Plant Growth-Promoting Bacteria Influence Microbial Community Composition and Metabolic Function to Enhance the Efficiency of Hybrid pennisetum Remediation in Cadmium-Contaminated Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Pot Experiment
2.3. Determination of Cd Content and Analysis of Soil Physical-Chemical Properties
2.4. High-Throughput Sequencing
2.5. Metabolomic Analysis
2.6. Data Analysis
3. Results
3.1. Effects of Tested Strains on Growth and Cd Accumulation of Hybrid pennisetum
3.2. Effects of Tested Strains on Soil Physicalchemical Properties
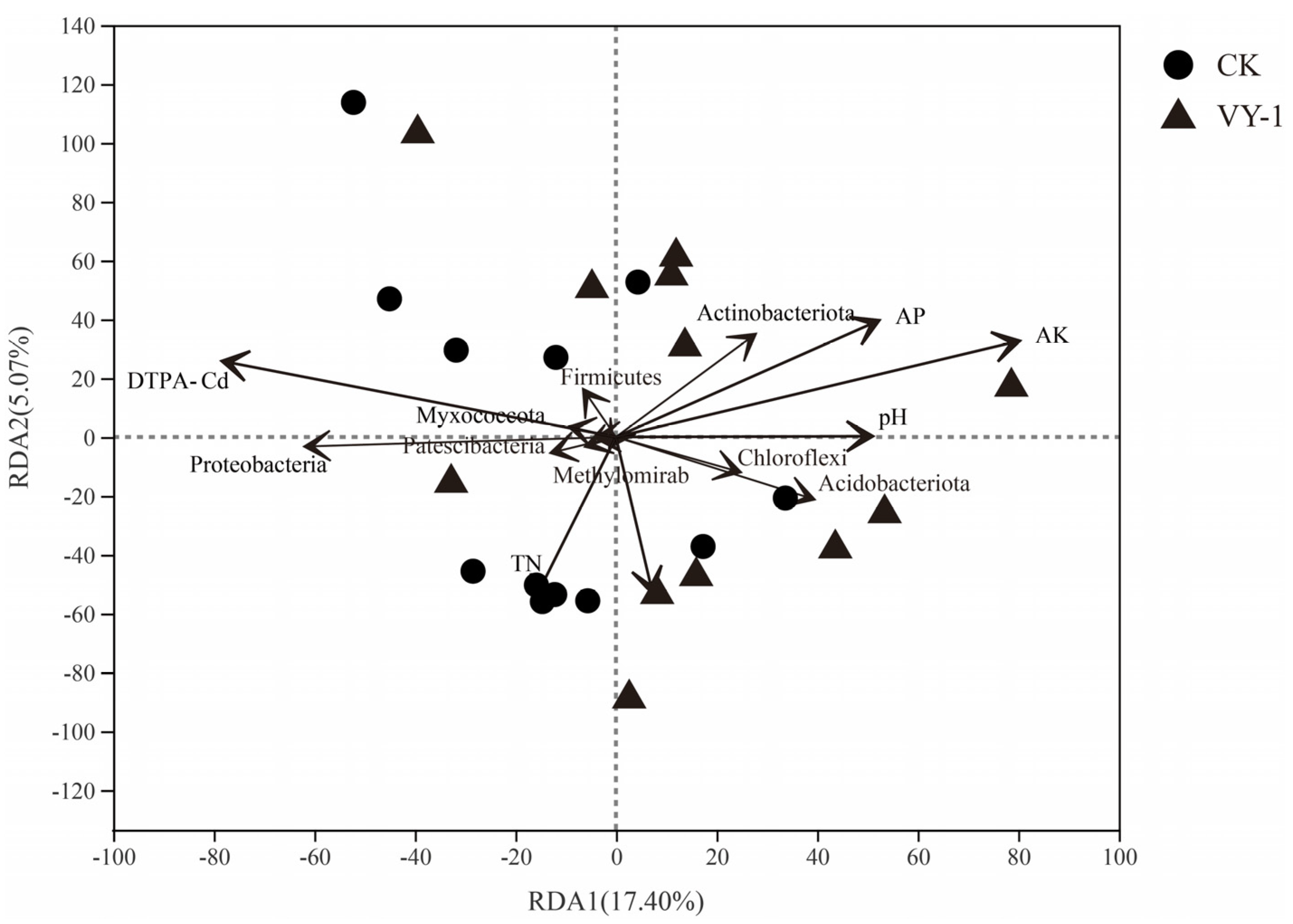
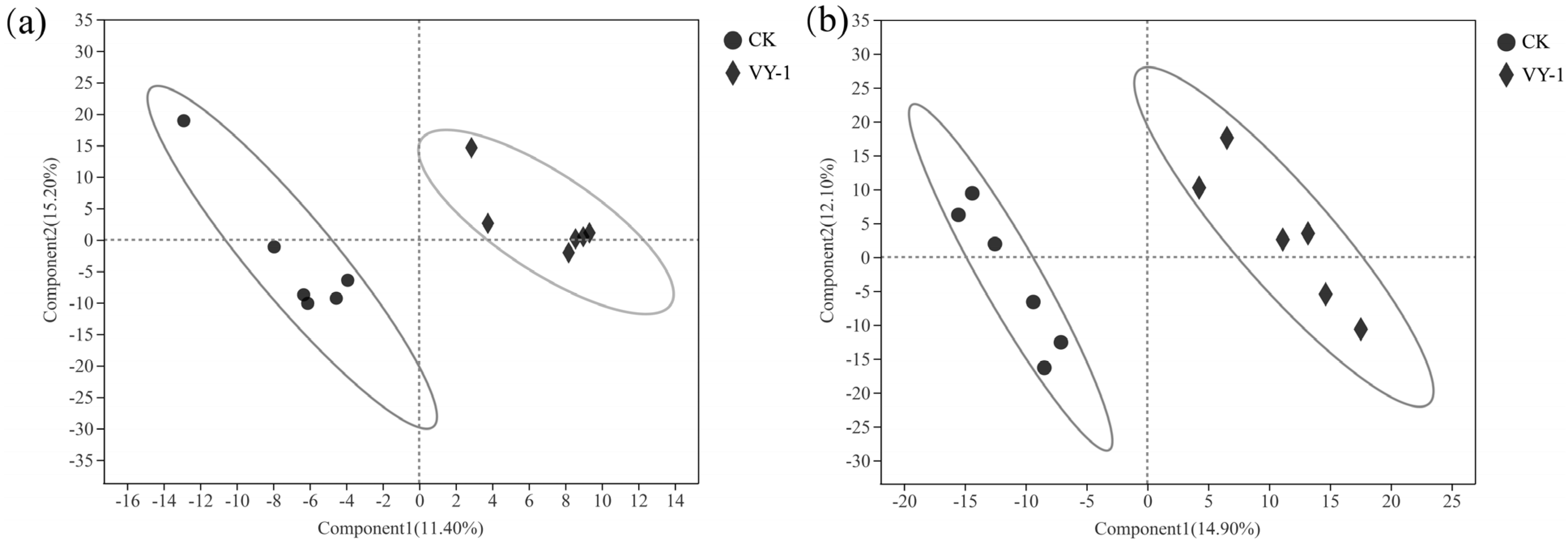
3.3. Effect of Tested Strains on Bacterial Community Composition
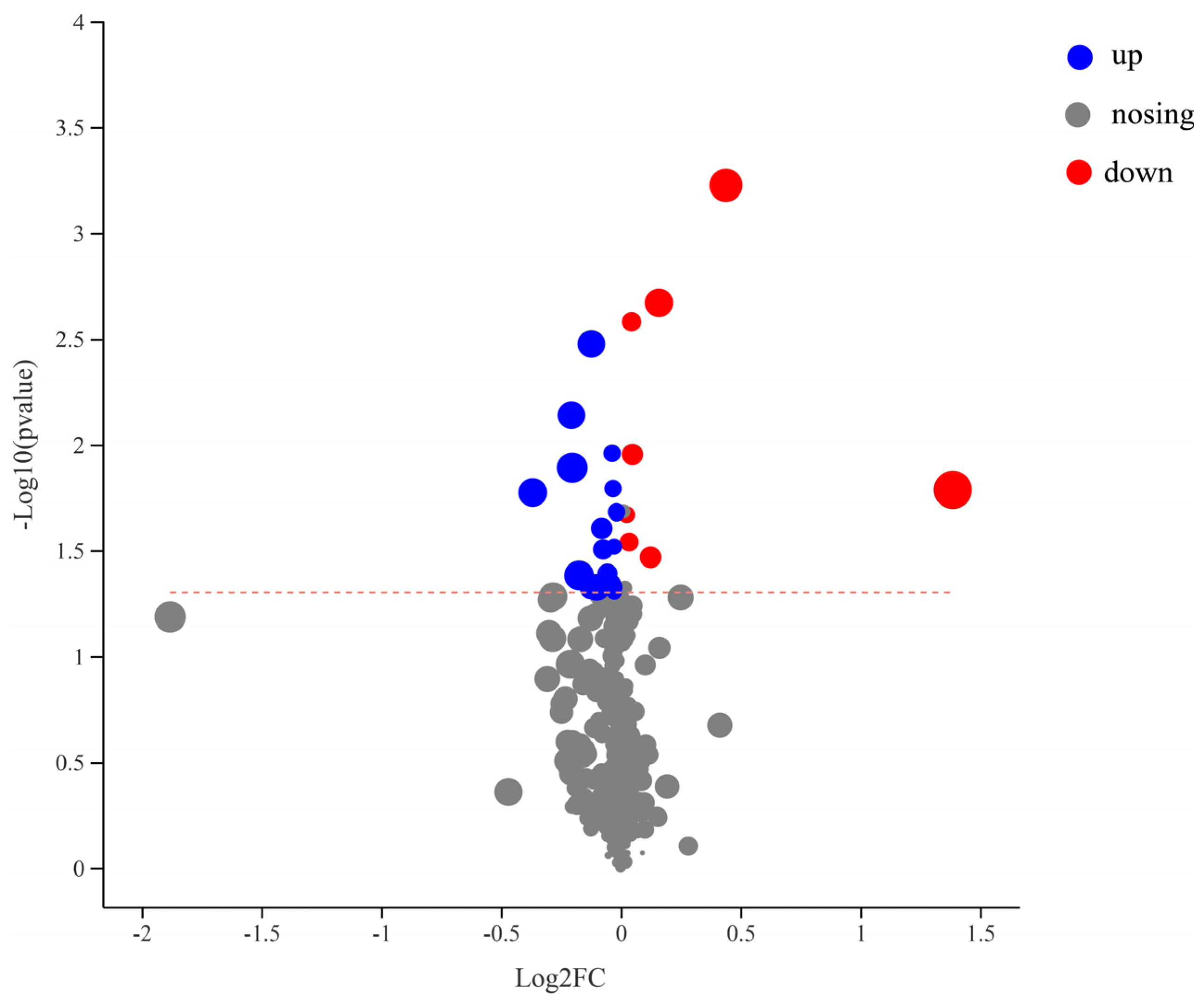
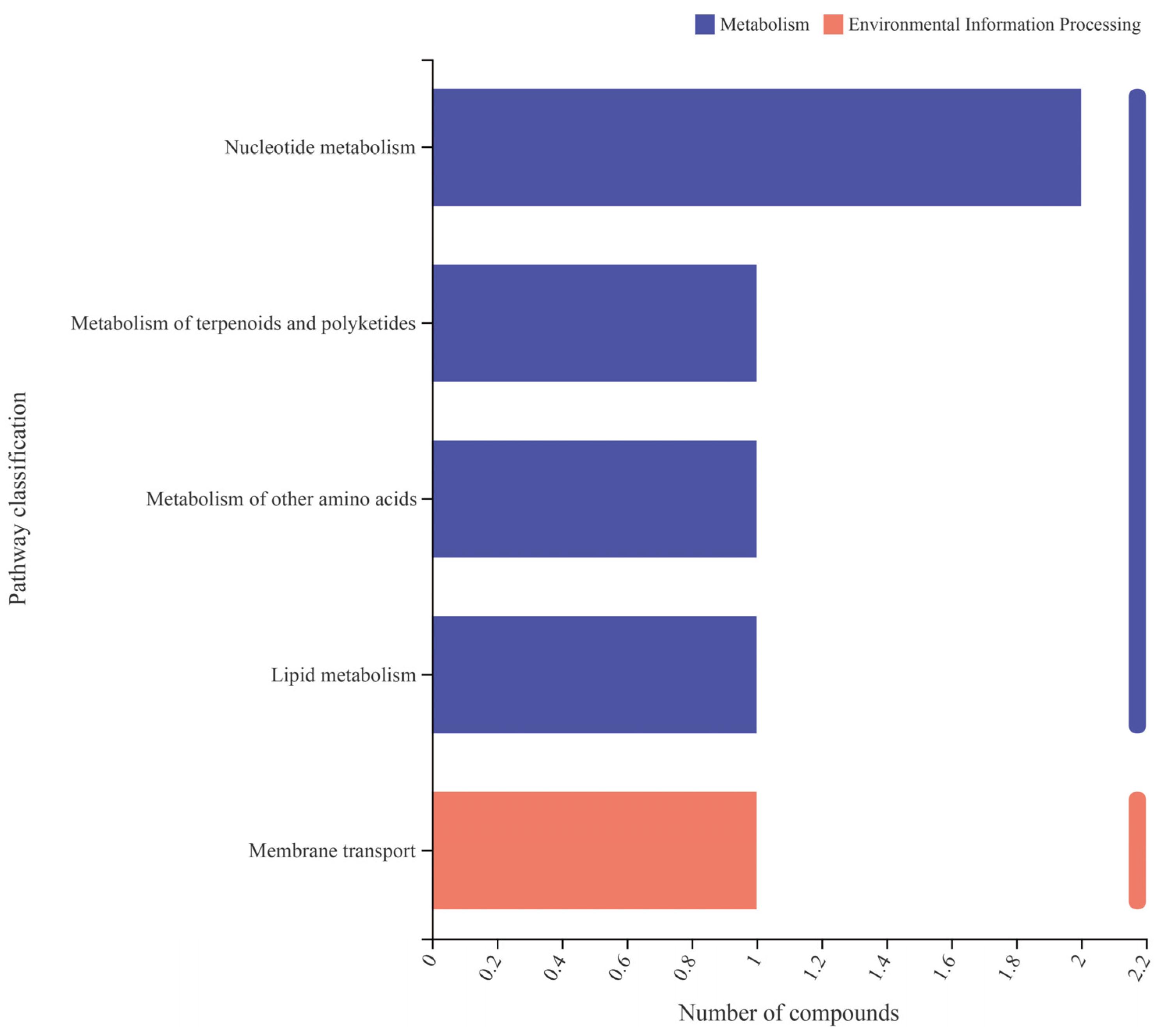
3.4. Effect of Test Strains on Metabolic Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, G.; Niu, Z.; Yu, J.; Li, Z.; Ma, J.; Xiang, P. Soil heavy metal pollution and food safety in China: Effects, sources and removing technology. Chemosphere 2021, 267, 129205. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S. Heavy metal pollution in China: Origin, pattern and control. Environ. Sci. Pollut. Res. 2003, 10, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Ma, Z.; Gingerich, D.B.; Zhao, X.; Zhao, D. Heavy metals in agricultural soil in China: A systematic review and meta-analysis. Eco-Environ. Health 2022, 1, 219–228. [Google Scholar] [CrossRef]
- Pilon-Smits, E. phytoremediation. Annu. Rev. Plant Biol. 2005, 56, 15–39. [Google Scholar] [CrossRef]
- Gavrilescu, M. Enhancing phytoremediation of soils polluted with heavy metals. Curr. Opin. Biotechnol. 2022, 74, 21–31. [Google Scholar] [CrossRef]
