Abstract
Previous studies on the early interference of gut microbiota by Bacillus siamensis (B. siamensis) in weaned piglets are rarely reported, and the present trial is a preliminary study. This experiment was conducted to investigate the effects of B. siamensis supplementation on the growth performance, serum biochemistry, immune response, fecal short-chain fatty acids and microbiota of weaned piglets. Sixty weaned piglets were randomly divided into a control group (CON) and a B. siamensis group (BS), which were fed a basal diet and the basal diet supplemented with 5 × 1010 CFU B. siamensis per kg, respectively. Each group had 3 replicates and 10 piglets per replicate. The trial lasted for 28 days. The results showed that B. siamensis significantly increased the serum growth hormone (GH) and insulin-like growth factor (IGF) in piglets. Compared with the CON group, the levels of serum immunoglobulin and inflammatory factors in the BS group were significantly improved. In addition, the serum concentrations of zonulin and endotoxin (ET) in the BS group were lower. The dietary addition of B. siamensis significantly increased fecal short-chain fatty acid (SCFA) levels in piglets. Notably, B. siamensis improved the microbial composition by increasing beneficial genera, including Weissella, Lachnospiraceae_NK4A136_group and Bifidobacterium, and decreasing pathogenic genera, including Pantoea, Fusobacterium and Gemella, in piglet feces. Correlation analysis showed that the benefits of dietary B. siamensis supplementation were closely related to its improved microbial composition. In summary, the addition of B. siamensis can improve the immunity function, inflammatory response, gut permeability and SCFA levels of weaned piglets, which may be achieved through the improvement of their microbiota.
1. Introduction
Weaning is a stressful phase of the pig growth cycle and often has a negative impact on the growth performance and gut health of piglets. Especially with the early weaning strategies implemented in modern farming, piglets are challenged by environmental, dietary and psychological emergencies that can lead to immune dysfunction, impaired gut barrier function and microbiota homeostasis, resulting in impaired growth and even death [1]. In recent decades, antibiotics have been widely used as feed additives to reduce the damage caused by weaning stress [2]. Antibiotics, as growth promoters, improve feed utilization and reduce bacterial pathogen infections, largely improving economic returns for producers [3]. However, antibiotics have the potential to jeopardize human health by increasing the tolerance of pathogenic bacteria and residues in livestock products [4]. Reducing the use of antibiotics is an inevitable trend, and since 2006, the European Union and many countries around the world have officially banned the use of antibiotics in feed [5]. Therefore, finding safe and effective antibiotic-replacement strategies is important for the healthy and sustainable development of the pig industry.
Probiotics are known to be an effective and safe alternative to antibiotics [6]. The composition and metabolic activity of the gut microbiota co-evolves with the host from the time it leaves the mother’s body and is influenced by the host’s diet and lifestyle [7]. Gut microbes interact with the host and influence the host’s physiology, nutrition and health [8]. Furthermore, gut microbes can positively influence the host’s immune response, anti-inflammatory capacity and gut barrier through the production of metabolites and short-chain fatty acids (SCFAs) [9]. Therefore, the early intervention of the gut microbiota is an effective way to relieve weaning stress. Studies have shown that probiotics can improve the composition of the gut microbiota, increase colon SCFA levels and promote growth in weaned piglets [10]. Recent research evidence suggests that probiotics can alter the gut microbiota and alleviate systemic inflammatory responses [11]. Therefore, we hypothesize that probiotics can effectively alleviate piglet weaning stress by intervening in the composition of the gut microbiota at an early stage, which ultimately positively affects the growth and health status of piglets.
Given the direct and indirect contribution of probiotics to piglet health, their potential to optimize piglet health is currently being investigated [12]. Probiotics, such as Bacillus subtilis, Lactobacillus plantarum and Clostridium butyricum, have been shown to have a positive effect on growth performance, immune levels, the integrity of gut barrier function and the gut microbiota in weaned piglets [13,14,15]. Bacillus siamensis (B. siamensis) is a kind of probiotic with good resistance to acids, alkalis, bile salt and high temperatures. However, there are limited studies on the effects of B. siamensis on the health of weaned piglets. Therefore, the aim of this study was to investigate the effects of the supplementation of diets with B. siamensis on the growth performance, immunity level, gut permeability, and fecal SCFAs and microbiota of weaned piglets.
2. Material and Methods
The Northeast Agricultural University Ethical Committee for Animal Experiments (Grant Number: NEAU-[2013]-9) approved all animal experiments conducted at the Bayan Kangrun Husbandry Co. (Harbin, China).
2.1. Animals and Diets
Sixty crossbred castrated male and female (Duroc × Large White × Landrace) weaned piglets (30 days of age) with an initial body weight (BW) of 8.63 ± 0.1 kg were randomly divided into control and experimental groups according to sex and BW. Piglets were weaned on the evening of the 30th day after birth. There were 3 replicates per treatment group and 10 pigs per replicate. The control group (CON) was fed a basal diet, and the B. siamensis group (BS) was fed the basal diet supplemented with B. siamensis. The experimental diet was prepared by mixing the basal diet with B. siamensis bacterial solution at a concentration of 5 × 1010 CFU/kg. This concentration is based on previous experimental studies conducted by the laboratory team. The B. siamensis strain was stored in the Chinese Typical Culture Preservation Center (CCTCC NO: M 202297). The piglet basal diets were formulated to meet or exceed the nutritional requirements recommended by the National Research Council (NRC, 2012) [16]. The trial period lasted for 28 days from early on the 31st day to the evening of the 58th day after the birth of piglets. All weaned piglets in this trial were used for this study only. The ingredients and chemical composition of the basal diets are detailed in Table 1. All piglets were housed in pens (2.5 m × 4.0 m) in which the temperature was maintained at 26–28 °C and the relative humidity at 55–65%. All piglets were allowed to feed and drink at will during the experimental period.

Table 1.
Dietary ingredients and nutrient contents of the basal diets (from 30 to 58 days of age).
2.2. Sample and Data Collection
The feed intake of the piglets was recorded once a week, and the fasting BW of piglets was recorded on days 1 and 28 to determine the average daily gain (ADG), average daily feed intake (ADFI) and feed conversion ratio (FCR) of piglets. On day 28 of the experiment, two piglets per replicate close to the mean BW were selected and blood was obtained by puncturing their anterior vena cava with a sterile needle. The blood samples were then centrifuged at 4 °C for 10 min at 3000× g to obtain the supernatant serum, which was stored at −80 °C for the determination of serum biochemical, immunological, inflammatory, gut permeability and growth hormone-related parameters. Fresh feces from piglets (the same batch of pigs from which serum was collected) were collected by massaging the rectum and stored at −80 °C for the analysis of microbial community and SCFAs.
2.3. Determination of Serum Biochemistry
Serum biochemical markers included Urea nitrogen (UN), total protein (TP), albumin (ALB), globulin (GLB), urea nitrogen (UN), creatinine (CREA), aspartate transaminase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total cholesterol (TCHO), triglyceride (TG), glucose (GLU), high-density lipoprotein cholesterol (HDL) and low-density lipoprotein cholesterol (LDL). Analyses were performed using an Automatic Biochemical Analyzer (7160 autoanalyzer; Hitachi, Tokyo, Japan). The kit was purchased from the Nanjing Institute of Construction Bioengineering (Nanjing, China), and the trial steps were performed following the manufacturer’s notes.
2.4. Determination of Immunity, Inflammation, Gut Permeability and Growth Factors
The levels of immunoglobulin A (IgA), immunoglobulin G (IgG), immunoglobulin M (IgM), interleukin-6 (IL-6), interleukin-10 (IL-10), tumor necrosis factor-alpha (TNF-α), diamine oxidase (DAO), D-lactic acid (D-LA), endotoxin (ET), zonulin, growth hormone (GH) and insulin-like growth factor (IGF) were measured using Enzyme-Linked Immunosorbent Assay (ELISA) kits from Shanghai Enzyme-linked Biotechnology Co., Ltd. (Shanghai, China). The experimental procedures were performed in strict accordance with the manufacturer’s instructions.
2.5. Analysis of SCFAs
SCFA analysis of piglet fecal samples was performed using a GC-FID system (model 7890A; Agilent Technologies, Santa Clara, CA, USA) equipped with an AOC-20s autosampler, a capillary column (HP-INNOWAX (19091N-133), 30 m × 0.53 mm × 1.0 µm) and a flame ionization detector. Specifically, 2 g of piglet feces was macerated in 2 mL of ultrapure water in a freezer at 4 °C for 48 h. The feces were then centrifuged at 10,000 rpm/min for 10 min at 4 °C, and this step was repeated twice. Filtration of the supernatant was carried out using a 0.22 μm filter membrane. Finally, the filtrate was combined with an internal standard solution (25% metaphosphate solution containing crotonic acid) at a volume ratio of 5:1. The injector temperature, detector temperature and oven temperature were set to 230 °C, 240 °C and 180 °C, respectively, and the pressure was set to 90kPa. The flow rates of nitrogen, air and hydrogen were 20, 400 and 30 mL/min, respectively.
