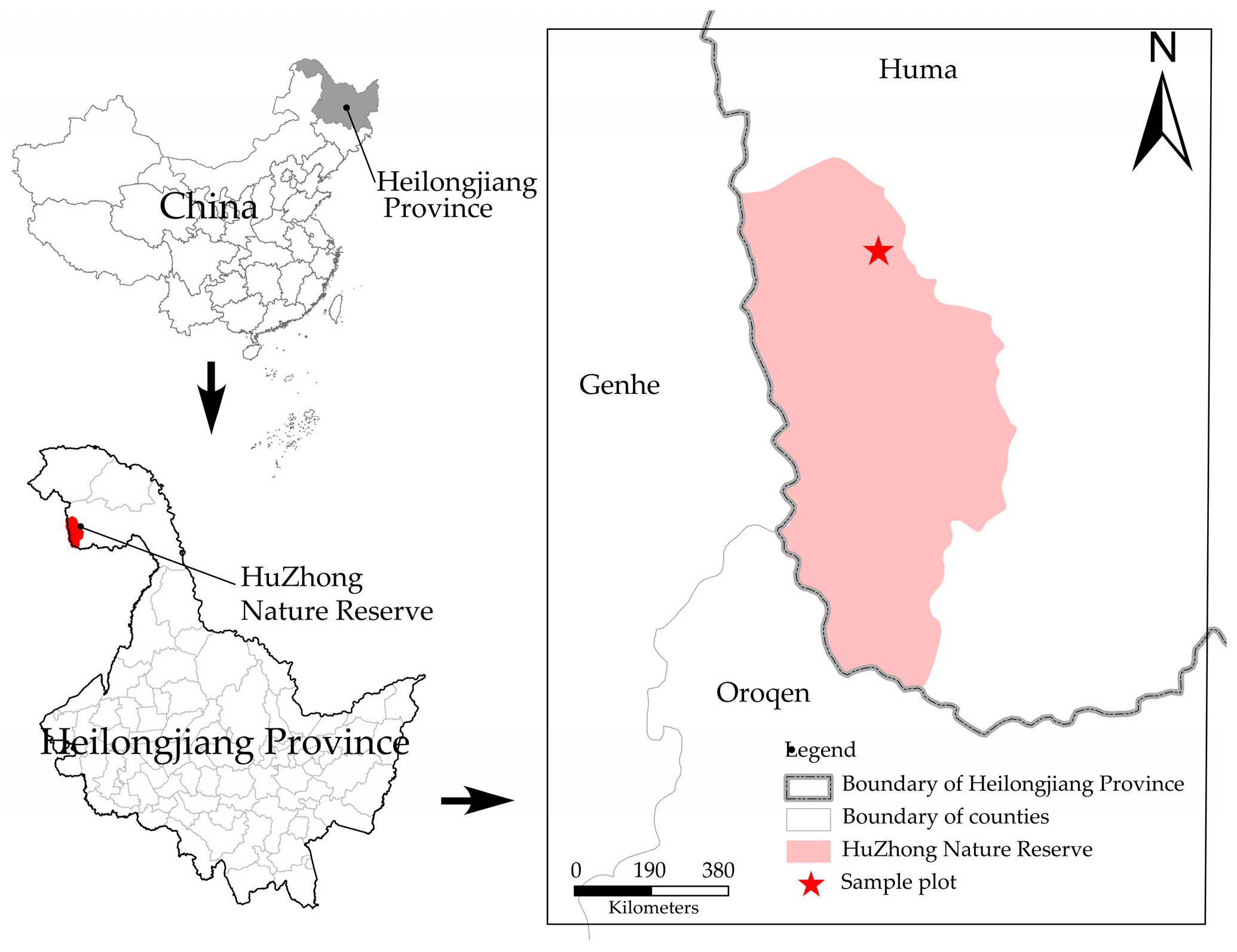
2.1. Overview of the Study Area
2.2. Experimental Design and Soil Sample Collection
2.3. Analyzing Soil Chemical and Physical Properties
2.4. Features of Soil Microbial Carbon Source Utilization
2.5. Data Processing and Analysis
3. Results
3.1. Differences in Soil Physical and Chemical Properties
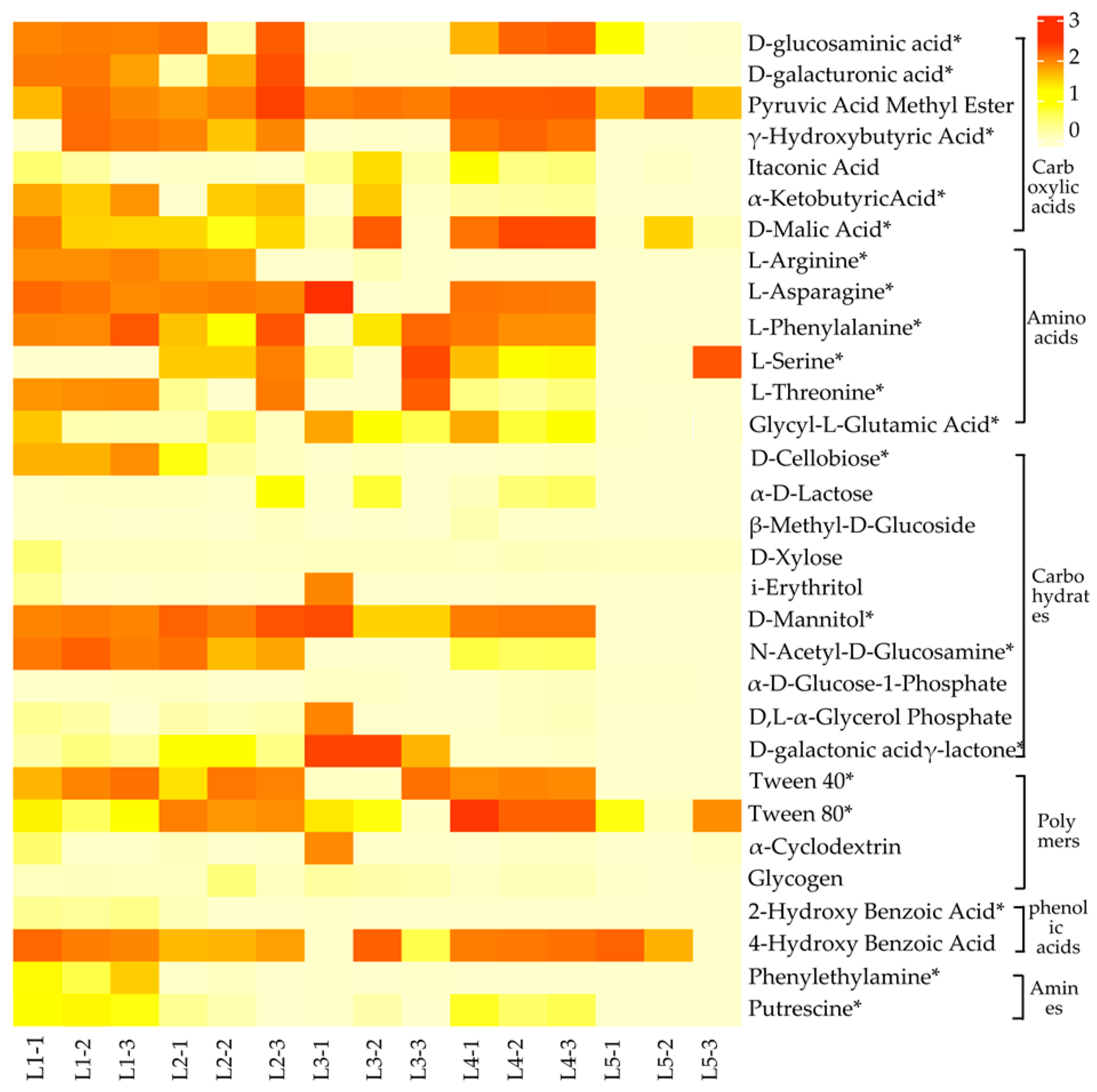
3.2. Differences in the Utilization of Different Carbon Sources by Soil Microorganisms
3.3. Analysis of Soil Microbial Functional Diversity
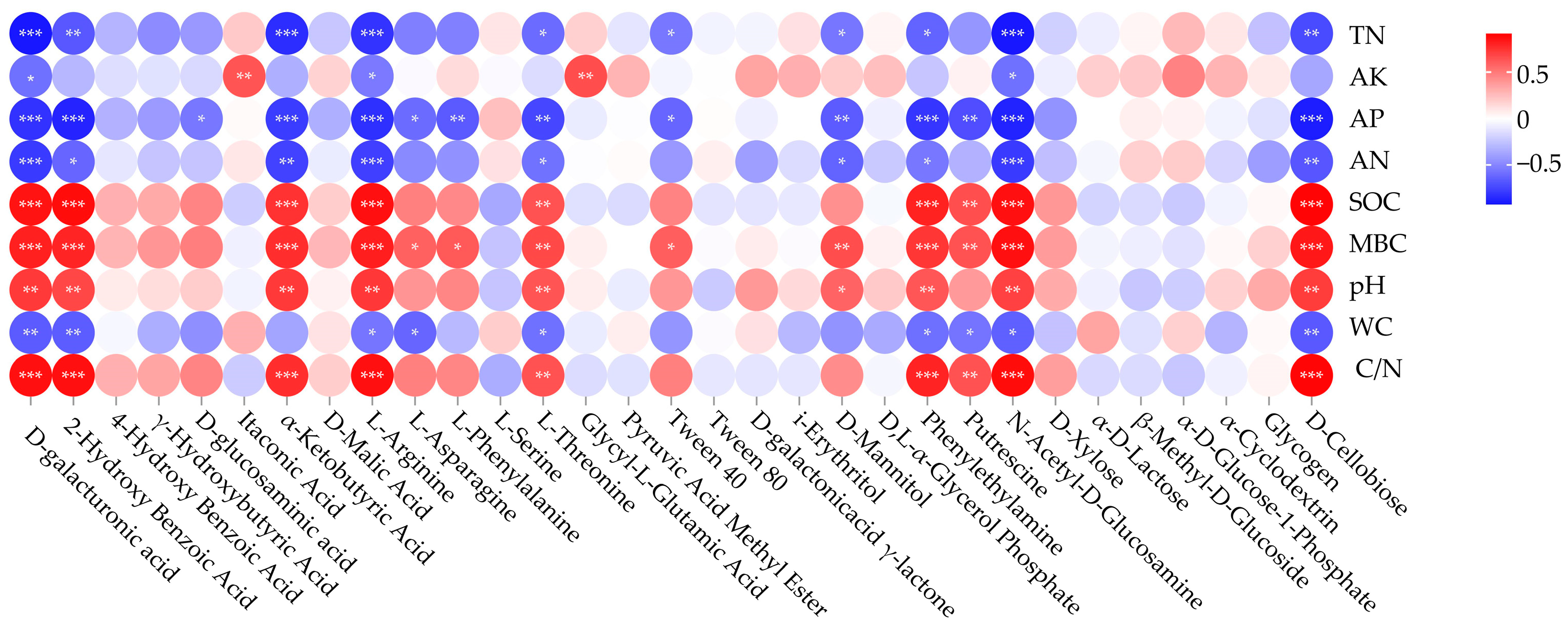
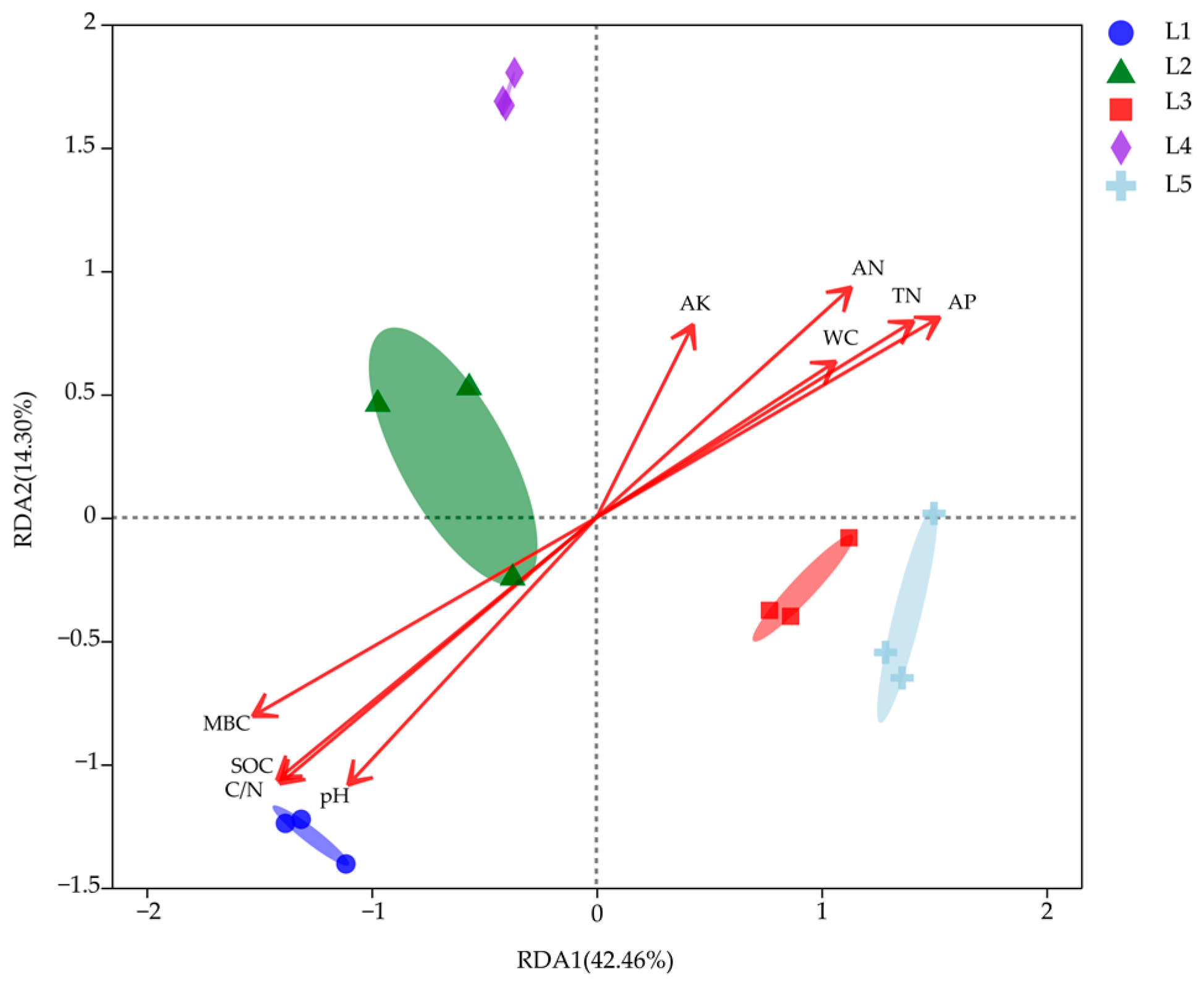
3.4. Correlation between Soil Microbial Functional Diversity and Soil Physical and Chemical Properties
4. Discussion
4.1. Effects of Heterogeneous Habitats on the Ability of Soil Microbial Carbon Source Utilization in Taiga Forests
4.2. Effects of Heterogeneous Habitats on Soil Microbial Functional Diversity in Taiga Forests
5. Conclusions
- (1)
- The capacity of soil microbes to utilize carbon sources in taiga forests with different important values was significantly different, with higher utilization of carboxylic acids, amino acids, and carbohydrates than polymers, phenolic acids, and amines.
- (2)
- The soil microbial functional diversity decreased significantly with the increase in the important value of Larix gmelinii, and soil TN, AP, AN, SOC, MBC, pH, C/N, and WC were the main influencing factors.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lohbeck, M.; Bongers, F.; Martinez-Ramos, M.; Poorter, L. The importance of biodiversity and dominance for multiple ecosystem functions in a human-modified tropical landscape. Ecology 2016, 97, 2772–2779. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Li, H.Q.; Zhang, L.; Zhu, J.B.; He, H.D.; Wei, Y.X.; Li, Y.N. Characteristics of soil water percolation and dissolved organic carbon leaching and their response to long-term fencing in an alpine meadow on the Tibetan Plateau. Environ. Earth Sci. 2016, 75, 1471. [Google Scholar] [CrossRef]