- Kamal, N.; Liu, Z.; Qian, C.; Wu, J.; Zhong, X. Improving hybrid Pennisetum growth and cadmium phytoremediation potential by using Bacillus megaterium BM18-2 spores as biofertilizer. Microbiol. Res. 2021, 242, 126594. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Kamal, N.; Hao, H.; Qian, C.; Liu, Z.; Shao, Y.; Zhong, X.; Xu, B. Endophytic Bacillus megaterium BM18-2 mutated for cadmium accumulation and improving plant growth in Hybrid pennisetum. Biotechnol. Rep. 2019, 24, e00374. [Google Scholar] [CrossRef]
- He, L.; Zhu, Q.; Wang, Y.; Chen, C.; He, M.; Tan, F. Irrigating digestate to improve cadmium phytoremediation potential of Pennisetum hybridum. Chemosphere 2021, 279, 130592. [Google Scholar] [CrossRef]
- Weyens, N.; van der Lelie, D.; Taghavi, S.; Newman, L.; Vangronsveld, J. Exploiting plant-microbe partnerships to improve biomass production and remediation. Trends Biotechnol. 2009, 27, 591–598. [Google Scholar] [CrossRef]
- Kisvarga, S.; Hamar-Farkas, D.; Ördögh, M.; Horotán, K.; Neményi, A.; Kovács, D.; Orlóci, L. The Role of the Plant–Soil Relationship in Agricultural Production—With Particular Regard to PGPB Application and Phytoremediation. Microorganisms 2023, 11, 1616. [Google Scholar] [CrossRef]
- Alves, A.R.A.; Yin, Q.; Oliveira, R.S.; Silva, E.F.; Novo, L.A.B. Plant growth-promoting bacteria in phytoremediation of metal-polluted soils: Current knowledge and future directions. Sci. Total Environ. 2022, 838, 156435. [Google Scholar] [CrossRef]