2.6. DNA Extraction, PCR and Library Construction and Sequencing
Total genomic DNA was extracted from the piglet feces sample by the CTAB/SDS method. DNA concentration and purity were monitored on 1% agarose gel. Primes 341F (5′-CCTAYGGGRBGCASCAG-3′) and 805R (5′-GGACTACNNGGGTATCTAAT-3′) were used to construct PCR amplification for the V3-V4 region of the bacterial 16S rDNA gene. The amplified products were purified by using a gel extraction kit (Darmstadt, Germany). Purified PCR products were evaluated using an Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA, USA) and Illumina’s (Kapa Biosciences, Woburn, MA, USA) library quantification kit with qualified library concentrations above 2 nM. Finally, the libraries were sequenced on an Illumina NovaSeq platform to generate 250 bp paired-end reads.
For the double-ended data obtained by sequencing, the sample needed to be data-split according to barcode information, and the joint and barcode sequences were removed. Then, the data were spliced and filtered, and then, DADA2 was invoked with qiime DADA2 denoise-paired for length filtering and denoising. The Amplicon Sequence Variant (ASV) feature sequence and an ASV abundance table were obtained, and singleton ASVs were removed. The SILVA and NCBI databases were used to annotate species according to the ASV sequence files, and the abundance of species of each level in each sample was counted according to the ASV abundance table. The diversity index was calculated using the R language vegan package (V.2.5–7), and the rank sum was used to test the significance of the differences between the two groups. The Mann–Whitney U test was used to screen phylum- and genus-level differential species.
2.7. Analysis of Data
All data were statistically analyzed by IBM SPSS 26.0 statistics using a two-tailed unpaired Student’s t-test. Data are expressed as mean ± SEM. p < 0.05 indicates statistically significant differences.
3. Results
3.1. Growth Performance
The growth performance results are summarized in Table 2. Compared with the CON group, dietary B. siamensis supplementation had no significant effects (p > 0.05) on the initial weight, final weight, ADG, ADFI and FCR of piglets.

Table 2.
Effects of B. siamensis supplementation on growth performance of weaned piglets.
3.2. Serum Biochemistry
As shown in Table 3, the serum levels of TCHO (p < 0.001) and HDL (p = 0.001) in the BS group were significantly reduced. Moreover, the levels of UN, CREA, GLU, AST, ALT, TP, ALB, GLB, TG and LDL were not significantly affected (p > 0.05).

Table 3.
Effects of B. siamensis supplementation on serum biochemical parameters of weaned piglets.
3.3. Serum Growth Factor
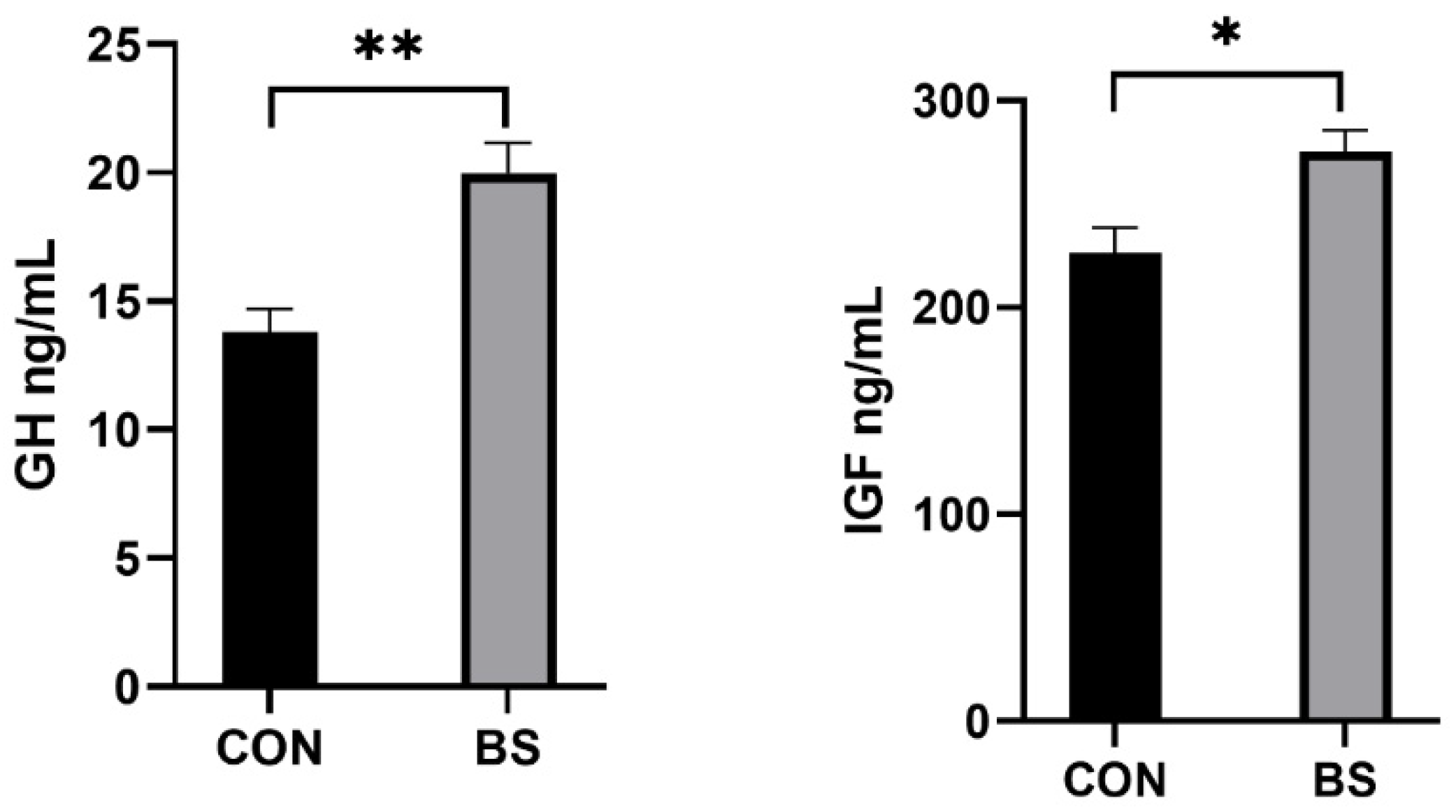
As shown in Figure 1, the concentrations of GH (p < 0.01) and IGF (p < 0.05) in the serum of piglets in the BS group were significantly higher than in the CON group.
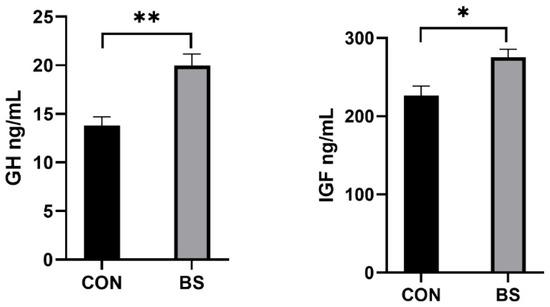
Figure 1.
Effects of supplemental B. siamensis on serum growth hormone levels of weaned piglets. Data are expressed as mean ± SEM (n = 6). * p < 0.05, ** p < 0.01. CON: control; BS: B. siamensis.
3.4. Serum Immunity and Inflammation
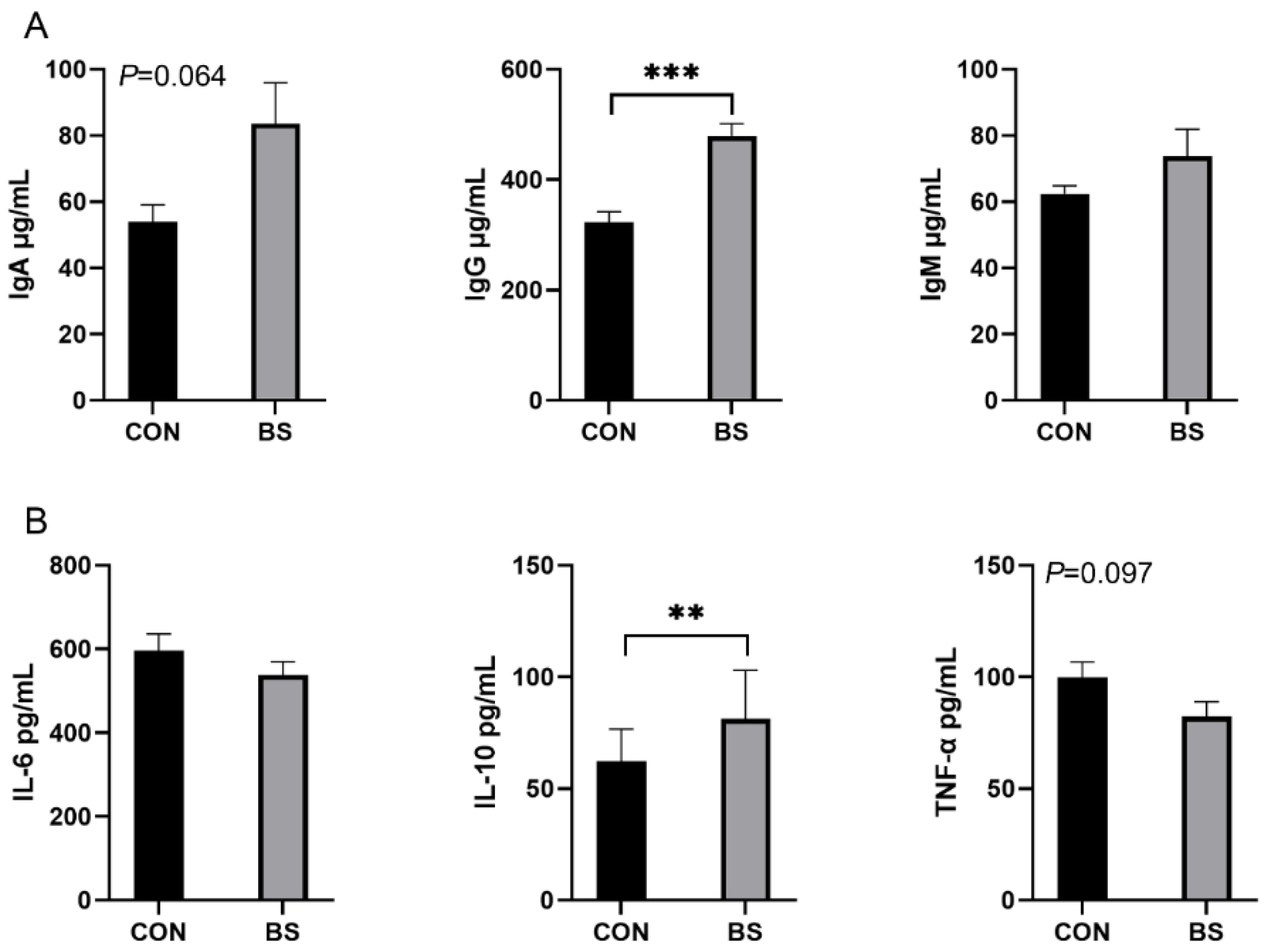
The effects of B. siamensis on the serum immune and inflammatory levels of piglets are shown in Figure 2. Compared with the CON group, the serum levels of IgG were significantly higher (p < 0.001) in piglets in the BS group, and there was a tendency (p = 0.064) for IgA to be elevated, with no significant difference (p > 0.05) in IgM (Figure 2A). Compared with the CON group, the serum levels of IL-10 were significantly higher (p < 0.01) in piglets in the BS group, and there was a tendency (p = 0.097) for TNF-α to be decreased, with no significant difference (p > 0.05) in IL-6 (Figure 2B).