- Mishra, A.K.; Sudalaimuthuasari, N.; Hazzouri, K.M.; Saeed, E.E.; Shah, I.; Amiri, K.M.A. Tapping into plant-microbiome interactions through the lens of multi-omics techniques. Cells 2022, 11, 3254. [Google Scholar] [CrossRef]
- Li, H.L.; Xu, Z.W.; Yang, S.; Li, X.B.; Top, E.M.; Wang, R.Z.; Zhang, Y.G.; Cai, J.P.; Yao, F.; Han, X.G.; et al. Responses of soil bacterial communities to nitrogen deposition and precipitation increment are closely linked with aboveground community variation. Microb. Ecol. 2016, 71, 974–989. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, R.D.; Manning, P.; Morriën, E.; De Vries, F.T. Hierarchical responses of plant-soil interactions to climate change: Consequences for the global carbon cycle. J. Ecol. 2013, 101, 334–343. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.A.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Bever, J.D.; Dickie, I.A.; Facelli, E.; Klironomos, J.; Moora, M.; Rillig, M.C.; Stock, W.D.; Tibbett, M.; Zobel, M. Rooting theories of plant community ecology in microbial interactions. Trends Ecol. Evol. 2010, 25, 468–478. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Van Der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Steinauer, K.; Chatzinotas, A.; Eisenhauer, N. Root exudate cocktails: The link between plant diversity and soil microorganisms? Ecol. Evol. 2016, 6, 7387–7396. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Ma, S.Q.; Jiang, H.M.; Hu, Y.; Lu, X.Y. Influences of litter diversity and soil moisture on soil microbial communities in decomposing mixed litter of alpine steppe species. Geoderma 2020, 377, 114577. [Google Scholar] [CrossRef]
- Liu, Z.F.; Liu, G.H.; Fu, B.J.; Zheng, X.X. Relationship between plant species diversity and soil microbial functional diversity along a longitudinal gradient in temperate grasslands of Hulunbeir, Inner Mongolia, China. Ecol. Res. 2008, 23, 511–518. [Google Scholar] [CrossRef]
- Stephan, A.; Meyer, A.H.; Schmid, B. Plant diversity affects culturable soil bacteria in experimental grassland communities. J. Ecol. 2000, 22, 988–998. [Google Scholar] [CrossRef]