- Ren, X.-M.; Guo, S.-J.; Tian, W.; Chen, Y.; Han, H.; Chen, E.; Li, B.-L.; Li, Y.-Y.; Chen, Z.-J. Effects of Plant Growth-Promoting Bacteria (PGPB) Inoculation on the Growth, Antioxidant Activity, Cu Uptake, and Bacterial Community Structure of Rape (Brassica napus L.) Grown in Cu-Contaminated Agricultural Soil. Front. Microbiol. 2019, 10, 1455. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Zhang, X.; Zhu, Y.; Zhuo, R. Recent advances in phyto-combined remediation of heavy metal pollution in soil. Biotechnol. Adv. 2024, 72, 108337. [Google Scholar] [CrossRef]
- Yadav, R.; Singh, G.; Santal, A.R.; Singh, N.P. Omics approaches in effective selection and generation of potential plants for phytoremediation of heavy metal from contaminated resources. J. Environ. Manag. 2023, 336, 117730. [Google Scholar] [CrossRef]
- Li, X.-Q.; Liu, Y.-Q.; Li, Y.-J.; Han, H.; Zhang, H.; Ji, M.-F.; Chen, Z.-J. Enhancing Mechanisms of the Plant Growth-Promoting Bacterial Strain Brevibacillus sp. SR-9 on Cadmium Enrichment in Sweet Sorghum by Metagenomic and Transcriptomic Analysis. Int. J. Environ. Res. Public Health 2022, 19, 16309. [Google Scholar] [CrossRef] [PubMed]
- Rane, N.R.; Tapase, S.; Kanojia, A.; Watharkar, A.; Salama, E.-S.; Jang, M.; Kumar Yadav, K.; Amin, M.A.; Cabral-Pinto, M.M.S.; Jadhav, J.P.; et al. Molecular insights into plant–microbe interactions for sustainable remediation of contaminated environment. Bioresour. Technol. 2022, 344, 126246. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Q.; Chen, Y.; Li, Y.-Y.; Ding, C.-Y.; Li, B.-L.; Han, H.; Chen, Z.-J. Plant growth-promoting bacteria improve the Cd phytoremediation efficiency of soils contaminated with PE–Cd complex pollution by influencing the rhizosphere microbiome of sorghum. J. Hazard. Mater. 2024, 469, 134085. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, W.; Shao, Y.; Li, Y.-J.; Lin, L.-A.; Zhang, Y.-J.; Han, H.; Chen, Z.