Fermented Rapeseed and Soybean Alone and in Combination with Macro Algae Inhibit Human and Pig Pathogenic Bacteria In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Extracts of Fermented Products for Investigation of Antimicrobial Activity
2.2. LC-MS Analysis
2.3. Bacterial Strains and Propagation Conditions
2.4. Plate Well Diffusion Assay
2.5. Thermal Stability and Resistance to Proteolytic and Lipolytic Enzymes of Antimicrobial Compounds
2.6. Statistics and Data Visualization
3. Results
3.1. Extracts of Fermented Plant Material Contain Higher Amounts of Secondary Metabolites, Relative to Unfermented Control Products
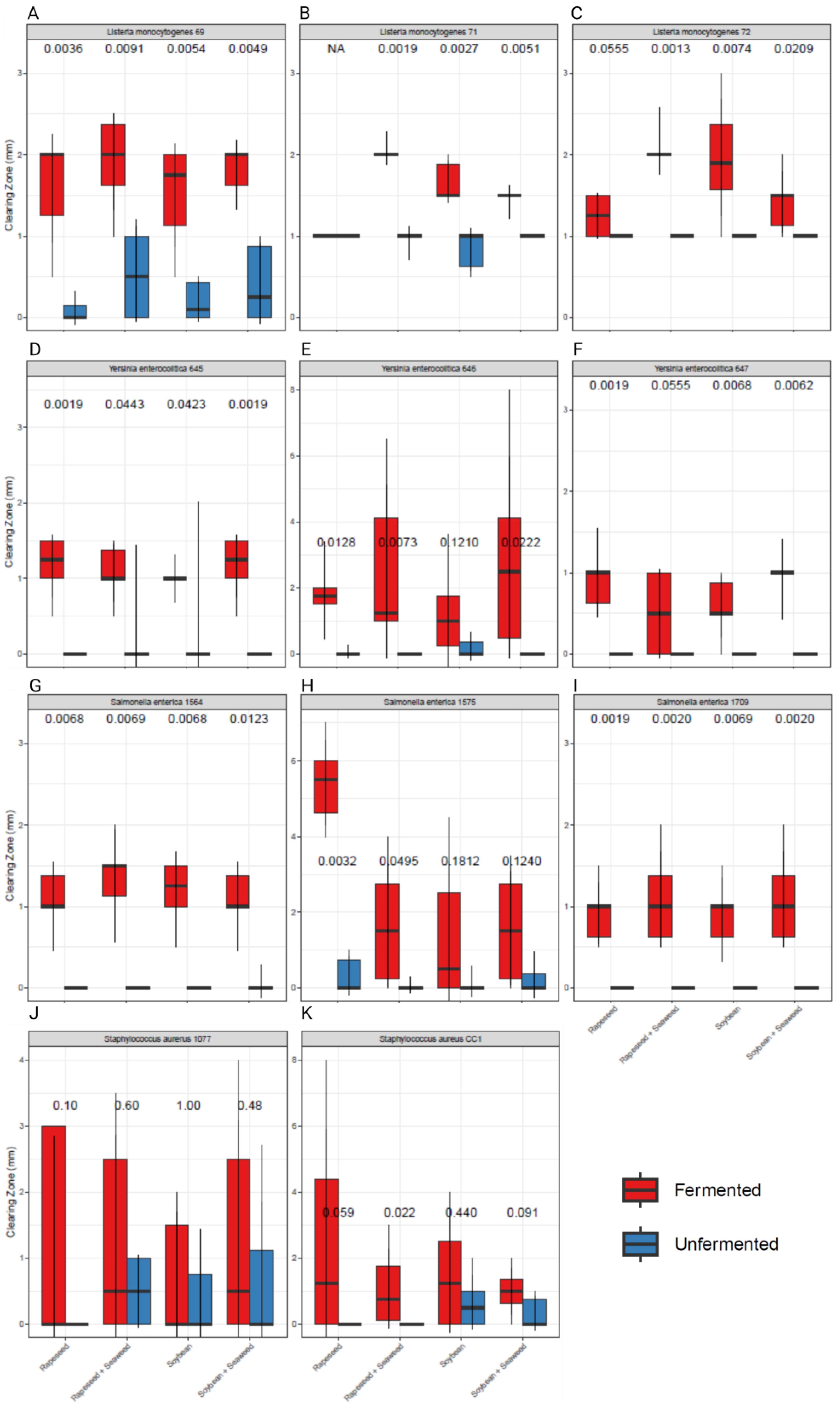
3.2. Fermented Soybean- and Rapeseed-Based Supplements Exhibit Greater Inhibition of Microbial Pathogens Compared to Unfermented Controls
3.3. Heat Treatment of Extracted Metabolites Lowers the Antimicrobial Potential
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Anadón, A.; Martínez-Larrañaga, M.R.; Martínez, M.A. Probiotics for animal nutrition in the European Union. Regulation and safety assessment. Regul. Toxicol. Pharmacol. 2006, 45, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Larsen, M.M.; Bak, J. National monitoring study in Denmark finds increased and critical levels of copper and zinc in arable soils fertilized with pig slurry. Environ. Pollut. 2016, 214, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Ciesinski, L.; Guenther, S.; Pieper, R.; Kalisch, M.; Bednorz, C.; Wieler, L.H. High dietary zinc feeding promotes persistence of multi-resistant E. coli in the swine gut. PLoS ONE 2018, 13, e0191660. [Google Scholar] [CrossRef] [PubMed]
- Heinzl, I.; Barbosa, F.F.; Nutrition, E.W. The Zinc Oxide Ban: What Led to It, What Are the Alternatives? Zinc Oxide Disadvantages Outweigh Advantages. 2022. Available online: https://ew-nutrition.com/en-uk/page/3/?s=how+to+get+cash+from+paypal+instantlyVisit+Site+Sig8.comhow+to+transfer+paypal+to+gcashhow+to+transfer+money+from+bank+account+to+paypal+instantlysending+money+through+paypalhow+to+pay+someone+on+paypalpaypal+flipstransfer+money+from+credit+card+to+paypalpaypal+wire+transferpay+someone+with+paypalhow+to+transfer+money+from+paypal+to+bankhow+to+receive+money+on+paypal+from+a+friend....a09f&print=print-search (accessed on 1 February 2020).
- Ming, D.; Wang, W.; Huang, C.; Wang, Z.; Shi, C.; Ding, J.; Wang, F. Effects of Weaning Age at 21 and 28 Days on Growth Performance, Intestinal Morphology and Redox Status in Piglets. Animals 2021, 11, 2169. [Google Scholar] [CrossRef] [PubMed]