- Loranger-Merciris, G.; Barthes, L.; Gastine, A.; Leadley, P. Rapid effects of plant species diversity and identity on soil microbial communities in experimental grassland ecosystems. Soil Biol. Biochem. 2006, 38, 2336–2343. [Google Scholar] [CrossRef]
- Powell, J.R.; Karunaratne, S.; Campbell, C.D.; Yao, H.Y.; Robinson, L.; Singh, B.K. Deterministic processes vary during community assembly for ecologically dissimilar taxa. Nat. Commun. 2015, 6, 8444. [Google Scholar] [CrossRef] [PubMed]
- Khlifa, R.; Paquette, A.; Messier, C.; Reich, P.B.; Munson, A.D. Do temperate tree species diversity and identity influence soil microbial community function and composition? Ecol. Evol. 2017, 7, 7965–7974. [Google Scholar] [CrossRef]
- Cheng, Z.C.; Wu, S.; Du, J.; Pan, H.; Lu, X.M.; Liu, Y.Z.; Yang, L.B. Variations in the Diversity and Biomass of Soil Bacteria and Fungi under Different Fire Disturbances in the Taiga Forests of Northeastern China. Forests 2023, 14, 2063. [Google Scholar] [CrossRef]
- Vuong, T.M.D.; Zeng, J.Y.; Man, X.L. Soil fungal and bacterial communities in southern boreal forests of the Greater Khingan Mountains and their relationship with soil properties. Sci. Rep. 2020, 10, 22025. [Google Scholar] [CrossRef] [PubMed]
- Zhai, K.T.; Hua, Y.C.; Liang, J.W.; Li, J.; Wang, Z.R.; Liu, L.; Gao, M.L.; Sa, R.L.; Zhao, M.M. Soil microbial diversity under different types of interference in birch secondary forest in the Greater Khingan Mountains in China. Front. Microbiol. 2023, 14, 1267746. [Google Scholar] [CrossRef]
- Jiang, S.; Xing, Y.J.; Liu, G.C.; Hu, C.Y.; Wang, X.C.; Yan, G.Y.; Wang, Q.G. Changes in soil bacterial and fungal community composition and functional groups during the succession of boreal forests. Soil Biol. Biochem. 2021, 161, 108393. [Google Scholar] [CrossRef]
- Zhu, J.J.; Mao, Z.H.; Hu, L.L.; Zhang, J.X. Plant diversity of secondary forests in response to anthropogenic disturbance levels in montane regions of northeastern China. J. For. Res. 2007, 12, 403–416. [Google Scholar] [CrossRef]