-J. Miscanthus cultivation shapes rhizosphere microbial community structure and function as assessed by Illumina MiSeq sequencing combined with PICRUSt and FUNGUIld analyses. Arch. Microbiol. 2020, 202, 1157–1171. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, S.; Liu, X.; Ren, X.; Wang, S.; Gao, Z. The Effects of Trichoderma viride T23 on Rhizosphere Soil Microbial Communities and the Metabolomics of Muskmelon under Continuous Cropping. Agronomy 2023, 13, 1092. [Google Scholar] [CrossRef]
- Hou, X.C.; Fan, X.F.; Wu, J.Y.; Zhu, Y.; Zhang, Y.X. Potentiality of herbaceous bioenergy plants of remediation of soil contaminated by heavy metals soi. Chin. J. Grassl. 2012, 34, 59–76. [Google Scholar]
- Chen, Z.-J.; Tian, W.; Li, Y.-J.; Sun, L.-N.; Chen, Y.; Zhang, H.; Li, Y.-Y.; Han, H. Responses of rhizosphere bacterial communities, their functions and their network interactions to Cd stress under phytostabilization by Miscanthus spp. Environ. Pollut. 2021, 287, 117663. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Zheng, Y.; Ding, C.Y.; Ren, X.M.; Yuan, J.; Sun, F.; Li, Y.Y. Integrated metagenomics and molecular ecological network analysis of bacterial community composition during the phytoremediation of cadmium-contaminated soils by bioenergy crops. Ecotoxicol. Environ. Saf. 2017, 145, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Brookes, P.C.; Shan, S.; Xu, J.; Liu, X. Effects of remediation agents on microbial community structure and function in soil aggregates contaminated with heavy metals. Geoderma 2022, 425, 116030. [Google Scholar] [CrossRef]
