Comparative Analysis of Bisexual and Parthenogenetic Populations in Haemaphysalis Longicornis
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sample Collection
2.3. Identification of Tick Species and Phylogenetic Analysis
2.4. Polyploid Analysis of Ticks by FACS
2.5. Phylogenetic Tree and Genetic Distance
2.6. Animal Experiment
2.7. HRTV and SFTSV RNA Extraction and Real-Time RT-PCR
2.8. Virus and Cells
2.9. Virus Titration
3. Results
3.1. Subsection Geographical Distribution of H. longicornis
3.2. Geographical Distribution of Bisexual and Parthenogenetic H. longicornis
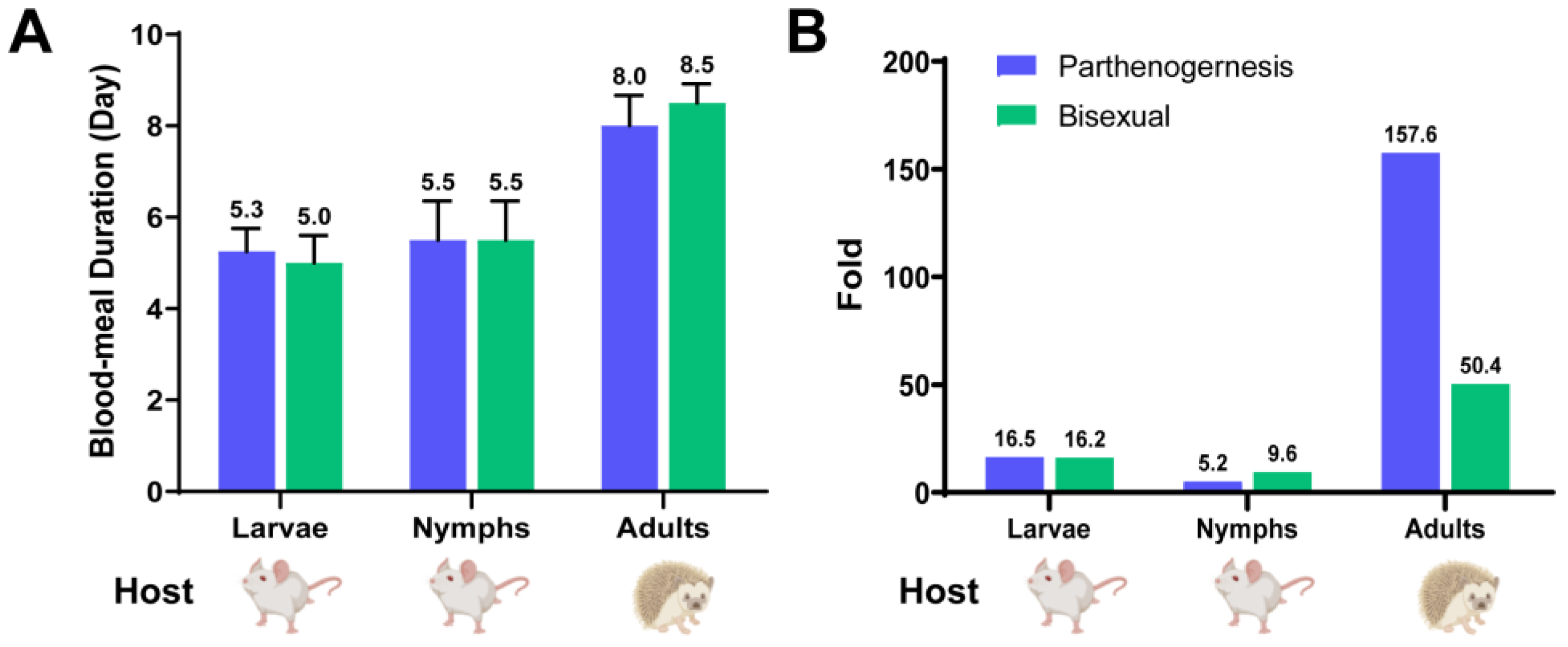
3.3. Base Features and Blood-Sucking Time
3.4. Ploidy, Molecular, and Phylogenetic Analysis
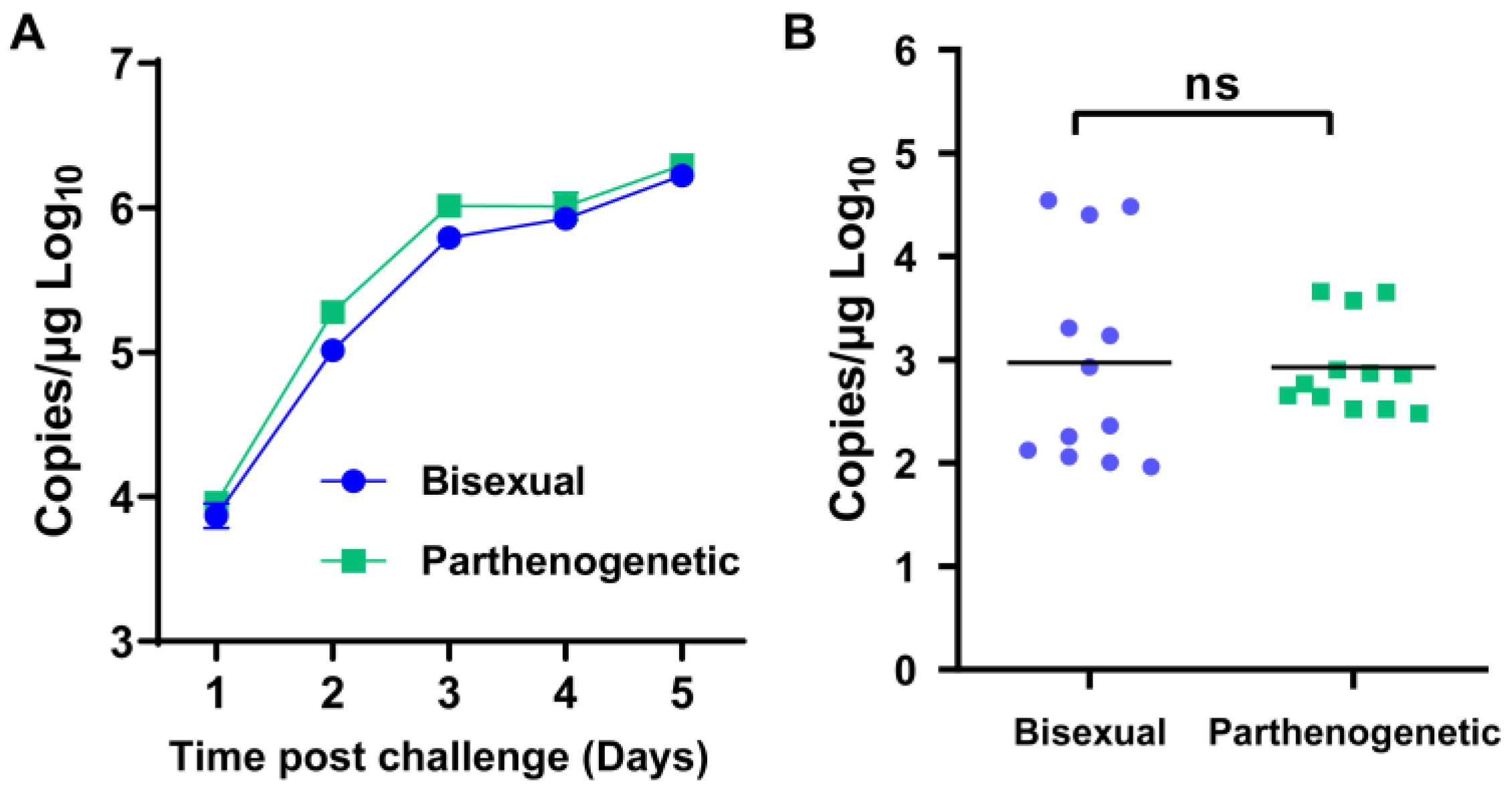
3.5. Susceptibility to HRTV and SFTS Virus
3.6. Resistance to Ivermectin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, G.P.; Wang, Y.X.; Fan, Z.W.; Ji, Y.; Liu, M.J.; Zhang, W.H.; Li, X.L.; Zhou, S.X.; Li, H.; Liang, S.; et al. Mapping ticks and tick-borne pathogens in China. Nat. Commun. 2021, 12, 1075. [Google Scholar] [CrossRef] [PubMed]
- Horak, I.G.; Camicas, J.L.; Keirans, J.E. The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida): A world list of valid tick names. Exp. Appl. Acarol. 2002, 28, 27–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Liu, J. Ticks (Acari: Ixodoidea) in China: Geographical distribution, host diversity, and specificity. Arch. Insect Biochem. Physiol. 2019, 102, e21544. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, X.; Bu, F.; Yang, X.; Yang, X.; Liu, J. Ticks (acari: Ixodoidea: Argasidae, ixodidae) of China. Exp. Appl. Acarol. 2010, 51, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Eisen, L.; Eisen, R.J. Critical Evaluation of the Linkage Between Tick-Based Risk Measures and the Occurrence of Lyme Disease Cases. J. Med. Entomol. 2016, 53, 1050–1062. [Google Scholar] [CrossRef] [PubMed]