- Campos, P.V.; Villa, P.M.; Nunes, J.A.; Schaefer, C.E.G.R.; Porembski, S.; Neri, A.V. Plant diversity and community structure of Brazilian Páramos. J. Mt. Sci. 2018, 15, 1186–1198. [Google Scholar] [CrossRef]
- Cheng, Z.C.; Wu, S.; Du, J.; Liu, Y.Z.; Sui, X.; Yang, L.B. Reduced arbuscular mycorrhizal fungi (AMF) diversity in light and moderate fire sites in Taiga forests, Northeast China. Microorganisms 2023, 11, 1836. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.B.; Yang, L.B.; Wu, S.; Zhou, T. Warming changes the composition and diversity of fungal communities in permafrost. Ann. Microbiol. 2023, 73, 7. [Google Scholar] [CrossRef]
- Jiang, P.; Ye, J.; Deng, H.B.; Cui, G.F. Variations of population structure and important value of the main edificators along the elevation gradient on the northern slope of Changbai Mountain. J. For. Res. 2003, 14, 117–121. [Google Scholar] [CrossRef]
- Zhou, T.; Wu, S.; Pan, H.; Lu, X.M.; Du, J.; Yang, L.B. Heterogeneous habitats in Taiga Forests with different important values of constructive species changes bacterial beta diversity. Microorganisms 2023, 11, 2609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Fan, S.H.; Guan, F.Y.; Yan, X.R.; Yin, Z.X. Soil bacterial community structure of mixed bamboo and broad-leaved forest based on tree crown width ratio. Sci. Rep. 2020, 10, 6522. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.A.; Lei, X.M.; Zhao, L.X.; Brookes, P.C.; Wang, F.; Chen, C.R.; Yang, W.H.; Xing, S.H. Fungal communities and functions response to long-term fertilization in paddy soils. Appl. Soil Ecol. 2018, 130, 251–258. [Google Scholar] [CrossRef]
- Liu, X.L.; Yang, W.P.; Li, W.G.; Ali, A.; Chen, J.; Sun, M.; Gao, Z.Q.; Yang, Z.P. Moderate organic fertilizer substitution for partial chemical fertilizer improved soil microbial carbon source utilization and bacterial community composition in rain-fed wheat fields: Current year. Front. Microbiol. 2023, 14, 1190052. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Li, C.S.; Feng, Q.; Wei, Y.P.; Zheng, H.; Zhao, Y.; Feng, Y.J.; Li, H.Y. Shifts in soil microbial metabolic activities and community structures along a salinity gradient of irrigation water in a typical arid region of China. Sci. Total Environ. 2017, 598, 64–70. [Google Scholar] [CrossRef]