- Kong, Z.; Wu, Z.; Glick, B.R.; He, S.; Huang, C.; Wu, L. Co-occurrence patterns of microbial communities affected by inoculants of plant growth-promoting bacteria during phytoremediation of heavy metal-contaminated soils. Ecotoxicol. Environ. Saf. 2019, 183, 109504. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, Q.; Huang, L.; Xu, S.a.; Fu, Y.; Hou, D.; Feng, Y.; Yang, X. Cadmium phytoextraction through Brassica juncea L. under different consortia of plant growth-promoting bacteria from different ecological niches. Ecotoxicol. Environ. Saf. 2022, 237, 113541. [Google Scholar] [CrossRef] [PubMed]
- Bourceret, A.; Cébron, A.; Tisserant, E.; Poupin, P.; Bauda, P.; Beguiristain, T.; Leyval, C. The Bacterial and Fungal Diversity of an Aged PAH- and Heavy Metal-Contaminated Soil is Affected by Plant Cover and Edaphic Parameters. Microb. Ecol. 2016, 71, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, Q.; Wang, B.; Hou, J.; Luo, Y.; Tang, C.; Franks, A.E. Plant growth-promoting rhizobacteria enhance the growth and Cd uptake of Sedum plumbizincicola in a Cd-contaminated soil. J. Soils Sediments 2015, 15, 1191–1199. [Google Scholar] [CrossRef]
- Boukhatem, Z.F.; Merabet, C.; Tsaki, H. Plant Growth Promoting Actinobacteria, the Most Promising Candidates as Bioinoculants? Front. Agron. 2022, 4, 849911. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Chen, Y.; Ding, Q.; Chao, Y.; Wei, X.; Wang, S.; Qiu, R. Structural development and assembly patterns of the root-associated microbiomes during phytoremediation. Sci. Total Environ. 2018, 644, 1591–1601. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, Y.; Wang, Z.; Zhang, H.; Chen, M.; Chen, Y.; Wang, J.; Liu, B. Effects of a novel bio-organic fertilizer on the composition of rhizobacterial communities and bacterial wilt outbreak in a continuously mono-cropped tomato field. Appl. Soil Ecol. 2020, 156, 103717. [Google Scholar] [CrossRef]