- Satessa, G.D.; Tamez-Hidalgo, P.; Hui, Y.; Cieplak, T.; Krych, L.; Kjærulff, S.; Nielsen, M.O. Impact of Dietary Supplementation of Lactic Acid Bacteria Fermented Rapeseed with or without Macroalgae on Performance and Health of Piglets Following Omission of Medicinal Zinc from Weaner Diets. Animals 2020, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Satessa, G.D.; Tamez-Hidalgo, P.; Kjærulff, S.; Vargas-Bello-Pérez, E.; Dhakal, R.; Nielsen, M.O. Effects of increasing doses of Lactobacillus pre-fermented rapeseed product with or without inclusion of macroalgae product on Weaner piglet performance and intestinal development. Animals 2020, 10, 559. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Tamez-Hidalgo, P.; Cieplak, T.; Satessa, G.D.; Kot, W.; Kjærulff, S.; Krych, L. Supplementation of a lacto-fermented rapeseed-seaweed blend promotes gut microbial-and gut immune-modulation in weaner piglets. J. Anim. Sci. Biotechnol. 2021, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Liu, X.; Xu, Z.R.; Lu, Y.P.; Liu, Y.Y. Effect of fermented soybean meal on intestinal morphology and digestive enzyme activities in weaned piglets. Dig. Dis. Sci. 2007, 52, 1845–1850. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, Y.; Yin, Y.; Wang, C.; Lu, Z.; Wang, F.; Wang, Y. Amino acid and phosphorus digestibility of fermented corn-soybean meal mixed feed with Bacillus subtilis and Enterococcus faecium fed to pigs1. J. Anim. Sci. 2017, 95, 3996–4004. [Google Scholar] [CrossRef]
- Tajima, K.; Ohmori, H.; Aminov, R.I.; Kobashi, Y.; Kawashima, T. Fermented liquid feed enhances bacterial diversity in piglet intestine. Anaerobe 2010, 16, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Ahluwalia, V.; Saran, S.; Kumar, J.; Patel, A.K.; Singhania, R.R. Recent developments on solid-state fermentation for production of microbial secondary metabolites: Challenges and solutions. Bioresour. Technol. 2021, 323, 124566. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Henika, P.R.; Mandrell, R.E. Antibacterial Activities of Phenolic Benzaldehydes and Benzoic Acids against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2003, 66, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, L.; Armstrong, F.; Stewart, J. Inhibition of Listeria monocytogenes and Salmonella enteriditis by combinations of plant oils and derivatives of benzoic acid: The development of synergistic antimicrobial combinations1The work in this manuscript is the subject of a patent application.1. Int. J. Antimicrob. Agents 1998, 9, 195–199. [Google Scholar] [CrossRef]
- Hadian, M.; Rajaei, A.; Mohsenifar, A.; Tabatabaei, M. Encapsulation of Rosmarinus officinalis essential oils in chitosan-benzoic acid nanogel with enhanced antibacterial activity in beef cutlet against Salmonella typhimurium during refrigerated storage. LWT 2017, 84, 394–401. [Google Scholar] [CrossRef]