- Kumar, U.; Shahid, M.; Tripathi, R.; Mohanty, S.; Kumar, A.; Bhattacharyya, P.; Lal, B.; Gautam, P.; Raja, R.; Panda, B.B.; et al. Variation of functional diversity of soil microbial community in sub-humid tropical rice-rice cropping system under long-term organic and inorganic fertilization. Ecol. Indic. 2017, 73, 536–543. [Google Scholar] [CrossRef]
- Seaton, F.M.; George, P.B.L.; Lebron, I.; Jones, D.L.; Creer, S.; Robinson, D.A. Soil textural heterogeneity impacts bacterial but not fungal diversity. Soil Biol. Biochem. 2020, 144, 107766. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Benavent-González, A.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K.; Fierer, N. A global atlas of the dominant bacteria found in soil. Science 2018, 359, 320–325. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, P.; Wang, Y.H.; Li, B.; Wang, Y.X. Effects of roxarsone on the functional diversity of soil microbial community. Int. Biodeterior. Biodegrad. 2013, 76, 32–35. [Google Scholar] [CrossRef]
- Ji, L.; Yang, Y.C.; Yang, L.X. Seasonal variations in soil fungal communities and co-occurrence networks along an altitudinal gradient in the cold temperate zone of China: A case study on Oakley Mountain. Catena 2021, 204, 105448. [Google Scholar] [CrossRef]
- Zhang, W.W.; Yang, K.; Lyu, Z.T.; Zhu, J.J. Microbial groups and their functions control the decomposition of coniferous litter: A comparison with broadleaved tree litters. Soil Biol. Biochem. 2019, 133, 196–207. [Google Scholar] [CrossRef]
- Wang, S.Q.; Li, T.X.; Zheng, Z.C.; Chen, H.Y.H. Soil aggregate-associated bacterial metabolic activity and community structure in different aged tea plantations. Sci. Total Environ. 2019, 654, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.L.; Li, J.X.; Jiang, Q.; Zhao, H.Y. Soil microbial carbon metabolism and enzyme activity of Larix olgensis along an altitudinal gradient on the eastern slope of Changbai Mountain, Northeast China. Ecol. Environ. Sci. 2019, 28, 652–660. [Google Scholar]