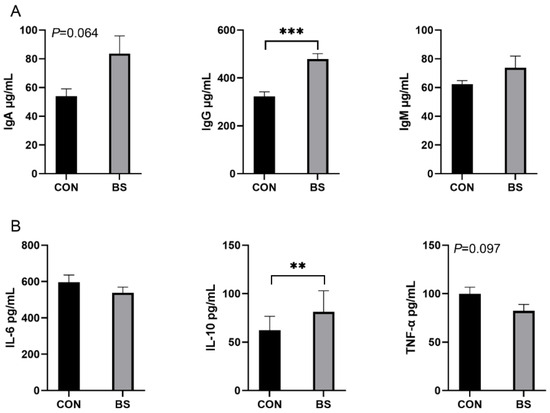
Figure 2.
Effects of B. siamensis supplementation on serum immunity (A) and inflammation (B) levels of weaned piglets. Data are expressed as mean ± SEM (n = 6). ** p < 0.01, *** p < 0.001. CON: control; BS: B. siamensis.
3.5. Gut Permeability
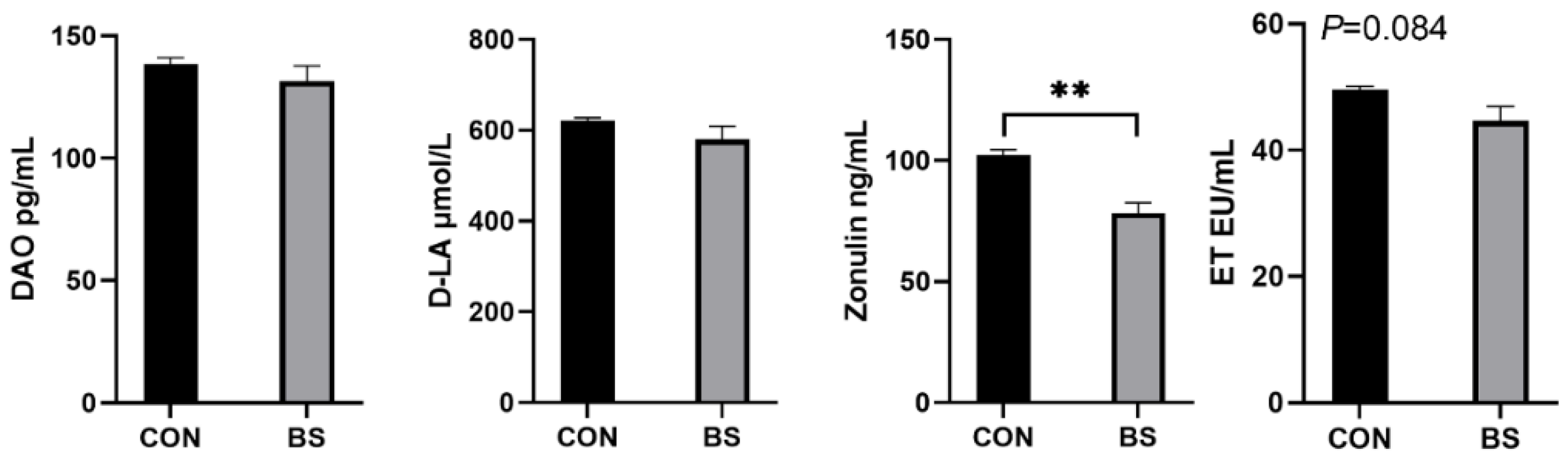
As illustrated in Figure 3, the serum zonulin levels of piglets in the BS group were significantly decreased (p < 0.01), ET had a tendency (p = 0.084) to be decreased, and DAO and D-LA were not significantly affected (p > 0.05).
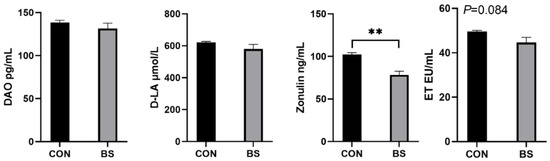
Figure 3.
Effects of B. siamensis supplementation on serum levels of intestinal-permeability markers in weaned piglets. Data are expressed as mean ± SEM (n = 6). ** p < 0.01. CON: control; BS: B. siamensis.
3.6. Fecal SCFA Content
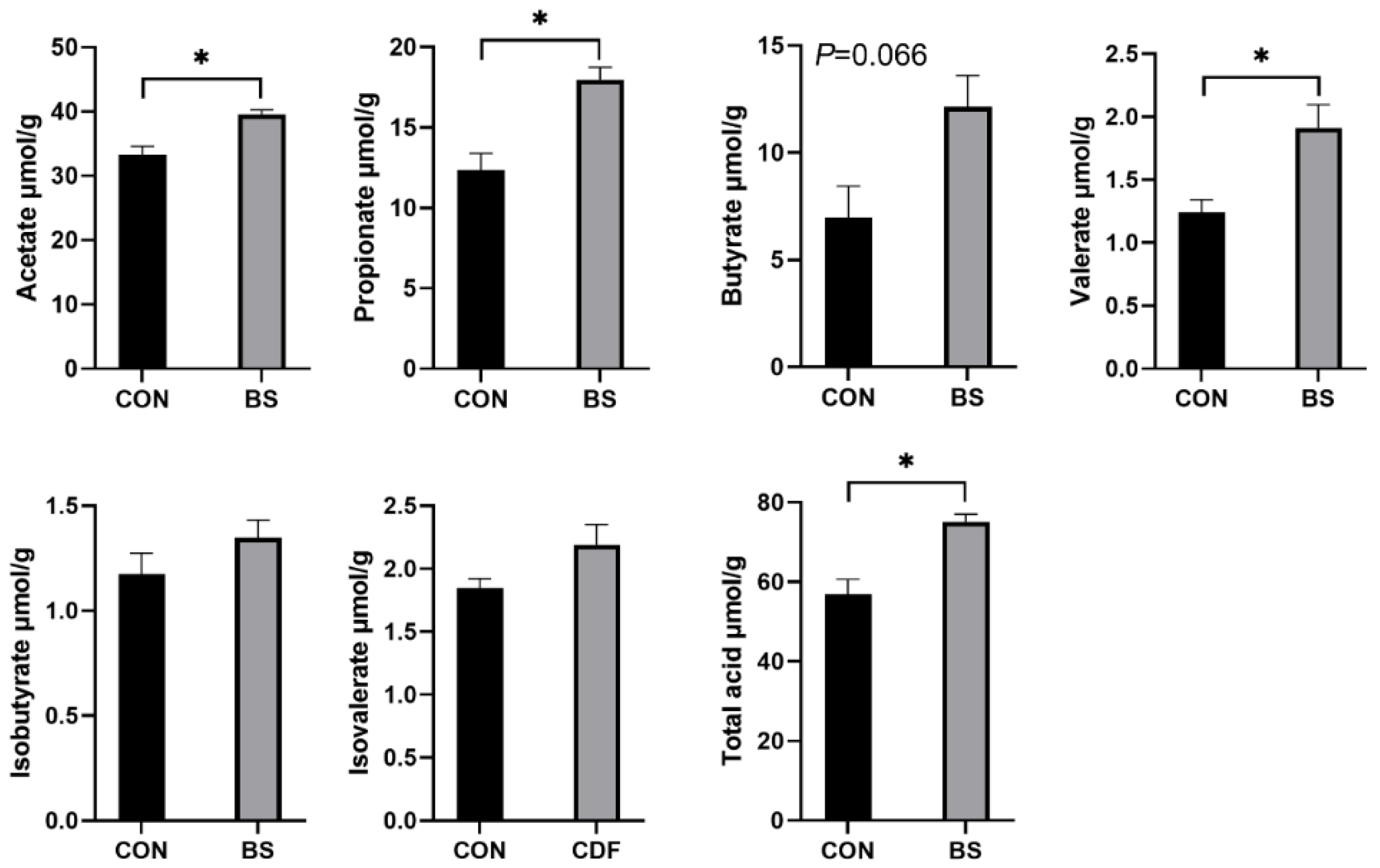
The effects of B. siamensis on the fecal SCFA content of piglets are shown in Figure 4. Dietary B. siamensis supplementation significantly increased (p < 0.05) the contents of acetate, propionate, valerate and total acid in piglets. Compared with the control group, butyrate in the BS group had a tendency (p = 0.066) to increase. In addition, isobutyrate and isovalerate did not differ significantly (p > 0.05) between the two groups.