- Diuk-Wasser, M.A.; Vannier, E.; Krause, P.J. Coinfection by Ixodes Tick-Borne Pathogens: Ecological, Epidemiological, and Clinical Consequences. Trends Parasitol. 2016, 32, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Wang, J.; Shi, W.; Du, L.; Ye, R.Z.; Zhao, F.; Cao, W.C. Haemaphysalis longicornis. Trends Genet. TIG 2021, 37, 292–293. [Google Scholar] [CrossRef] [PubMed]
- Yabsley, M.J.; Thompson, A.T. Haemaphysalis longicornis (Asian longhorned tick). Trends Parasitol. 2023, 39, 305–306. [Google Scholar] [CrossRef] [PubMed]
- Tufts, D.M.; VanAcker, M.C.; Fernandez, M.P.; DeNicola, A.; Egizi, A.; Diuk-Wasser, M.A. Distribution, Host-Seeking Phenology, and Host and Habitat Associations of Haemaphysalis longicornis Ticks, Staten Island, New York, USA. Emerg. Infect. Dis. 2019, 25, 792–796. [Google Scholar] [CrossRef]
- Hoogstraal, H.; Roberts, F.H.; Kohls, G.M.; Tipton, V.J. Review of Haemaphysalis (kaiseriana) Longicornis Neumann (resurrected) of Australia, New Zealand, New Caledonia, Fiji, Japan, Korea, and Northeastern China and USSR, and its parthenogenetic and bisexual populations (Ixodoidea, Ixodidae). J. Parasitol. 1968, 54, 1197–1213. [Google Scholar] [CrossRef]
- Beard, C.B.; Occi, J.; Bonilla, D.L.; Egizi, A.M.; Fonseca, D.M.; Mertins, J.W.; Backenson, B.P.; Bajwa, W.I.; Barbarin, A.M.; Bertone, M.A.; et al. Multistate Infestation with the Exotic Disease-Vector Tick Haemaphysalis longicornis—United States, August 2017-September 2018. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 1310–1313. [Google Scholar] [CrossRef] [PubMed]
- Rainey, T.; Occi, J.L.; Robbins, R.G.; Egizi, A. Discovery of Haemaphysalis longicornis (Ixodida: Ixodidae) Parasitizing a Sheep in New Jersey, United States. J. Med. Entomol. 2018, 55, 757–759. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, J.; Cui, X.; Jia, N.; Wei, J.; Xia, L.; Wang, H.; Zhou, Y.; Wang, Q.; Liu, X.; et al. Distribution of Haemaphysalis longicornis and associated pathogens: Analysis of pooled data from a China field survey and global published data. Lancet Planet. Health 2020, 4, e320–e329. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, K.L.; Cook, C.; Ellis, R.J.; Bell-Sakyi, L.; Johnson, N.; Alberdi, P.; de la Fuente, J.; Fooks, A.R. Tick-borne pathogens induce differential expression of genes promoting cell survival and host resistance in Ixodes ricinus cells. Parasites Vectors 2017, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.H., Jr. Parthenogenesis in mites and ticks (Arachnida: Acari). Am. Zool. 1971, 11, 283–299. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, J. The Vector Competence of Asian Longhorned Ticks in Langat Virus Transmission. Viruses 2024, 16, 304. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Mukhopadhayay, S.K. Tick-borne ehrlichiosis infection in human beings. J. Vector Borne Dis. 2008, 45, 273–280. [Google Scholar] [PubMed]
- Kang, J.G.; Ko, S.; Smith, W.B.; Kim, H.C.; Lee, I.Y.; Chae, J.S. Prevalence of Anaplasma, Bartonella and Borrelia Species in Haemaphysalis longicornis collected from goats in North Korea. J. Vet. Sci. 2016, 17, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, S.; Yu, Z.; Guo, L.; Yang, S.; Liu, L.; Yang, X.; Liu, J. Multiple lines of evidence on the genetic relatedness of the parthenogenetic and bisexual Haemaphysalis longicornis (Acari: Ixodidae). Infect. Genet. Evol. 2014, 21, 308–314. [Google Scholar] [CrossRef]
- Egizi, A.; Bulaga-Seraphin, L.; Alt, E.; Bajwa, W.I.; Bernick, J.; Bickerton, M.; Campbell, S.R.; Connally, N.; Doi, K.; Falco, R.C.; et al. First glimpse into the origin and spread of the Asian longhorned tick, Haemaphysalis longicornis, in the United States. Zoonoses Public Health 2020, 67, 637–650. [Google Scholar] [CrossRef]