- Brackin, R.; Robinson, N.; Lakshmanan, P.; Schmidt, S. Microbial function in adjacent subtropical forest and agricultural soil. Soil Biol. Biochem. 2013, 57, 68–77. [Google Scholar] [CrossRef]
- Nayyar, A.; Hamel, C.; Lafond, G.; Gossen, B.D.; Hanson, K.; Germida, J. Soil microbial quality associated with yield reduction in continuous-pea. Appl. Soil Ecol. 2009, 43, 115–121. [Google Scholar] [CrossRef]
- Chen, X.L.; Wang, D.; Chen, X.; Wang, J.; Diao, J.J.; Zhang, J.Y.; Guan, Q.W. Soil microbial functional diversity and biomass as affected by different thinning intensities in a Chinese fir plantation. Appl. Soil Ecol. 2015, 92, 35–44. [Google Scholar] [CrossRef]
- Bonner, M.T.L.; Allen, D.E.; Brackin, R.; Smith, T.E.; Lewis, T.; Shoo, L.P.; Schmidt, S. Tropical rainforest restoration plantations are slow to restore the soil biological and organic carbon characteristics of old growth rainforest. Microb. Ecol. 2020, 79, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.L.; Tang, Z.Y.; Liu, J.M.; Tong, B.L. Rhizosphere microbial community structure and its correlation with soil nutrients in Cinnamomum migao. J. Northeast For. Univ. 2023, 51, 92–97+105. [Google Scholar]
- Escalas, A.; Hale, L.; Voordeckers, J.W.; Yang, Y.F.; Firestone, M.K.; Alvarez-Cohen, L.; Zhou, J.Z. Microbial functional diversity: From concepts to applications. Ecol. Evol. 2019, 9, 12000–12016. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.C.; Yang, L.B.; Sui, X.; Zhang, T.; Wang, W.H.; Yin, W.P.; Li, G.F.; Song, F.Q. Soil microbial functional diversity responses to different revegetation types in Heilongjiang Zhongyangzhan Black-Billed Capercaillie Nature Reserve. Res. Environ. Sci. 2021, 34, 1177–1186. [Google Scholar]
- Wang, X.; Gao, S.H.; Chen, J.Q.; Yao, Z.W.; Zhang, L.; Wu, H.L.; Shu, Q.; Zhang, X.D. Response of functional diversity of soil microbial community to forest cutting and regeneration methodology in a Chinese fir plantation. Forests 2022, 13, 360. [Google Scholar] [CrossRef]