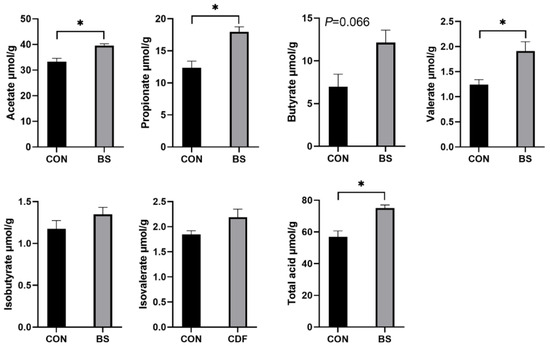
Figure 4.
Effects of B. siamensis supplementation on fecal SCFA levels of weaned piglets. Data are expressed as mean ± SEM (n = 6). * p < 0.05. CON: control; BS: B. siamensis.
3.7. Fecal Microbiota
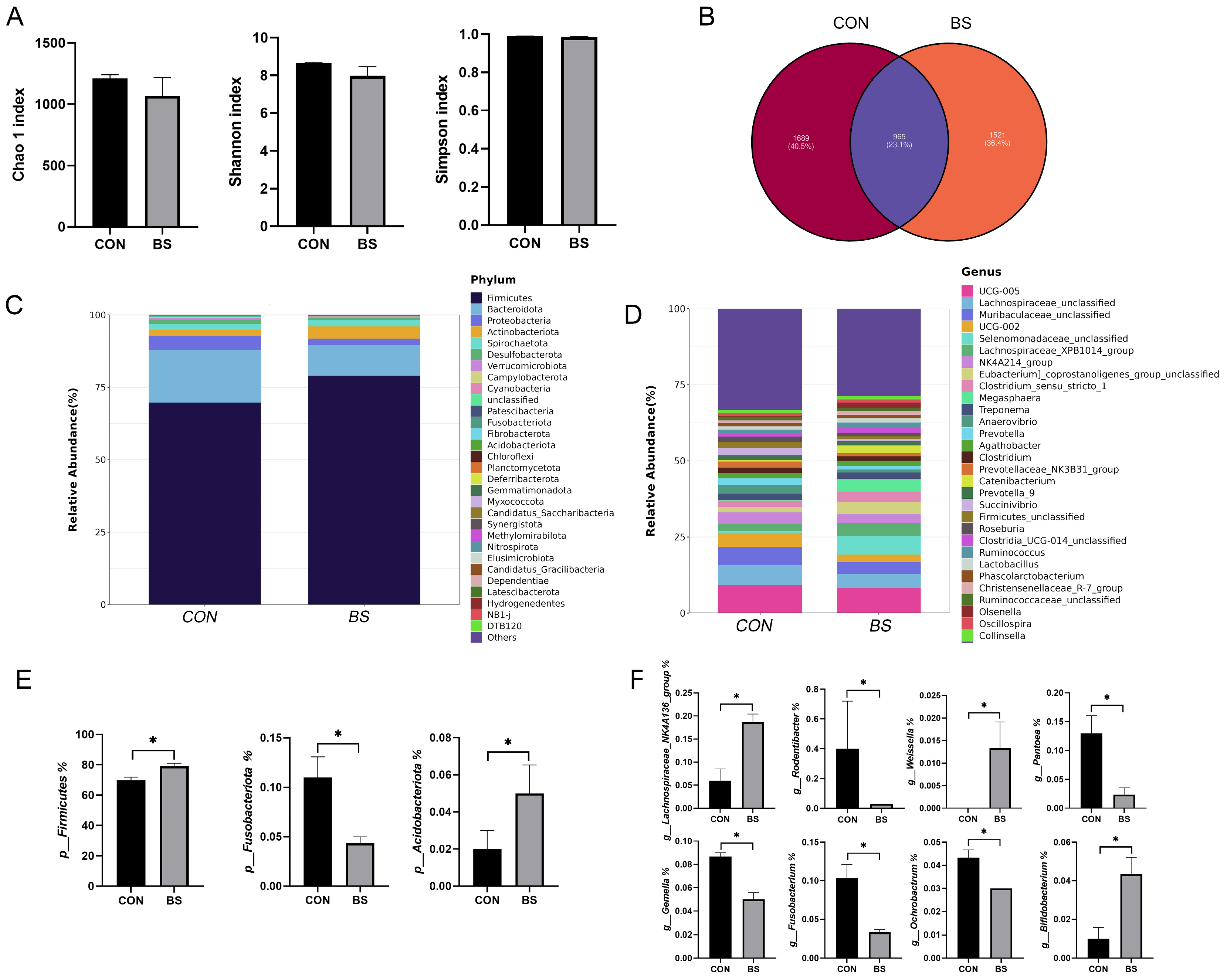
To investigate the effects of B. siamensis addition on the fecal microbiota of piglets, we sequenced the 16SrRNA gene in the fecal samples. The result is shown in Figure 5. Compared with the control group, there were no significant changes in the Chao 1, Shannon and Simpson indexes in the BS group (Figure 5A). This showed that B. siamensis supplementation did not affect fecal microbiota diversity. By observing the Venn diagram, it was found that there were 965 shared ASVs in both groups and 1689 and 1521 specific ASVs in the CON and BS groups, respectively (Figure 5B). At the phylum level, Firmicutes and Bacteroidetes were the dominant phyla, accounting for more than 85% of the totality (Figure 5C). At the genus level, the dominant genera were UCG-005, Lachnospiraceae_unclassified, Muribaculaceae_unclassified, UCG-002, Selenomonadaceae_unclassified, Lachnospiraceae_XPB1014_group and NK4A214_group (Figure 5D). Subsequently, the difference in microbial composition between the two treatments was further analyzed. At the phylum level, the abundance of Firmicutes and Acidobacteriota was significantly increased (p < 0.05) in the BS group and the abundance of Fusobacteriota was significantly reduced (p < 0.05) compared with the CON group (Figure 5E). At the genus level, B. siamensis supplementation significantly increased (p < 0.05) the abundance of Weissella, Lachnospiraceae_NK4A136_group and Bifidobacterium. Meanwhile, the abundance of Rodentibacter, Pantoea, Gemella, Fusobacterium and Ochrobactrum was significantly reduced (p < 0.05) (Figure 5F).
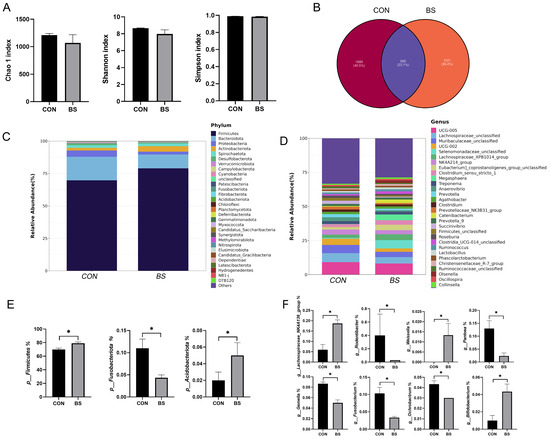
Figure 5.
Effects of B. siamensis supplementation on fecal microbiota composition of weaned piglets. Comparison of the observed Chao 1, Shannon and Simpson indices (A), and Venn diagram of shared and specific ASVs in the fecal microbiota between CON and BS groups (B). Relative abundance of fecal microbiota at the phylum (C,E) and genus (D,F) levels. Data are expressed as mean ± SEM (n = 3). * p < 0.05. CON: control; BS: B. siamensis.