- Wang, T.; Wang, T.; Zhang, M.; Shi, X.; Zhang, M.; Wang, H.; Yang, X.; Yu, Z.; Liu, J. The Ovarian Development Genes of Bisexual and Parthenogenetic Haemaphysalis longicornis Evaluated by Transcriptomics and Proteomics. Front. Vet. Sci. 2021, 8, 783404. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, X.; Bu, F.; Yang, X.; Liu, J. Morphological, biological and molecular characteristics of bisexual and parthenogenetic Haemaphysalis longicornis. Vet. Parasitol. 2012, 189, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.H., Jr.; Tanaka, K.; Sawada, M. Cytogenetics of ticks (Acari: Ixodoidea). 12. Chromosome and hybridization studies of bisexual and parthenogenetic Haemaphysalis longicornis races from Japan and Korea. Chromosoma 1973, 42, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, C.; Cheng, C.; Zhang, G.; Yu, T.; Lawrence, K.; Li, H.; Sun, J.; Yang, Z.; Ye, L.; et al. Rapid Spread of Severe Fever with Thrombocytopenia Syndrome Virus by Parthenogenetic Asian Longhorned Ticks. Emerg. Infect. Dis. 2022, 28, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, B.H.; Tao, F.; Duan, X.Z. Two-phase optimization method of multi-period reactive power and voltage control. Gaodianya Jishu/High Volt. Eng. 2007, 33, 104–109. [Google Scholar]
- Zhou, J.L.; Zhou, Y.Z.; Gong, H.Y.; Chen, L.Z.; Cao, J. Discovery of parthenogenesis population of Haemaphysalis longicornis in China and its biological characteristic. Chin. J. Vector Biol. Control 2004, 15, 173–174. [Google Scholar]
- Liu, J.; Jiang, Z. Biological characteristics of Haemaphysalis longicornis under laboratory conditions. Acta Entomol. Sin. 1998, 41, 280–283. [Google Scholar]
- Herrinf, C.S.; James, H.; Oliver, J. Numerical taxonomic studies of parthenogenetic and bisexual populations of Haemaphysalis longicornis and related species. J. Parasitol. 1974, 60, 1025–1036. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, S.; Pei, T.; Yu, Z.; Liu, J. The Complete Mitochondrial Genome and Expression Profile of Mitochondrial Protein-Coding Genes in the Bisexual and Parthenogenetic Haemaphysalis longicornis. Front. Physiol. 