- Kuk, J.-H.; Ma, S.-J.; Park, K.-H. Isolation and characterization of benzoic acid with antimicrobial activity from needle of Pinus densiflora. Korean J. Food Sci. Technol. 1997, 29, 204–210. [Google Scholar]
- Xie, Z.; Hu, L.; Li, Y.; Geng, S.; Cheng, S.; Fu, X.; Han, X. Changes of gut microbiota structure and morphology in weaned piglets treated with fresh fermented soybean meal. World J. Microbiol. Biotechnol. 2017, 33, 213. [Google Scholar] [CrossRef]
- Leonel, A.J.; Alvarez-Leite, J.I. Butyrate: Implications for intestinal function. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 474–479. Available online: https://journals.lww.com/co-clinicalnutrition/Fulltext/2012/09000/Butyrate__implications_for_intestinal_function.13.aspx (accessed on 1 September 2012). [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.-J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Lallès, J.-P.; Bosi, P.; Smidt, H.; Stokes, C.R. Nutritional management of gut health in pigs around weaning. Proc. Nutr. Soc. 2007, 66, 260–268. [Google Scholar] [CrossRef]
- Genovese, K.J.; Anderson, R.C.; Harvey, R.B.; Nisbet, D.J. Competitive exclusion treatment reduces the mortality and fecal shedding associated with enterotoxigenic Escherichia coli infection in nursery-raised neonatal pigs. Can. J. Vet. Res. Rev. Can. Rech. Vet. 2000, 64, 204–207. [Google Scholar]
- Genovese, K.J.; Anderson, R.C.; Harvey, R.B.; Callaway, T.R.; Poole, T.L.; Edrington, T.S.; Nisbet, D.J. Competitive Exclusion of Salmonella from the Gut of Neonatal and Weaned Pigs. J. Food Prot. 2003, 66, 1353–1359. [Google Scholar] [CrossRef][Green Version]
- Choct, M. Managing gut health through nutrition. Br. Poult. Sci. 2009, 50, 9–15. [Google Scholar] [CrossRef]
- McCullough, J.; Ratcliffe, B.; Mandir, N.; Carr, K.; Goodlad, R. Dietary fibre and intestinal microflora: Effects on intestinal morphometry and crypt branching. Gut 1998, 42, 799. [Google Scholar] [CrossRef]
- Van Der Hulst, R.R.; Von Meyenfeldt, M.F.; Van Kreel, B.K.; Thunnissen, F.B.; Brummer RJ, M.; Arends, J.W.; Soeters, P.B. Gut permeability, intestinal morphology, and nutritional depletion. Nutrition 1998, 14, 1–6. [Google Scholar] [CrossRef]
- Tenorio-Rodríguez, P.A.; Esquivel-Solis, H.; Murillo-Álvarez, J.I.; Ascencio, F.; Campa-Córdova, Á.I.; Angulo, C. Biosprospecting potential of kelp (Laminariales, Phaeophyceae) from Baja California Peninsula: Phenolic content, antioxidant properties, anti-inflammatory, and cell viability. J. Appl. Phycol. 2019, 31, 3115–3129. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- Vásquez, V.; Martínez, R.; Bernal, C. Enzyme-assisted extraction of proteins from the seaweeds Macrocystis pyrifera and Chondracanthus chamissoi: Characterization of the extracts and their bioactive potential. J. Appl. Phycol. 2019, 31, 1999–2010. [Google Scholar] [CrossRef]