3.8. Spearman’s Correlation Analysis
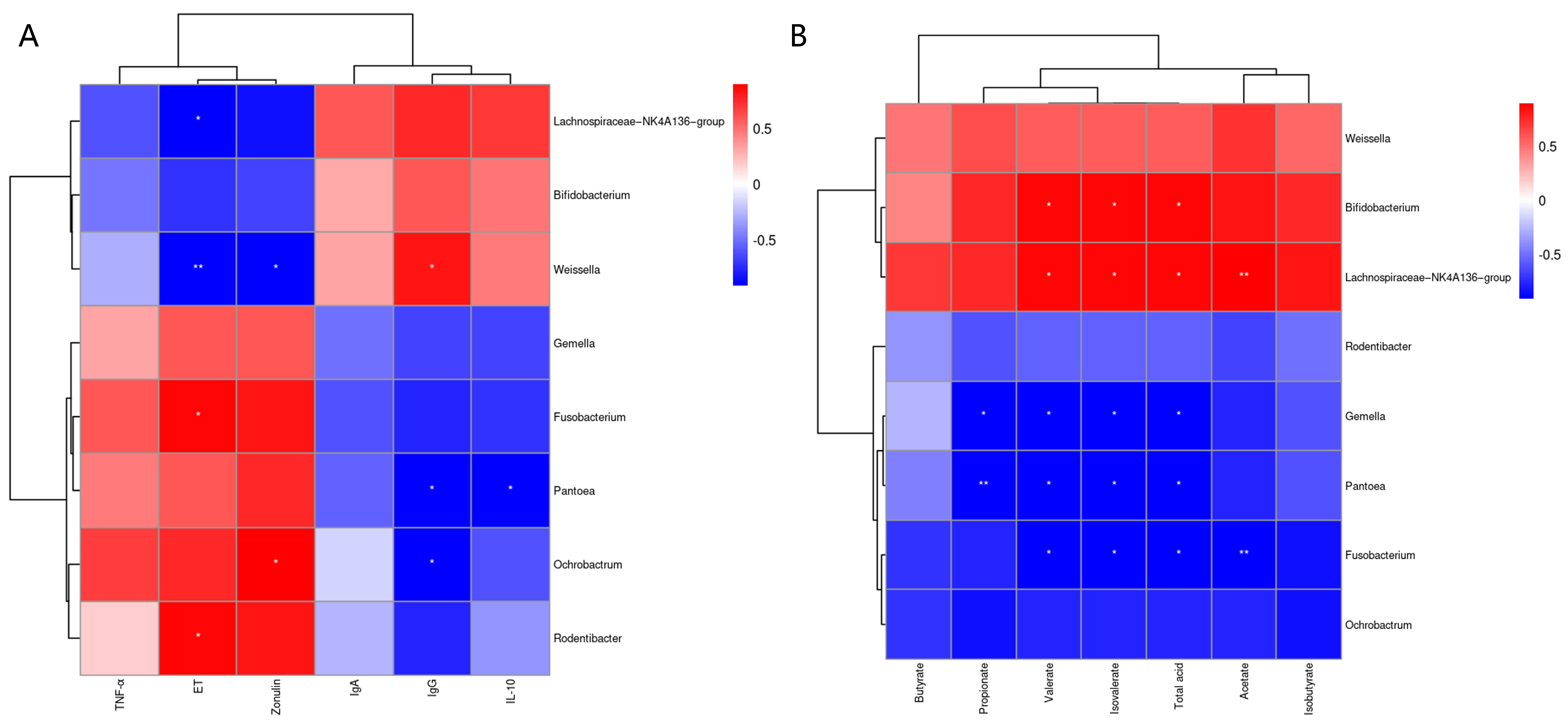
The Pearson correlation analysis of fecal microbiota with serum immune and inflammatory factors and gut permeability markers is shown in Figure 6A. The results showed that serum ET was positively correlated (p < 0.05) with Rodentibacter and Fusobacterium, and negatively correlated with Weissella (p < 0.01) and Lachnospiraceae_NK4A136_group (p < 0.05). The serum zonulin levels were positively correlated (p < 0.05) with Ochrobactrum and negatively correlated (p < 0.05) with Weissella. The serum IgG levels were positively correlated (p < 0.05) with Weissella and negatively correlated (p < 0.05) with Ochrobactrum and Pantoea. There was a significant negative correlation (p < 0.05) between serum IL-10 and Pantoea. Pearson correlation analysis of fecal microbiota and fecal SCFAs is shown in Figure 6B. The results showed that Propionate was significantly negatively correlated with Pantoea (p < 0.01) and Gemella (p < 0.05). The levels of fecal valerate, isovalerate and total acid were significantly negatively correlated (p < 0.05) with Fusobacterium, Pantoea and Gemella, and significantly positively correlated (p < 0.05) with Lachnospiraceae_NK4A136_group and Bifidobacterium. The level of acetate in stool was negatively correlated (p < 0.01) with that of Fusobacterium, and significantly positively correlated (p < 0.01) with Lachnospiraceae_NK4A136_group.
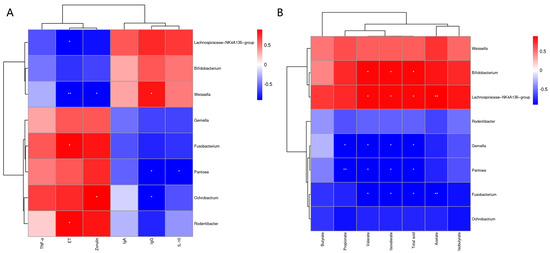
Figure 6.
The Pearson correlation analysis of fecal microbiota with serum immune and inflammatory factors and intestinal-permeability markers (A). Spearman correlation analysis of fecal microbiota with fecal SCFAs (B). The red and blue indicate positive and negative correlations, respectively: * p < 0.05, ** p < 0.01.
4. Discussion
Piglets are challenged by dietary and environmental stresses and harmful bacteria during the weaning period, which often causes dysfunctions of the gut and immune systems and disturbances in the gut microbial ecology, ultimately negatively affecting the growth performance and health status of the piglets [17,18]. Therefore, effective mitigation or elimination of the stress associated with weaning is necessary. Probiotics are widely used in animal feed due to their advantages of regulating the host immune system, maintaining the balance of microbiota and inhibiting the proliferation of pathogens in the gut [19,20]. Therefore, this study investigated the relieving effect of B. siamensis on the weaning stress of piglets.
The growth performance of piglets is often limited by weaning stress [21]. Many studies have previously shown that probiotic supplementation at the weaning stage improves piglet growth performance [22,23]. However, the results of this study showed that B. siamensis had no significant effect on the growth performance of piglets. This is consistent with the results of Cui et al. [24]. These differences in results may be explained by inconsistencies in diet composition, piglet strain and dosage. Duddeck et al. supplemented the diet of their experimental group with 1.875 × 105 CFU/g and 1.875 × 106 CFU/g B. subtilis, respectively, and the test results showed that the ADG of piglets in the lower-dose probiotic group was significantly higher than that in the control group and the higher-dose probiotic group [25]. Nevertheless, in order to more deeply explore the effect of B. siamensis on piglet growth, we measured serum GH and IGF levels. The results showed that the addition of B. siamensis to the diet significantly increased the serum GH and IGF levels in piglets. Meanwhile, the GH/IGF axis has been shown to be a master regulator for stimulating the growth of animal cells and somatic cells [26]. In addition, the higher levels of serum GH and IGF in the BS group indicated that piglets had higher anabolic activity, which had a positive effect on body growth and development [27]. Studies have shown that high-protein diets significantly increase growth hormone and IGF levels [28]. Therefore, the upregulation of growth hormone and IGF secretion may be closely related to the fact that B. siamensis improved the composition of the intestinal microbiota in piglets and thus increased nutrient intake [29].
The concentration of blood biochemical parameters is a standard index to evaluate nutrient metabolism and function [30]. Serum TCHO concentration can be used as a marker of lipid metabolism [31]. In this study, we found that the serum TCHO levels of piglets supplemented with B. siamensis were significantly reduced. This decrease in serum TCHO concentration reflected the improvement in lipid metabolism [32]. The reason may be that the supplementation of probiotics can effectively reduce serum cholesterol levels by increasing bile salt synthesis and bile acidolysis binding [33]. It is very interesting that the results of this trial showed that the serum HDL level was significantly reduced in the BS group.
Immunoglobulins are secreted by b-cell-activated plasma cells and play an important role in humoral immunity [34]. The main functions of immunoglobulins include activation of the complement system, which is responsible for inhibiting the attachment of microbiota, and inhibiting bacterial metabolism by blocking enzymes, antimicrobials and neutralizing viruses, thereby preventing pathogenic effects [35]. In this study, we showed that the addition of B. siamensis to the diet increased IgA and IgG levels in weaned piglets. This result is consistent with that reported by Zong et al. [36]. In addition, in the present study we found that B. siamensis supplementation increased IL-10 and decreased TNF-α. IL-10, as a master anti-inflammatory regulator with multiple functions, can antagonize pro-inflammatory factors and inhibit the migration of inflammatory cells [37]. Meanwhile, TNF-α is an inflammation-initiating factor that can initiate TNFR2 signaling through the activation of TNFR1, causing an inflammatory response in the body [38]. Overall, B. siamensis alleviated the inflammatory response and improved immunity levels in piglets.
A well-functioning gut barrier effectively prevents pathogens, toxins and antigens from entering the circulation through the gut mucosa [39]. Therefore, we measured the levels of four serum gut-permeability-related biomarkers (DAO, D-LA, ET and zonulin). Although the differences in serum DAO and D-LA were not significant, this study showed that serum zonulin and ET concentrations were reduced in piglets fed B. siamensis. The levels of gap connexin zonulin and endotoxin were significantly reduced, indicating reduced gut permeability [40,41]. It has also been previously reported that probiotics play a positive role in maintaining the integrity of gut epithelial cells and improving gut permeability [42]. This may be attributed to the fact that B. siamensis regulates the composition of the gut microbiota and promotes the secretion of SCFAs.