2019, 10, 982. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, G.-Y.; Bian, Y. Karyotypic analyses of the bisexual and parthenogenetic tick (Haemaphysalis concinna). Int. J. Acarol. 2012, 38, 206–213. [Google Scholar] [CrossRef]
- McMullan, L.K.; Folk, S.M.; Kelly, A.J.; MacNeil, A.; Goldsmith, C.S.; Metcalfe, M.G.; Batten, B.C.; Albarino, C.G.; Zaki, S.R.; Rollin, P.E.; et al. A new phlebovirus associated with severe febrile illness in Missouri. N. Engl. J. Med. 2012, 367, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, C.A.; Ebihara, H.; Yamaoka, S. Immune Modulation and Immune-Mediated Pathogenesis of Emerging Tickborne Banyangviruses. Vaccines 2019, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- van der Kooi, C.J.; Matthey-Doret, C.; Schwander, T. Evolution and comparative ecology of parthenogenesis in haplodiploid arthropods. Evol. Lett. 2017, 1, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Stanley, H.M.; Ford, S.L.; Snellgrove, A.N.; Hartzer, K.; Smith, E.B.; Krapiunaya, I.; Levin, M.L. The Ability of the Invasive Asian Longhorned Tick Haemaphysalis longicornis (Acari: Ixodidae) to Acquire and Transmit Rickettsia rickettsii (Rickettsiales: Rickettsiaceae), the Agent of Rocky Mountain Spotted Fever, Under Laboratory Conditions. J. Med. Entomol. 2020, 57, 1635–1639. [Google Scholar] [CrossRef]
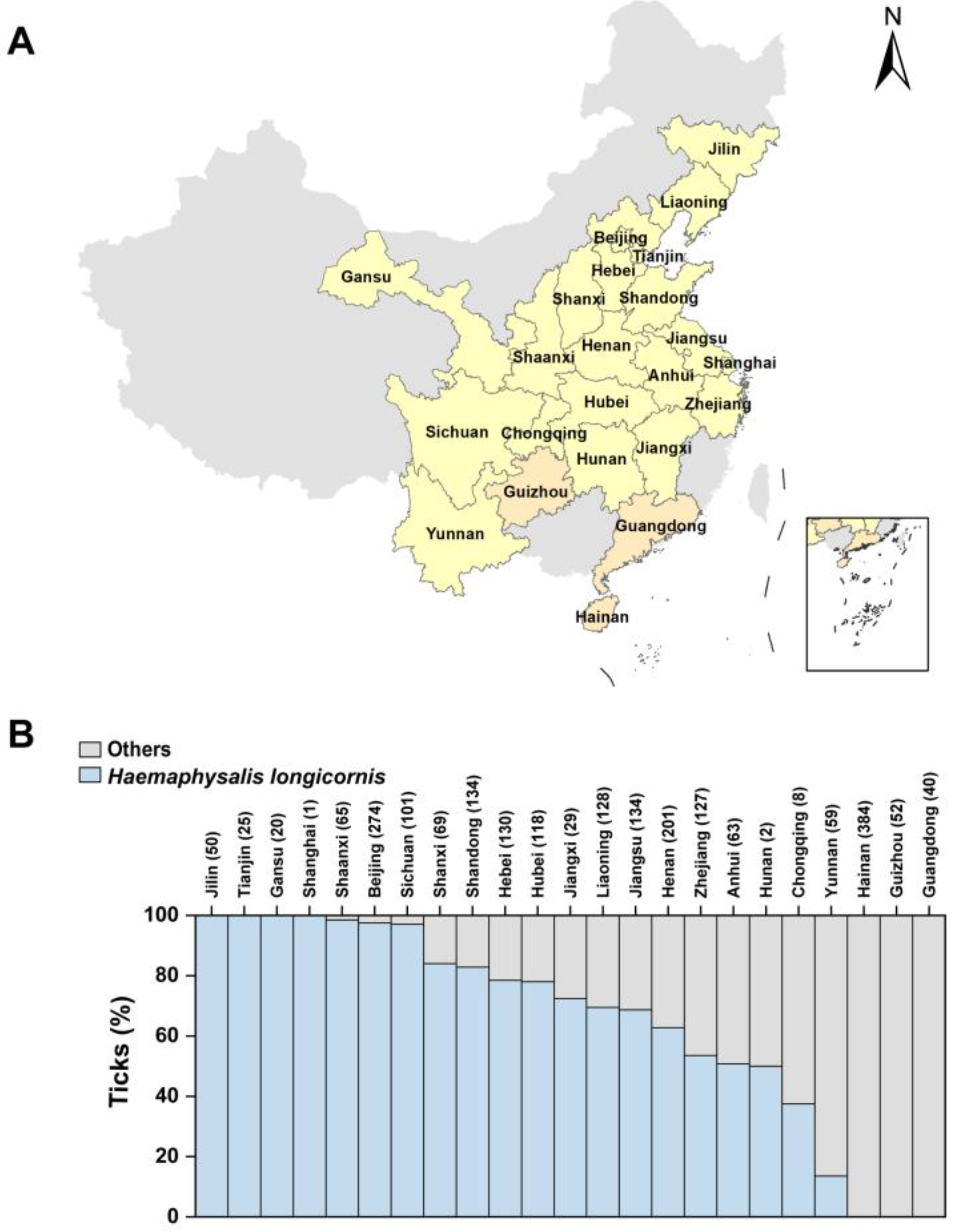




| Province/Region | Number of Ticks | Number of H. longicornis | Percentage of Total Ticks | Percentage of Total H. longicornis | H. longicornis Percentage of Ticks |
|---|---|---|---|---|---|
| Jilin | 50 | 50 | 2.26% | 3.77% | 100.00% |
| Tianjin | 25 | 25 | 1.13% | 1.88% | 100.00% |
| Gansu | 20 | 20 | 0.90% | 1.51% | 100.00% |
| Shanghai | 1 | 1 | 0.05% | 0.08% | 100.00% |
| Shaanxi | 65 | 64 | 2.94% | 4.82% | 98.46% |