- Mazid, M.; Khan, T.A.; Mohammad, F. Role of secondary metabolites in defense mechanisms of plants. Biol. Med. 2011, 3, 232–249. [Google Scholar]
- Kilic, N.K.; Erdem, K.; Donmez, G. Bioactive Compounds Produced by Dunaliella species, Antimicrobial Effects and Optimization of the Efficiency. Turk. J. Fish. Aquat. Sci. 2018, 19, 923–933. [Google Scholar]
- Lelario, F.; Scrano, L.; De Franchi, S.; Bonomo, M.G.; Salzano, G.; Milan, S.; Bufo, S.A. Identification and antimicrobial activity of most representative secondary metabolites from different plant species. Chem. Biol. Technol. Agric. 2018, 5, 13. [Google Scholar] [CrossRef]
- Bishehsari, F.; Engen, P.A.; Preite, N.Z.; Tuncil, Y.E.; Naqib, A.; Shaikh, M.; Keshavarzian, A. Dietary Fiber Treatment Corrects the Composition of Gut Microbiota, Promotes SCFA Production, and Suppresses Colon Carcinogenesis. Genes 2018, 9, 102. [Google Scholar] [CrossRef]
- Hong, K.-J.; Lee, C.-H.; Kim, S.W. Aspergillus oryzae GB-107 fermentation improves nutritional quality of food soybeans and feed soybean meals. J. Med. Food 2004, 7, 430–435. [Google Scholar] [CrossRef]
- Jamil, A.; Rofida, S.; Priyani, D.; Nabila, W.; Wulandari, E. Phytochemical Screening and Antimicrobial Activity of Limonia acidissima Ethanol Extract against Microbes from Clinical Isolates. 2020. Available online: https://www.scitepress.org/PublishedPapers/2019/91260/91260.pdf (accessed on 1 February 2020).
- IHolder, A.; Boyce, S.T. Agar well diffusion assay testing of bacterial susceptibility to various antimicrobials in concentrations non-toxic for human cells in culture. Burns 1994, 20, 426–429. [Google Scholar] [CrossRef]
- Wickham, H.; Chang, W.; Wickham, M.H. Package ‘ggplot2’. Creat. Elegant Data Vis. Using Gramm. Graphics. Version 2016, 2, 1–189. [Google Scholar]
- Wickham, H.; Wickham, M.H. Package Tidyverse. Easily Install Load ‘Tidyverse’. 2017. Available online: http://gwmodel.whu.edu.cn/mirrors/CRAN/web/packages/tidyverse/tidyverse.pdf (accessed on 1 February 2020).
- Wilke, C.O.; Wickham, H.; Wilke, M.C.O. Package ‘cowplot’. Streamlined Plot Theme Plot Annot. ‘ggplot2’. 2019. Available online: https://mirror.lyrahosting.com/CRAN/web/packages/cowplot/cowplot.pdf (accessed on 1 February 2020).
- Kassambara, A.; Kassambara, M.A. Package ‘ggpubr’, R Package Version 0.1; R Foundation: Vienna, Austria, 2020.