- Soumare, A.; Boubekri, K.; Lyamlouli, K.; Hafidi, M.; Ouhdouch, Y.; Kouisni, L. Efficacy of phosphate solubilizing Actinobacteria to improve rock phosphate agronomic effectiveness and plant growth promotion. Rhizosphere 2021, 17, 100284. [Google Scholar] [CrossRef]
- Kalam, S.; Basu, A.; Ahmad, I.; Sayyed, R.Z.; El-Enshasy, H.A.; Dailin, D.J.; Suriani, N.L. Recent Understanding of Soil Acidobacteria and Their Ecological Significance: A Critical Review. Front. Microbiol. 2020, 11, 580024. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Liu, C.; Li, B.; Dong, Y. Trifolium repens L. regulated phytoremediation of heavy metal contaminated soil by promoting soil enzyme activities and beneficial rhizosphere associated microorganisms. J. Hazard. Mater. 2021, 402, 123829. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, M.; Yang, W.; Di, H.J.; Ma, L.; Liu, W.; Li, B. Effects of microbial inoculants on phosphorus and potassium availability, bacterial community composition, and chili pepper growth in a calcareous soil: A greenhouse study. J. Soils Sediments 2019, 19, 3597–3607. [Google Scholar] [CrossRef]
- Bell, T.H.; Joly, S.; Pitre, F.E.; Yergeau, E. Increasing phytoremediation efficiency and reliability using novel omics approaches. Trends Biotechnol. 2014, 32, 271–280. [Google Scholar] [CrossRef]
- Alzate Zuluaga, M.Y.; Milani, K.M.L.; Miras-Moreno, M.B.; Lucini, L.; Valentinuzzi, F.; Mimmo, T.; Pii, Y.; Cesco, S.; Rodrigues, E.P.; de Oliveira, A.L.M. The adaptive metabolomic profile and functional activity of tomato rhizosphere are revealed upon PGPB inoculation under saline stress. Environ. Exp. Bot. 2021, 189, 104552. [Google Scholar] [CrossRef]