SCFAs, as end products of gut microbial fermentation [43], have been shown to have a variety of beneficial effects on immunity and metabolism [44]. Meanwhile, previous studies have shown that the supplementation of probiotics can increase the content of gut SCFAs in piglets, thereby alleviating the negative effects of weaning stress [45]. Similarly, our study showed that B. siamensis significantly increased SCFAs in piglet feces. Furthermore, SCFAs have been shown to be involved in various physiological processes and have positive effects on host health and nutrition [46]. Butyric acid, in particular, is thought to be a source of metabolic energy for gut cells and has anti-inflammatory properties that help the host maintain healthy gut barrier function [47]. Thus, the increased concentration of SCFAs may be one of the reasons why immunity, inflammation and gut permeability are improved in B. siamensis-fed piglets.
The gastrointestinal microbiota is critical for the metabolism of nutrients, the growth and maturation of immune responses and protection against pathogens [48]. The gut microbiota is a dynamic ecosystem that is influenced by a variety of factors, including diet, lifestyle, age and genotype. Diet is particularly important [49]. In this study, we investigated the effects of the addition of B. siamensis on microbial composition at the phylum and genus levels. At the phylum level, the abundance of Firmicutes in the feces of piglets from the BS group was significantly higher than that of the CON group, while the abundance of Bacteroidetes was lower, suggesting that the ratio of Firmicutes to Bacteroidetes was increased in the CON group. It has been reported that the increased ratio of Firmicutes to Bacteroidetes may be related to increased energy production and nutrient intake [50,51]. This may be due to improved nutrient utilization and absorption by dietary B. siamensis supplementation. In addition, the increased abundance of Firmicutes contributes to the synthesis of butyrate and propionate [52]. At the genus level, we found that the abundance of Weissella, Lachnospiraceae_NK4A136_group and Bifidobacterium in the BS group was significantly higher than that in the CON group. Weissella belongs to the facultative anaerobic lactic acid bacteria that can control foodborne pathogens by producing bacteriocins and organic acids [53]. Lachnospiraceae_NK4A136_group has been suggested to have multiple benefits, including the improvement of gut permeability and the production of SCFAs [54,55]. In addition, it has been shown that Lachnospiraceae_NK4A136_group is negatively correlated with inflammation [56]. Bifidobacterium, as a typical probiotic, can produce key anti-inflammatory SCFA propionate and inhibit the reproduction of harmful bacteria [57,58]. In addition, this study also found that the abundance of Pantoea, Fusobacterium and Gemella was significantly reduced. Pantoea is a Gram-negative bacterium that can cause associated infections that harm host health [59]. Fusobacterium is well known as an aggressive and pro-inflammatory disease-causing bacterium [60,61]. Gemella is a catalase-negative, Gram-positive bacterium and a potential pathogenic agent in the gastrointestinal tract [62,63]. According to this, dietary B. siamensis can improve the gut microbial composition of piglets by increasing the abundance of beneficial bacteria and inhibiting the abundance of pathogenic bacteria.
Many studies have shown a strong link between gut microbiota and host health [64,65]. Spillman correlation analysis showed that harmful bacteria (Rodentibacter, Pantoea, Fusobacterium and Ochrobactrum) with significantly lower abundance were negatively correlated with host immune levels, anti-inflammatory factors and SCFAs, and positively correlated with gut permeability markers. The increased abundance of beneficial bacteria (Weissella and Lachnospiraceae_NK4A136_group) was positively correlated with immune levels and SCFAs, and negatively correlated with gut permeability markers. Previous studies have shown that the gut microbiota has many benefits for the host, including activating the immune system, producing beneficial metabolites in gut fermentation, and preventing the proliferation of pathogenic bacteria [66]. In addition, SCFAs are the main source of energy for gut epithelial cells and are known for their antioxidant and anti-inflammatory properties [67]. Overall, B. siamensis improved immunity, inflammation, gut permeability and SCFAs in piglets by increasing the abundance of beneficial bacteria and decreasing the abundance of harmful bacteria.
5. Conclusions
Our results suggest that the supplementation of B. siamensis in the diet improves growth hormones, immunity, inflammatory factors, gut permeability and SCFAs in weaned piglets, and that all of these benefits are closely linked to the positive modulation of the microbiota by B. siamensis. More importantly, this study provides a new potential nutritional strategy for the alleviation of weaning stress in piglets. Meanwhile, this study also has certain limitations, which require experiments under different conditions to increase the sample size, such as different concentrations of probiotics or pigs with different growth periods. In addition, it is necessary to optimize the administration form of probiotics and determine the exact administration amount in test piglets to further verify the effectiveness of B. siamensis.
Author Contributions
H.L. (Huawei Liu): investigation, experiments, data analysis, data curation and writing—original draft. X.L.: experiments, data analysis and writing—original draft. H.L. (Haiyang Liu): experiments, software and visualization. J.T. and W.H.: methodology and software. T.X. and B.C.: experiments. J.H. and B.S.: funding acquisition, project administration, conceptualization, resources, supervision, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Major Program of Heilongjiang Province of China (2021ZX12B08).
Institutional Review Board Statement
The animal use protocol was reviewed and approved by the Ethics Committee for Animal Experiments of Northeast Agricultural University (NEAU-[2013]-9).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within the article.
Conflicts of Interest
The authors have no conflicts of interest to report.
References
- Chen, J.; Hu, N.Q.; Mao, Y.Q.; Hu, A.M.; Jiang, W.J.; Huang, A.M.; Wang, Y.; Meng, P.Y.; Hu, M.W.; Yang, X.B.; et al. Traditional Chinese medicine prescriptions (XJZ, JSS) ameliorate spleen inflammatory response and antioxidant capacity by synergistically regulating NF-κB and Nrf2 signaling pathways in piglets. Front. Vet. Sci. 2022, 9, 993018. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tan, X.; Wang, H.R.; Wang, Q.Y.; Huang, P.F.; Li, Y.L.; Li, J.Z.; Huang, J.; Yang, H.S.; Yin, Y.L. Effects of varying dietary folic acid during weaning stress of piglets. Anim. Nutr. 2021, 7, 101–110. [Google Scholar] [CrossRef]
- Gilani, S.M.H.; Rashid, Z.; Galani, S.; Ilyas, S.; Sahar, S.; Hassan, Z.U.; Al-Ghanim, K.; Zehra, S.; Azhar, A.; Al-Misned, F.; et al. Growth performance, intestinal histomorphology, gut microflora and ghrelin gene expression analysis of broiler by supplementing natural growth promoters: A nutrigenomics approach. Saudi J. Biol. Sci. 2021, 28, 3438–3447. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wang, A.; Fu, M.; Wang, A.; Chen, K.; Jia, Q.; Huang, Z.Y. Investigation of Incidents and Trends of Antimicrobial Resistance in Foodborne Pathogens in Eight Countries from Historical Sample Data. Int. J. Environ. Res. Public Health 2020, 17, 472. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yin, Y.X.; Wang, F.; Bao, X.T.; Long, L.N.; Tan, B.; Yin, Y.L.; Chen, J.S. Effects of dietary rosemary extract supplementation on growth performance, nutrient digestibility, antioxidant capacity, intestinal morphology, and microbiota of weaning pigs. J. Anim. Sci. 2021, 99, skab237. [Google Scholar] [CrossRef]
- Hu, S.L.; Cao, X.F.; Wu, Y.P.; Mei, X.Q.; Xu, H.; Wang, Y.; Zhang, X.P.; Gong, L.; Li, W.F. Effects of Probiotic Bacillus as an Alternative of Antibiotics on Digestive Enzymes Activity and Intestinal Integrity of Piglets. Front. Microbiol. 2018, 9, 2427. [Google Scholar] [CrossRef] [PubMed]