| Beijing | 274 | 267 | 12.38% | 20.11% | 97.45% |
| Sichuan | 101 | 98 | 4.56% | 7.38% | 97.03% |
| Shanxi | 69 | 58 | 3.12% | 4.37% | 84.06% |
| Shandong | 134 | 111 | 6.05% | 8.36% | 82.84% |
| Hebei | 130 | 102 | 5.87% | 7.68% | 78.46% |
| Hubei | 118 | 92 | 5.33% | 6.93% | 77.97% |
| Jiangxi | 29 | 21 | 1.31% | 1.58% | 72.41% |
| Liaoning | 128 | 89 | 5.78% | 6.70% | 69.53% |
| Jiangsu | 134 | 92 | 6.05% | 6.93% | 68.66% |
| Henan | 201 | 126 | 9.08% | 9.49% | 62.69% |
| Zhejiang | 127 | 68 | 5.74% | 5.12% | 53.54% |
| Anhui | 63 | 32 | 2.85% | 2.41% | 50.79% |
| Hunan | 2 | 1 | 0.09% | 0.08% | 50.00% |
| Chongqing | 8 | 3 | 0.36% | 0.23% | 37.50% |
| Yunnan | 59 | 8 | 2.66% | 0.60% | 13.56% |
| Hainan | 384 | 0 | 17.34% | 0.00% | 0.00% |
| Guizhou | 52 | 0 | 2.35% | 0.00% | 0.00% |
| Guangdong | 40 | 0 | 1.81% | 0.00% | 0.00% |
| Total | 2214 | 1328 | 100.00% | 100.00% | 59.98% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Cai, G.; Zhang, X.; Liu, X.; Wang, P.; Zheng, A. Comparative Analysis of Bisexual and Parthenogenetic Populations in Haemaphysalis Longicornis. Microorganisms 2024, 12, 823. https://doi.org/10.3390/microorganisms12040823
Zhao C, Cai G, Zhang X, Liu X, Wang P, Zheng A. Comparative Analysis of Bisexual and Parthenogenetic Populations in Haemaphysalis Longicornis. Microorganisms. 2024; 12(4):823. https://doi.org/10.3390/microorganisms12040823
Chicago/Turabian StyleZhao, Chaoyue, Guonan Cai, Xing Zhang, Xinyu Liu, Pengfei Wang, and Aihua Zheng. 2024. "Comparative Analysis of Bisexual and Parthenogenetic Populations in Haemaphysalis Longicornis" Microorganisms 12, no. 4: 823. https://doi.org/10.3390/microorganisms12040823
APA StyleZhao, C., Cai, G., Zhang, X., Liu, X., Wang, P., & Zheng, A. (2024). Comparative Analysis of Bisexual and Parthenogenetic Populations in Haemaphysalis Longicornis. Microorganisms, 12(4), 823. https://doi.org/10.3390/microorganisms12040823