- Kaur, S.; Suseela, V. Unraveling Arbuscular Mycorrhiza-Induced Changes in Plant Primary and Secondary Metabolome. Metabolites 2020, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhou, J.; Li, Q.; Gan, B.; Peng, W.; Zhang, X.; Tan, W.; Jiang, L.; Li, X. Manganese affects the growth and metabolism of Ganoderma lucidum based on LC-MS analysis. PeerJ 2019, 7, e6846. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, W.; Li, Y.; Xu, Y.; Teng, Y.; Christie, P.; Luo, Y. Nontargeted metabolomic analysis to unravel the impact of di (2-ethylhexyl) phthalate stress on root exudates of alfalfa (Medicago sativa). Sci. Total Environ. 2019, 646, 212–219. [Google Scholar] [CrossRef]
- Jung, Y.; Ha, M.; Lee, J.; Ahn, Y.G.; Kwak, J.H.; Ryu, D.H.; Hwang, G.-S. Metabolite Profiling of the Response of Burdock Roots to Copper Stress. J. Agric. Food Chem. 2015, 63, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Limami, A.M.; Glévarec, G.; Ricoult, C.; Cliquet, J.-B.; Planchet, E. Concerted modulation of alanine and glutamate metabolism in young Medicago truncatula seedlings under hypoxic stress. J. Exp. Bot. 2008, 59, 2325–2335. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Aboveground Biomass (g) | Root Biomass (g) | Aboveground Cd Content (mg·kg−1) | Root Cd Content (mg·kg−1) | Aboveground Cd Accumulation (μg) | Root Cd Accumulation (μg) | TF |
|---|---|---|---|---|---|---|---|
| CK | 5.39 ± 0.85 | 0.79 ± 0.15 | 2.48 ± 0.37 | 16.74 ± 1.83 | 13.38 ± 2.77 | 13.28 ± 2.80 | 0.15 |
| VY-1 | 6.75 ± 0.93 | 1.05 ± 0.08 | 3.36 ± 1.03 | 20.80 ± 2.60 | 21.99 ± 4.08 | 20.14 ± 1.62 | 0.16 |
| Treatment | pH | Total Nitrogen (mg·kg−1) | Total Phosphorus (mg·kg−1) | Available Phosphorus (mg·kg−1) | Available Potassium (mg·kg−1) |