- Faber, W.; Stolwijk-Swuste, J.; van Ginkel, F.; Nachtegaal, J.; Zoetendal, E.; Winkels, R.; Witteman, B. Faecal Microbiota in Patients with Neurogenic Bowel Dysfunction and Spinal Cord Injury or Multiple Sclerosis-A Systematic Review. J. Clin. Med. 2021, 10, 1598. [Google Scholar] [CrossRef] [PubMed]
- Vemuri, R.; Shinde, T.; Gundamaraju, R.; Gondalia, S.V.; Karpe, A.V.; Beale, D.J.; Martoni, C.J.; Eri, R. Lactobacillus acidophilus DDS-1 Modulates the Gut Microbiota and Improves Metabolic Profiles in Aging Mice. Nutrients 2018, 10, 1255. [Google Scholar] [CrossRef] [PubMed]
- Upreti, D.; Ishiguro, S.; Robben, N.; Nakashima, A.; Suzuki, K.; Comer, J.; Tamura, M. Oral Administration of Water Extract from Euglena gracilis Alters the Intestinal Microbiota and Prevents Lung Carcinoma Growth in Mice. Nutrients 2022, 14, 678. [Google Scholar] [CrossRef]
- Tian, Z.L.; Wang, X.D.; Duan, Y.H.; Zhao, Y.; Zhang, W.M.; Azad, M.A.K.; Wang, Z.B.; Blachier, F.; Kong, X.F. Dietary Supplementation With Bacillus subtilis Promotes Growth and Gut Health of Weaned Piglets. Front. Vet. Sci. 2021, 7, 600772. [Google Scholar] [CrossRef] [PubMed]
- Jinno, C.; Wong, B.D.; Klünemann, M.; Htoo, J.; Li, X.D.; Liu, Y.H. Effects of supplementation of Bacillus amyloliquefaciens on performance, systemic immunity, and intestinal microbiota of weaned pigs experimentally infected with a pathogenic enterotoxigenic E. coli F18. Front. Microbiol. 2023, 14, 1101457. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S.X.; Liu, H.; Zhang, D.Y.; Wang, Y.M.; Ji, H.F. Effects of oligosaccharides on the growth and stress tolerance of Lactobacillus plantarum ZLP001 in vitro, and the potential synbiotic effects of L. plantarum ZLP001 and fructo-oligosaccharide in post-weaning piglets. J. Anim. Sci. 2019, 97, 4588–4597. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.L.; Cao, G.T.; Zhang, H.R.; Li, Q.; Yang, C.M. Effects of Clostridium butyricum and Enterococcus faecalis on growth performance, immune function, intestinal morphology, volatile fatty acids, and intestinal flora in a piglet model. Food Funct. 2019, 10, 7844–7854. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.S.; Gu, W.; Liu, X.Y.; Zou, Y.W.; Wu, Y.J.; Xu, Y.H.; Han, D.D.; Wang, J.J.; Zhao, J.B. Joint Application of Lactobacillus plantarum and Bacillus subtilis Improves Growth Performance, Immune Function and Intestinal Integrity in Weaned Piglets. Vet. Sci. 2022, 9, 668. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Zhao, X.C.; Azad, M.A.; Ma, C.; Gao, Q.K.; He, J.H.; Kong, X.F. Dietary supplementation with Bacillus subtilis and xylo-oligosaccharides improves growth performance and intestinal morphology and alters intestinal microbiota and metabolites in weaned piglets. Food Funct. 2021, 12, 5837–5849. [Google Scholar] [CrossRef] [PubMed]
- Council, N.R. Nutrient Requirements of Swine: Eleventh Revised Edition; The National Academies Press: Washington, DC, USA, 2012; p. 420. [Google Scholar]
- González-Solé, F.; Solà-Oriol, D.; Ramayo-Caldas, Y.; Rodriguez-Prado, M.; Ortiz, G.G.; Bedford, M.R.; Pérez, J.F. Supplementation of xylo-oligosaccharides to suckling piglets promotes the growth of fiber-degrading gut bacterial populations during the lactation and nursery periods. Sci. Rep. 2022, 12, 11594. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.J.; Yang, Y.Z.; Bao, C.L.; Cao, Y.H. High-level expression of an acidic thermostable xylanase in Pichia pastoris and its application in weaned piglets. J. Anim. Sci. 2020, 98, skz364. [Google Scholar] [CrossRef]
- Chen, X.Y.; Zhao, X.; Wang, H.W.; Yang, Z.L.; Li, J.; Suo, H.Y. Prevent Effects of Lactobacillus Fermentum HY01 on Dextran Sulfate Sodium-Induced Colitis in Mice. Nutrients 2017, 9, 545. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Q.; Xia, Y.; Zhu, S.B.; Yang, J.; Yao, J.; Di, J.Z.; Liang, Y.; Gao, R.Y.; Wu, W.; Yang, Y.Z.; et al. Lactobacillus plantarum LP-Onlly alters the gut flora and attenuates colitis by inducing microbiome alteration in interleukin-10 knockout mice. Mol. Med. Rep. 2017, 16, 5979–5985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Ma, M.M.; Li, Z.Y.; Zhang, H.H.; He, X.; Song, Z.H. Protective Effects of L-Theanine on IPEC-J2 Cells Growth Inhibition Induced by Dextran Sulfate Sodium via p53 Signaling Pathway. Molecules 2021, 26, 7002. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.P.; Su, W.F.; Li, W.T.; Wen, C.Y.; Du, S.; He, H.; Zhang, Y.; Gong, T.; Wang, X.X.; Wang, Y.Z.; et al. Bacillus amyloliquefaciens 40 regulates piglet performance, antioxidant capacity, immune status and gut microbiota. Anim. Nutr. 2023, 12, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Jin, P.T.; Qin, S.Z.; Liu, J.Z.; Yang, Z.J.; Zhao, H.B.; Sheng, Q.K. Effects of dietary supplementation with S. platensis and probiotics on the growth performance, immune response and the fecal Lactobacillus spp. and E. coli contents of weaned piglets. Livest. Sci. 2019, 225, 32–38. [Google Scholar] [CrossRef]
- Cui, K.; Wang, Q.; Wang, S.Q.; Diao, Q.Y.; Zhang, N.F. The Facilitating Effect of Tartary Buckwheat Flavonoids and Lactobacillus plantarum on the Growth Performance, Nutrient Digestibility, Antioxidant Capacity, and Fecal Microbiota of Weaned Piglets. Animals 2019, 9, 986. [Google Scholar] [CrossRef] [PubMed]
- Duddeck, K.A.; Petersen, T.E.; Adkins, H.J.; Smith, A.H.; Hernandez, S.; Wenner, S.J.; Yao, D.; Chen, C.; Li, W.L.; Fregulia, P.; et al. Dose-Dependent Effects of Supplementing a Two-Strain Bacillus subtilis Probiotic on Growth Performance, Blood Parameters, Fecal Metabolites, and Microbiome in Nursery Pigs. Animals 2024, 14, 109. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Xiong, S.T.; Li, Z.; Wu, J.J.; Zhou, L.; Gui, J.F.; Mei, J. A feedback regulatory loop involving p53/miR-200 and growth hormone endocrine axis controls embryo size of zebrafish. Sci. Rep. 2015, 5, 15906. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.K.; Zhang, X.Y.; Wu, Y.J.; Che, D.S.; Ye, H.; Pi, Y.; Tao, S.Y.; Wang, J.J.; Han, D.D. Maternal Amino Acid Mixtures Supplementation during Late Gestation and Lactation Improved Growth Performance of Piglets through Improving Colostrum Composition and Antioxidant Capacity. Antioxidants 2022, 11, 2144. [Google Scholar] [CrossRef] [PubMed]
- Caputo, M.; Pigni, S.; Agosti, E.; Daffara, T.; Ferrero, A.; Filigheddu, N.; Prodam, F. Regulation of GH and GH Signaling by Nutrients. Cells 2021, 10, 1376. [Google Scholar] [CrossRef] [PubMed]
- Lavajoo, F.; Perelló-Amorós, M.; Vélez, E.J.; Sánchez-Moya, A.; Balbuena-Pecino, S.; Riera-Heredia, N.; Fernández-Borràs, J.; Blasco, J.; Navarro, I.; Capilla, E.; et al. Regulatory mechanisms involved in muscle and bone remodeling during refeeding in gilthead sea bream. Sci. Rep. 2020, 10, 184. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, C.; Wang, Z.S.; Cao, G.; Hu, R.; Wang, X.Y.; Zou, H.W.; Kang, K.; Peng, Q.H.; Xue, B.; et al. Active dry yeast supplementation improves the growth performance, rumen fermentation, and immune response of weaned beef calves. Anim. Nutr. 2021, 7, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.F.; Yue, S.Z.; Qiao, Y.H.; Sun, Z.J.; Wang, C.; Li, H.F. Dietary supplementation with selenium-enriched earthworm powder improves antioxidative ability and immunity of laying hens. Poult. Sci. 2020, 99, 5344–5349. [Google Scholar] [CrossRef] [PubMed]
- Huo, W.Y.; Li, M.; Wang, J.P.; Wang, Z.X.; Huang, Y.Q.; Chen, W. On growth performance, nutrient digestibility, blood T lymphocyte subsets, and cardiac antioxidant status of broilers. Anim. Nutr. 2019, 5, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. Probiotics, Prebiotics and Synbiotics-A Promising Strategy in Prevention and Treatment of Cardiovascular Diseases? Int. J. Mol. Sci. 2020, 21, 9737. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.Q.; Du, J.; Li, T.T.; Gao, N.; Yang, S.Y.; Zhang, Y.X.; Pan, L.L. Elevated serum immunoglobulin level predicts high risk of 1-year recurrence in patients with Takayasu arteritis. Arthritis Res. Ther. 2023, 25, 36. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Zhang, Y.; He, W.T.; Wei, Y.H.; Han, S.S.; Xia, L.; Tan, B.; Yu, J.; Kang, H.Y.; Ma, M.E.; et al. Effects of Small Peptide Supplementation on Growth Performance, Intestinal Barrier of Laying Hens During the Brooding and Growing Periods. Front. Immunol. 2022, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Wang, T.H.; Lu, Z.Q.; Song, D.G.; Zhao, J.; Wang, Y.Z. Effects of Clostridium butyricum or in combination with Bacillus licheniformis on the growth performance, blood indexes, and intestinal barrier function of weanling piglets. Livest. Sci. 2019, 220, 137–142. [Google Scholar] [CrossRef]