- Yue, X.J.; Ye, G.F.; Chen, M.Y.; Gao, W.; Li, D.; Zou, Y.H. Microbial functional diversity and corresponding impact factors of five typical forests on coast sandy land in South Asian subtropical zone. J. Cent. South Univ. For. Technol. 2019, 39, 1–10. [Google Scholar]
- Tamme, R.; Hiiesalu, I.; Laanisto, L.; Szava-Kovats, R.; Pärtel, M. Environmental heterogeneity, species diversity and co-existence at different spatial scales. J. Veg. Sci. 2010, 2, 796–801. [Google Scholar] [CrossRef]
- Tian, J.; McCormack, L.; Wang, J.Y.; Guo, D.L.; Wang, Q.F.; Zhang, X.Y.; Yu, G.R.; Blagodatskaya, E.; Kuzyakov, Y. Linkages between the soil organic matter fractions and the microbial metabolic functional diversity within a broad-leaved Korean pine forest. Eur. J. Soil Biol. 2015, 66, 57–64. [Google Scholar] [CrossRef]
- Su, S.F.; Wang, X.Y.; Lin, Z.P.; Jin, Y.H.; Xue, Y. The characteristics of soil microbial functional diversity of six types of vegetation in tropical regions. J. Yunnan Agric. Univ. (Nat. Sci.) 2022, 37, 505–514. [Google Scholar]
- Zhang, X.F.; Xu, S.J.; Li, C.M.; Zhao, L.; Feng, H.Y.; Yue, G.Y.; Ren, Z.W.; Cheng, G.D. The soil carbon/nitrogen ratio and moisture affect microbial community structures in alkaline permafrost-affected soils with different vegetation types on the Tibetan Plateau. Res. Microbiol. 2014, 165, 128–139. [Google Scholar] [CrossRef]
- Weng, X.H.; Sui, X.; Liu, Y.N.; Yang, L.B.; Zhang, R.T. Effect of nitrogen addition on the carbon metabolism of soil microorganisms in a Calamagrostis angustifolia wetland of the Sanjiang Plain, northeastern China. Ann. Microbiol. 2022, 72, 18. [Google Scholar] [CrossRef]
- Shen, C.C.; Xiong, J.B.; Zhang, H.Y.; Feng, Y.Z.; Lin, X.G.; Li, X.Y.; Liang, W.J.; Chu, H.Y. Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain. Soil Biol. Biochem. 2013, 57, 204–211. [Google Scholar] [CrossRef]
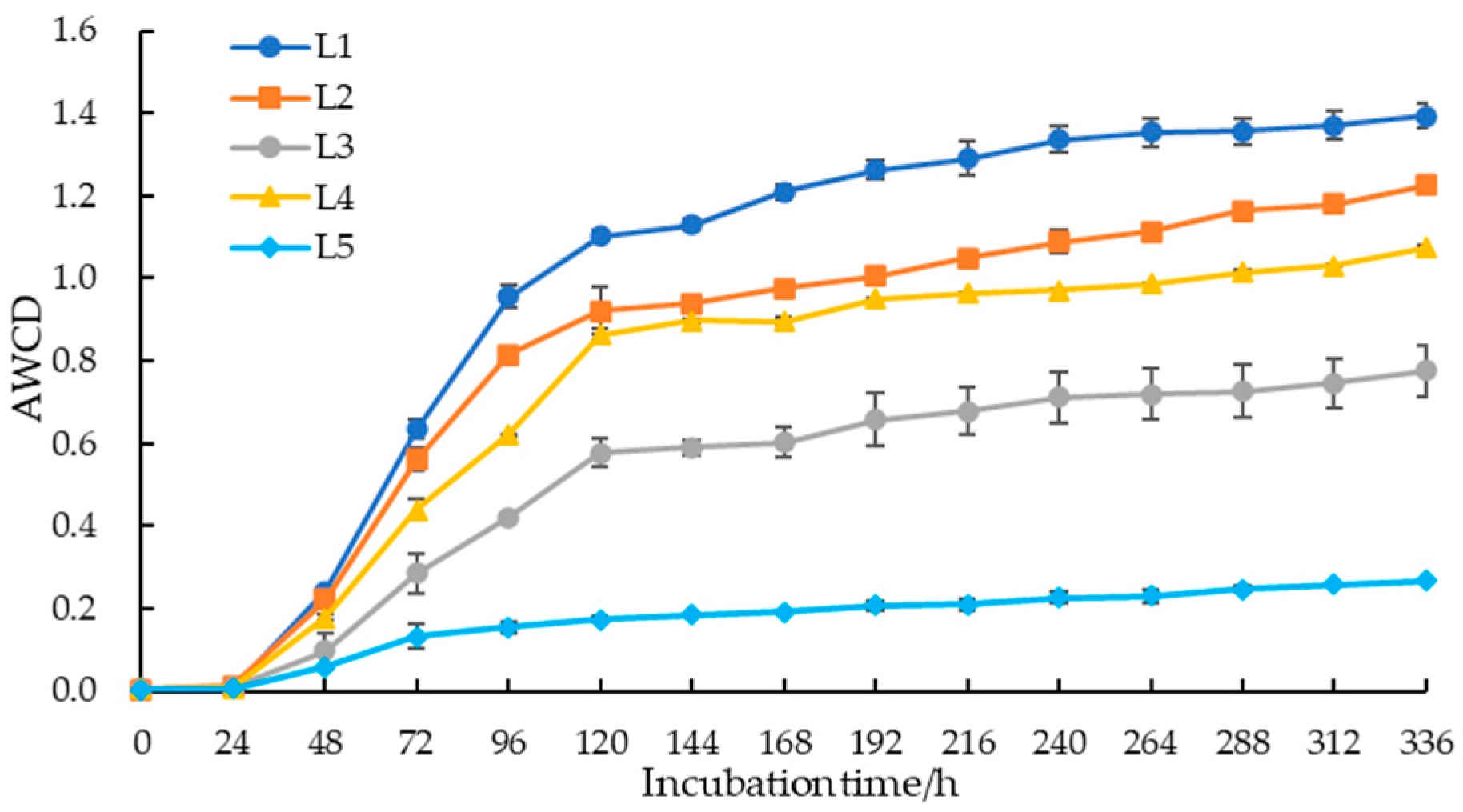
| Experimental Group | AWCD | Shannon Index | Simpson Index | McIntosh Index |
|---|---|---|---|---|