|---|---|---|---|---|---|
| CK | 7.09 ± 0.12 | 1.77 ± 0.10 | 243.07 ± 9.34 | 0.026 ± 0.001 | 108.25 ± 2.5 |
| VY-1 | 7.13 ± 0.09 | 1.87 ± 0.19 | 253.60 ± 19.57 | 0.031 ± 0.006 | 110.75 ± 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.-J.; Li, M.-L.; Gao, S.-S.; Sun, Y.-B.; Han, H.; Li, B.-L.; Li, Y.-Y. Plant Growth-Promoting Bacteria Influence Microbial Community Composition and Metabolic Function to Enhance the Efficiency of Hybrid pennisetum Remediation in Cadmium-Contaminated Soil. Microorganisms 2024, 12, 870. https://doi.org/10.3390/microorganisms12050870
Chen Z-J, Li M-L, Gao S-S, Sun Y-B, Han H, Li B-L, Li Y-Y. Plant Growth-Promoting Bacteria Influence Microbial Community Composition and Metabolic Function to Enhance the Efficiency of Hybrid pennisetum Remediation in Cadmium-Contaminated Soil. Microorganisms. 2024; 12(5):870. https://doi.org/10.3390/microorganisms12050870
Chicago/Turabian StyleChen, Zhao-Jin, Meng-Lu Li, Shan-Shan Gao, Yu-Bo Sun, Hui Han, Bai-Lian Li, and Yu-Ying Li. 2024. "Plant Growth-Promoting Bacteria Influence Microbial Community Composition and Metabolic Function to Enhance the Efficiency of Hybrid pennisetum Remediation in Cadmium-Contaminated Soil" Microorganisms 12, no. 5: 870. https://doi.org/10.3390/microorganisms12050870
APA StyleChen, Z.-J., Li, M.-L., Gao, S.-S., Sun, Y.-B., Han, H., Li, B.-L., & Li, Y.-Y. (2024). Plant Growth-Promoting Bacteria Influence Microbial Community Composition and Metabolic Function to Enhance the Efficiency of Hybrid pennisetum Remediation in Cadmium-Contaminated Soil. Microorganisms, 12(5), 870. https://doi.org/10.3390/microorganisms12050870