- Wang, W.H.; Zhang, J.S.; Feng, T.; Deng, J.; Lin, C.C.; Fan, H.; Yu, W.J.; Bao, H.Y.; Jia, W. Structural elucidation of a polysaccharide from &ITFlammulina velutipes &ITand its immunomodulation activities on mouse B lymphocytes. Sci. Rep. 2018, 8, 3120. [Google Scholar] [CrossRef] [PubMed]
- Dreschers, S.; Gille, C.; Haas, M.; Grosse-Ophoff, J.; Schneider, M.; Leiber, A.; Bühring, H.J.; Orlikowsky, T.W. Infection-induced Bystander-Apoptosis of Monocytes Is TNF-alpha-mediated. PLoS ONE 2013, 8, e53589. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.H.; Liu, H.S.; Liu, S.J.; He, T.F.; Piao, X.S. Effects of dietary fiber sources during late gestation and lactation on sow performance, milk quality, and intestinal health in piglets. J. Anim. Sci. 2019, 97, 4922–4933. [Google Scholar] [CrossRef] [PubMed]
- Dirajlal-Fargo, S.; El-Kamari, V.; Weiner, L.; Shan, L.P.; Sattar, A.; Kulkarni, M.; Funderburg, N.; Nazzinda, R.; Karungi, C.; Kityo, C.; et al. Altered Intestinal Permeability and Fungal Translocation in Ugandan Children With Human Immunodeficiency Virus. Clin. Infect. Dis. 2020, 70, 2413–2422. [Google Scholar] [CrossRef]
- de Punder, K.; Pruimboom, L. Stress induces endotoxemia and low-grade inflammation by increasing barrier permeability. Front. Immunol. 2015, 6, 223. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.Z.; Chen, H.Q.; Liang, Y.; Xia, Y.; Yang, Y.Z.; Yang, J.; Zhang, J.D.; Wang, S.H.; Liu, J.; Qin, H.L. Combined probiotic bacteria promotes intestinal epithelial barrier function in interleukin-10-gene-deficient mice. World J. Gastroenterol. 2014, 20, 4636–4647. [Google Scholar] [CrossRef] [PubMed]
- Emal, D.; Rampanelli, E.; Stroo, I.; Butter, L.M.; Teske, G.J.; Claessen, N.; Stokman, G.; Florquin, S.; Leemans, J.C.; Dessing, M.C. Depletion of Gut Microbiota Protects against Renal Ischemia-Reperfusion Injury. J. Am. Soc. Nephrol. 2017, 28, 1450–1461. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.J.; Yin, B.M.; Li, W.; Chai, T.J.; Liang, W.W.; Huang, Y.; Tan, X.M.; Zheng, P.; Wu, J.; Li, Y.F.; et al. Age-related changes in microbial composition and function in cynomolgus macaques. Aging-Us 2019, 11, 12080–12096. [Google Scholar] [CrossRef]
- Hsu, Y.J.; Huang, W.C.; Lin, J.S.; Chen, Y.M.; Ho, S.T.; Huang, C.C.; Tung, Y.T. Kefir Supplementation Modifies Gut Microbiota Composition, Reduces Physical Fatigue, and Improves Exercise Performance in Mice. Nutrients 2018, 10, 862. [Google Scholar] [CrossRef]
- Pereira, G.V.; Abdel-Hamid, A.M.; Dutta, S.; D’Alessandro-Gabazza, C.N.; Wefers, D.; Farris, J.A.; Bajaj, S.; Wawrzak, Z.; Atomi, H.; Mackie, R.I.; et al. Degradation of complex arabinoxylans by human colonic Bacteroidetes. Nat. Commun. 2021, 12, 459. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, Y.; Xiao, K.P.; Jiang, F.; Wang, H.C.; Tang, D.Z.; Liu, D.; Liu, B.; Liu, Y.S.; He, X.; et al. The chicken gut metagenome and the modulatory effects of plant-derived benzylisoquinoline alkaloids. Microbiome 2018, 6, 211. [Google Scholar] [CrossRef] [PubMed]
- Mach, N.; Fuster-Botella, D. Endurance exercise and gut microbiota: A review. J. Sport Health Sci. 2017, 6, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Vilchez-Vargas, R.; Skieceviciene, J.; Lehr, K.; Varkalaite, G.; Thon, C.; Urba, M.; Morkunas, E.; Kucinskas, L.; Bauraite, K.; Schanze, D.; et al. Gut microbial similarity in twins is driven by shared environment and aging. Ebiomedicine 2022, 79, 104011. [Google Scholar] [CrossRef] [PubMed]
- Carrothers, J.M.; York, M.A.; Brooker, S.L.; Lackey, K.A.; Williams, J.E.; Shafii, B.; Price, W.J.; Settles, M.L.; McGuire, M.A.; McGuire, M.K. Fecal Microbial Community Structure Is Stable over Time and Related to Variation in Macronutrient and Micronutrient Intakes in Lactating Women. J. Nutr. 2015, 145, 2379–2388. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.S.; Geng, S.J.; Li, Y.; Cheng, S.S.; Fu, X.F.; Yue, X.J.; Han, X.Y. Exogenous Fecal Microbiota Transplantation from Local Adult Pigs to Crossbred Newborn Piglets. Front. Microbiol. 2018, 8, 2663. [Google Scholar] [CrossRef] [PubMed]
- Kalkan, H.; Pagano, E.; Paris, D.; Panza, E.; Cuozzo, M.; Moriello, C.; Piscitelli, F.; Abolghasemi, A.; Gazzerro, E.; Silvestri, C.; et al. Targeting gut dysbiosis against inflammation and impaired autophagy in Duchenne muscular dystrophy. Embo Mol. Med. 2023, 15, e16225. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.G.; Fusieger, A.; Miliao, G.L.; Martins, E.; Drider, D.; Nero, L.A.; de Carvalho, A.F. Weissella: An Emerging Bacterium with Promising Health Benefits. Probiotics Antimicrob. Proteins 2021, 13, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.C.; Zhang, Y.; Ling, T.; Zhao, C.J.; Li, Y.R.; Geng, M.; Gai, S.L.; Qi, W.; Luo, X.G.; Chen, L.H.; et al. Chitosan Oligosaccharides Alleviate Colitis by Regulating Intestinal Microbiota and PPARγ/SIRT1-Mediated NF-κB Pathway. Mar. Drugs 2022, 20, 96. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.T.; Wang, Z.X.; Cao, J.; Dong, Y.L.; Chen, Y.X. Gut microbiota-derived metabolites mediate the neuroprotective effect of melatonin in cognitive impairment induced by sleep deprivation. Microbiome 2023, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.H.; Yang, W.L.; Su, C.Y.; Lu, X.H.; He, L.; Zhang, A.H. Relationship between gut microbiota and vascular calcification in hemodialysis patients. Ren. Fail. 2023, 45, 2148538. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Usyk, M.; Sollecito, C.C.; Qiu, Y.P.; Williams-Nguyen, J.; Hua, S.M.; Gradissimo, A.; Wang, T.; Xue, X.N.; Kurland, I.J.; et al. Altered Gut Microbiota and Host Metabolite Profiles in Women With Human Immunodeficiency Virus. Clin. Infect. Dis. 2020, 71, 2345–2353. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Fujita, Y.; Ren, Q.; Ma, M.; Dong, C.; Hashimoto, K. Bifidobacterium in the gut microbiota confer resilience to chronic social defeat stress in mice. Sci. Rep. 2017, 7, srep45942. [Google Scholar] [CrossRef] [PubMed]
- Susanto, M.; Dunning, J.; Chew, R. Pantoea abscess mimicking sarcoma in a HTLV-1-infected Indigenous Australian man: Case report and literature review. Clin. Case Rep. 2023, 11, e7351. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; McDowell, A.; Kim, E.K.; Seo, H.; Lee, W.H.; Moon, C.M.; Kym, S.M.; Lee, D.H.; Park, Y.S.; Jee, Y.K.; et al. Development of a colorectal cancer diagnostic model and dietary risk assessment through gut microbiome analysis. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yin, J.; Xu, K.; Han, H.; Liu, Z.M.; Wang, C.Y.; Li, T.J.; Yin, Y.L. Protein Level and Infantile Diarrhea in a Postweaning Piglet Model. Mediat. Inflamm. 2020, 2020, 1937387. [Google Scholar] [CrossRef] [PubMed]
- Maraki, S.; Pleyritaki, A.; Kofteridis, D.; Scoulica, E.; Eskitzis, A.; Gikas, A.; Panagiotakis, S.H. Bicuspid aortic valve endocarditis caused by Gemella sanguinis: Case report and literature review. J. Infect. Public Health 2019, 12, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Carmody, L.A.; Zhao, J.; Schloss, P.D.; Petrosino, J.F.; Murray, S.; Young, V.B.; Li, J.Z.; LiPuma, J.J. Changes in cystic fibrosis airway microbiota at pulmonary exacerbation. Ann. Am. Thorac. Soc. 2013, 10, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Vincent, C.; Miller, M.A.; Edens, T.J.; Mehrotra, S.; Dewar, K.; Manges, A.R. Bloom and bust: Intestinal microbiota dynamics in response to hospital exposures and Clostridium difficile colonization or infection. Microbiome 2016, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Rath, S.; Rud, T.; Karch, A.; Pieper, D.H.; Vital, M. Pathogenic functions of host microbiota. Microbiome 2018, 6, 174. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Xu, T.Q.; Sun, J.Y.; Shi, J.R.; Li, F.L.; Yin, Y.Q.; Wang, Z.Q.; Liu, Y. Nicotinamide Mononucleotide Ameliorates Sleep Deprivation-Induced Gut Microbiota Dysbiosis and Restores Colonization Resistance against Intestinal Infections. Adv. Sci. 2023, 10, e2207170. [Google Scholar] [CrossRef] [PubMed]
- Jaye, K.; Li, C.G.; Chang, D.; Bhuyan, D.J. The role of key gut microbial metabolites in the development and treatment of cancer. Gut Microbes 2022, 14, 2038865. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).