| L1 | 1.10 ± 0.02 a | 3.01 ± 0.03 a | 0.95 ± 0.00 a | 7.85 ± 0.05 a |
| L2 | 0.92 ± 0.06 b | 2.87 ± 0.01 b | 0.94 ± 0.00 a | 7.11 ± 0.41 b |
| L3 | 0.58 ± 0.04 c | 2.38 ± 0.07 c | 0.90 ± 0.01 b | 5.75 ± 0.23 c |
| L4 | 0.86 ± 0.01 b | 2.78 ± 0.01 b | 0.93 ± 0.00 a | 7.04 ± 0.07 b |
| L5 | 0.19 ± 0.00 d | 1.30 ± 0.06 d | 0.70 ± 0.01 c | 3.21 ± 0.10 d |
| TN | AK | AP | AN | SOC | MBC | pH | WC | C/N | |
|---|---|---|---|---|---|---|---|---|---|
| AWCD | −0.751 ** | −0.058 | −0.858 *** | −0.690 ** | 0.732 ** | 0.865 *** | 0.684 ** | −0.579 * | 0.738 ** |
| Shannon index | −0.595 ** | 0.196 | −0.729 *** | −0.653 ** | 0.521 * | 0.748 *** | 0.654 ** | −0.414 | 0.529 * |
| Simpson index | −0.668 * | 0.083 | −0.789 ** | −0.678 ** | 0.615 * | 0.806 ** | 0.670 ** | −0.463 | 0.622 * |
| McIntosh index | −0.692 ** | 0.054 | −0.812 *** | −0.662 ** | 0.650 ** | 0.818 *** | 0.657 ** | −0.550 * | 0.658 ** |
| Soil Factor | R2 | p-Value |
|---|---|---|
| TN | 0.806 | 0.001 |
| AK | 0.235 | 0.196 |
| AP | 0.920 | 0.001 |
| AN | 0.658 | 0.006 |
| SOC | 0.966 | 0.001 |
| MBC | 0.918 | 0.001 |
| pH | 0.730 | 0.003 |
| WC | 0.465 | 0.024 |
| C/N | 0.968 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, T.; Wu, S.; Gao, M.; Yang, L. The Heterogeneous Habitat of Taiga Forests Changes the Soil Microbial Functional Diversity. Microorganisms 2024, 12, 959. https://doi.org/10.3390/microorganisms12050959
Zhou T, Wu S, Gao M, Yang L. The Heterogeneous Habitat of Taiga Forests Changes the Soil Microbial Functional Diversity. Microorganisms. 2024; 12(5):959. https://doi.org/10.3390/microorganisms12050959
Chicago/Turabian StyleZhou, Tian, Song Wu, Mingliang Gao, and Libin Yang. 2024. "The Heterogeneous Habitat of Taiga Forests Changes the Soil Microbial Functional Diversity" Microorganisms 12, no. 5: 959. https://doi.org/10.3390/microorganisms12050959
APA StyleZhou, T., Wu, S., Gao, M., & Yang, L. (2024). The Heterogeneous Habitat of Taiga Forests Changes the Soil Microbial Functional Diversity. Microorganisms, 12(5), 959. https://doi.org/10.3390/microorganisms12050959