- Vettore, L.A.; Westbrook, R.L.; Tennant, D.A. Proline metabolism and redox; maintaining a balance in health and disease. Amino Acids 2021, 53, 1779–1788. [Google Scholar] [CrossRef]
- Lima, V.N.; Oliveira-Tintino, C.D.; Santos, E.S.; Morais, L.P.; Tintino, S.R.; Freitas, T.S.; Coutinho, H.D. Antimicrobial and enhancement of the antibiotic activity by phenolic compounds: Gallic acid, caffeic acid and pyrogallol. Microb. Pathog. 2016, 99, 56–61. [Google Scholar] [CrossRef]
- Mu, W.; Yu, S.; Zhu, L.; Zhang, T.; Jiang, B. Recent research on 3-phenyllactic acid, a broad-spectrum antimicrobial compound. Appl. Microbiol. Biotechnol. 2012, 95, 1155–1163. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Aliyu, H.N. Synthesis, structural characterization and antimicrobial potency of anthranilic acid based Mn (II) Schiff base complex. Chem. Res. J. 2019, 4, 54–61. [Google Scholar]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 32, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Morales-Mena, A.; Martínez-González, S.; Teague, K.D.; Graham, L.E.; Señas-Cuesta, R.; Vuong, C.N.; Tellez-Isaias, G. Assessment of Fermented Soybean Meal on Salmonella typhimurium Infection in Neonatal Turkey Poults. Animals 2020, 10, 1849. [Google Scholar] [CrossRef]
- Tolpeznikaite, E.; Ruzauskas, M.; Pilkaityte, R.; Bartkevics, V.; Zavistanaviciute, P.; Starkute, V.; Bartkiene, E. Influence of fermentation on the characteristics of Baltic Sea macroalgae, including microbial profile and trace element content. Food Control 2021, 129, 108235. [Google Scholar] [CrossRef]
- van Heel, A.J.; Montalban-Lopez, M.; Kuipers, O.P. Evaluating the feasibility of lantibiotics as an alternative therapy against bacterial infections in humans. Expert Opin. Drug Metab. Toxicol. 2011, 7, 675–680. [Google Scholar] [CrossRef]
- Perez, R.H.; Zendo, T.; Sonomoto, K. Circular and Leaderless Bacteriocins: Biosynthesis, Mode of Action, Applications, and Prospects. Front. Microbiol. 2018, 9, 410161. [Google Scholar] [CrossRef]
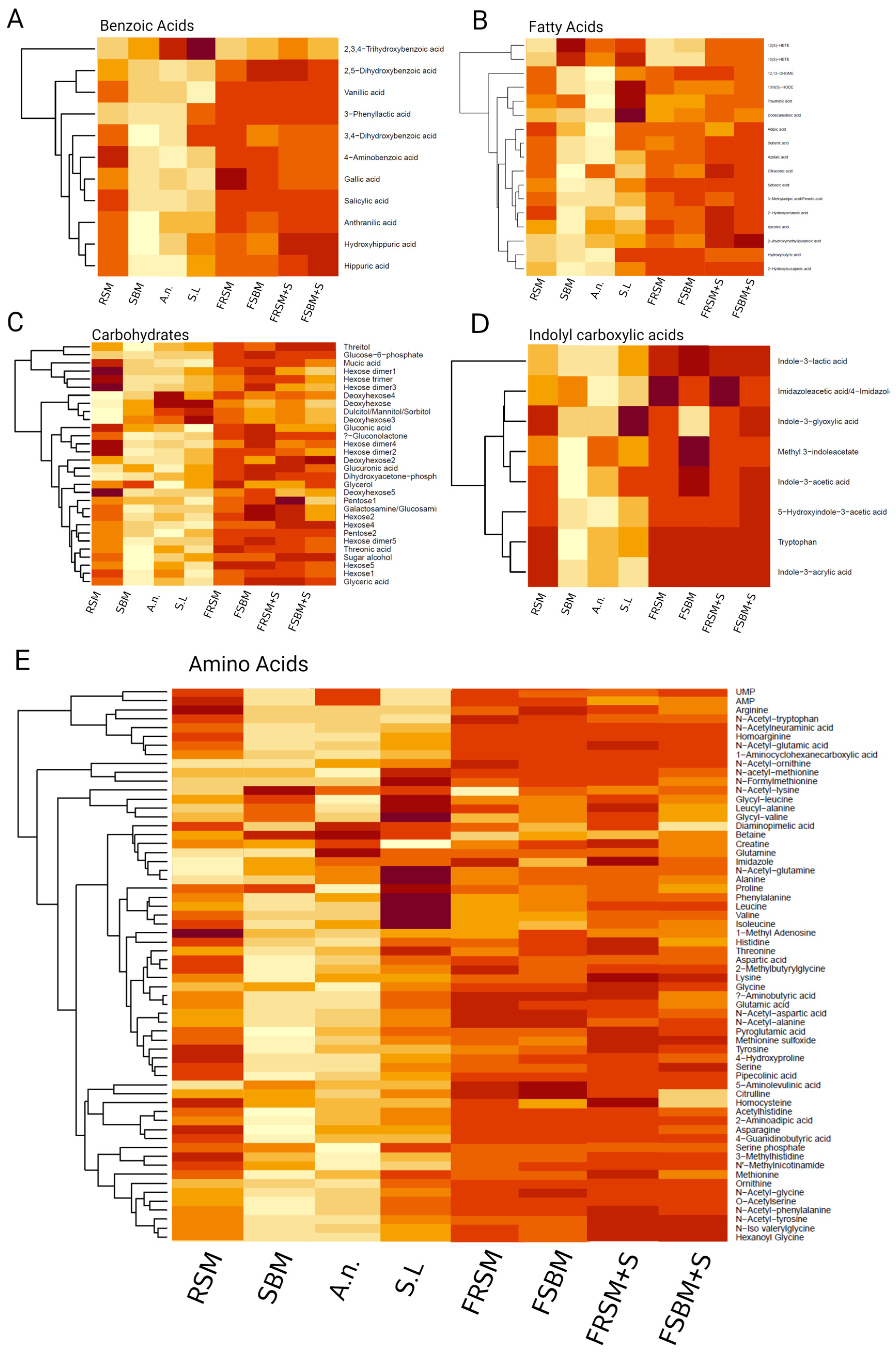

| Raw Material | Product | Content | Commercial Name |
|---|---|---|---|
| Soybean | FSBM | Fermented soybean meal | EP200 |
| Soybean | FSBM/S | Fermented soybean meal + 12% seaweed (6% S. latissima + 6% A. nodosum) | EP2299 |
| Soybean | SBM | Unfermented soybean meal | |
| Rapeseed | FRSM | Fermented rapeseed meal | EP100 |
| Rapeseed | FRSMS | Fermented rapeseed meal + 12% seaweed (6% S. latissima + 6% A. nodosum) | EP1199 |
| Rapeseed | RSM | Unfermented rapeseed meal | |
| Algae | AN | Ascophyllum nodosum | |
| Algae | SL | Saccharina latissima |
| Strain Number | Origin | Genus | Species | Subtype | Note |
|---|---|---|---|---|---|
| 1575 | Human | Salmonella | enterica | Typhimurium | Feces isolate from diseased individual |
| 1564 | Pig | Salmonella | enterica | Typhimurium | Feces isolate from diseased individual |
| 1709 | Pig | Salmonella | enterica | Typhimurium | Feces isolate from diseased individual |
| 69 | Human | Listeria | monocytogenes | Feces isolate from diseased individual | |
| 72 | Pig | Listeria | monocytogenes | Feces isolate from diseased individual | |
| 711 | Sheep | Listeria | monocytogenes | Feces isolate from diseased individual | |
| 645 | Human | Yersinia | enterocolitica | O:3 6A 28 | Feces isolate from diseased individual |
| 646 | Human | Yersinia | enterocolitica | O:5 27 8A 30 | Feces isolate from diseased individual |
| 647 | Human | Yersinia | enterocolitica | 7A 29 | Feces isolate from diseased individual |
| N1 | Pig | Escherichia | coli | F29 | Feces isolate from diseased individual |
| N2 | Pig | Escherichia | coli | F38 | Feces isolate from diseased individual |
| N3 | Pig | Escherichia | coli | F54 | Feces isolate from diseased individual |
| 144 | Lab0strain | Escherichia | coli | K12 | Experimental laboratory strain |
| N4 | Human | Staphylococcus | aureus | CC1 | Skin isolate from person suffering from atopic dermatitis |
| 1077 | Human | Staphylococcus | aureus | Skin isolate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beck, F.; Pedersen, N.R.; Nielsen, D.S. Fermented Rapeseed and Soybean Alone and in Combination with Macro Algae Inhibit Human and Pig Pathogenic Bacteria In Vitro. Microorganisms 2024, 12, 891. https://doi.org/10.3390/microorganisms12050891
Beck F, Pedersen NR, Nielsen DS. Fermented Rapeseed and Soybean Alone and in Combination with Macro Algae Inhibit Human and Pig Pathogenic Bacteria In Vitro. Microorganisms. 2024; 12(5):891. https://doi.org/10.3390/microorganisms12050891
Chicago/Turabian StyleBeck, Frederik, Ninfa Rangel Pedersen, and Dennis Sandris Nielsen. 2024. "Fermented Rapeseed and Soybean Alone and in Combination with Macro Algae Inhibit Human and Pig Pathogenic Bacteria In Vitro" Microorganisms 12, no. 5: 891. https://doi.org/10.3390/microorganisms12050891
APA StyleBeck, F., Pedersen, N. R., & Nielsen, D. S. (2024). Fermented Rapeseed and Soybean Alone and in Combination with Macro Algae Inhibit Human and Pig Pathogenic Bacteria In Vitro. Microorganisms, 12(5), 891. https://doi.org/10.3390/microorganisms12050891






