Abstract
Leizhou goats are famous for their delicious meat but have inferior growth performance. There is little information on rumen-protected fat (RPF) from the Leizhou goat. Hence, we observed the effects of RPF on growth, fecal short-chain fatty acids, and bacteria community with respect to Leizhou goats. Twelve goats (13.34 ± 0.024 kg) were selected and assigned randomly to one of two treatments: (1) a control diet (CON) and (2) 2.4% RPF with a control diet (RPF). The final body weight and average daily gain (ADG) were greater (p < 0.05), and the dry matter intake (DMI): ADG was lower (p < 0.05) in the RPF group than in the CON group. There were no differences in DMI between the CON and RPF groups. The concentrations of total short-chain fatty acids, acetate, propionate, and butyrate were lower (p < 0.05) in the RPF group than in the CON group. The relative abundances of Ruminococcus, Rikenellaceae_RC9_gut_group, Treponema, norank_f__norank_o__RF39, Eubacterium_siraeum_group, and Ruminococcus_torques_group were lower (p < 0.05) in the RPF group than in the CON group. The relative abundances of Bacteroides, norank_f__norank_o__Clostridia_UCG-014, norank_f__Eubacterium_coprostanoligenes_group, Eubacterium_ruminantium_group, norank_f__Oscillospirale-UCG-010, Oscillospiraceae_UCG-002, and Family_XIII_AD3011_group were greater (p < 0.05) in the RPF group than in the CON group. It was concluded that RPF could improve the goats’ growth performance by regulating their fecal bacteria communities.
1. Introduction
The indigenous Leizhou goat, also called the Hainan Black goat, was raised in the south of China (such as the Leizhou Peninsula and Hainan Island) and grazes in humid regions. In addition, it is the only dominant goat breed from Guangdong Province in the Catalogue of National Livestock and Poultry Genetic Resources of China [1]. Leizhou goats, with a population of 1 million, are vital for the livelihoods of local farmers and famous for their high-quality meat [2]. However, production efficiency for this goat is very low due to the insufficient forage availability between November and March in the next year. Hence, it is urgent to improve production efficiency for the Leizhou goat.
With regard to improving ruminant production, numerous studies have reported that supplementary amino acids or fatty acids could improve production for yaks, sheep, and goats [3,4,5]. Fat usage has become a common strategy for providing energy in animal production. However, fat is degraded by rumen microorganisms, whereas rumen-protected fat (RPF) can bypass the rumen and move to the small intestine, increasing the utilization of this fat. Numerous studies on RPF have focused on dairy cows [6], beef cattle [7], goats [5], and sheep [8], but there is little information on the Leizhou goat.
As gut microorganisms, bacteria play a crucial role in a variety of physiological processes in ruminants, such as nutrient metabolism, immune protection, homeostasis, and body development. Previous studies showed that rumen-protected products could change the bacteria communities in ruminants [9,10]. It was reported that a lower ratio of Firmicutes to Bacteroidetes was linked to an inhibition of fat deposition and a decreased ADG of the host animal [11]. In addition, the abundance of Porphyromonadaceae bacterium DJF B175 increased in the high-average-daily-gain group, while that of Lactobacillus reuteri decreased in pre-weaned beef calves [12]. However, there has been little research on changes in the gut microbiota with respect to fatty acids. Consequently, the objective of this experiment was to fill this gap by examining the effect of RPF on the growth performance and fecal bacteria communities of the Leizhou goat, providing a potential strategy for improving animal growth performance and increasing profits when raising Leizhou goats intensively.
2. Materials and Methods
This experiment was carried out from October to December 2023 at Zhanjiang Experiments Station of the Chinese Academy of Tropical Agricultural Sciences (21°16′12″ N, 110°21′27″ E), Zhanjiang City, Guangdong Province, China. All protocols and experimental procedures were approved by the Animal Care and Use Committee of Zhanjiang Experiments Station of the Chinese Academy of Tropical Agricultural Sciences (Protocol number: ZES 202306010).
2.1. Animals, Design, and Diets
Twelve goats (13.34 ± 0.024 kg), all of which were castrated males and 6 months of age, were held individually in cages (0.8 m × 1.2 m) equipped with feeders and automatic waterers. Within each block, the goats were assigned randomly to one of two treatments: (1) a control diet (CON) or a (2) 2.4% RPF with control diet. The RPF (consisting of 48% C16:0, 5% C18:0, 36% C18:1, 9% C18:2, and 2% C14:0) supplements were used according to the manufacturer’s recommendations (Yihai Kerry Arawana Holdings Co., Ltd., Shanghai, China). The goats were fed ad libitum a control diet of 500 g/kg of forage and 500 g/kg of concentrate on a dry-matter basis (Table 1; Guangxi Maosen Nongmu Co., Ltd., Nanning City, China).

Table 1.
Ingredients and chemical compositions of the diets offered to the goats.
The feedlot period lasted 56 days, comprising 14 days for diet and cage adaptation and 42 days for the experimental period. The average daily gain (ADG) was calculated throughout the experimental period. Feed was weighted and offered twice daily at 08:00 and 17:00 h. Orts were weighed daily, and DMI was calculated based on the records of the feed offered daily and the weight of the feed remaining the next day. The feed efficiency was calculated as the ratio of ADG to DMI.
2.2. Procedures and Sample Collection
Approximately 100 g of diet was collected daily between day 53 to 56. Before morning feeding on day 56, 20 g rectal fecal samples were taken from the animals and then loaded into 25 mL centrifuge tubes (Corning, Shanghai, China). All samples were immediately frozen after collection in liquid nitrogen and stored at −80 °C to analyze bacterial communities and short-chain fatty acids.
2.3. Feed Sample Analysis
Feed samples were dried at 65 °C in a forced-air oven for 80 h and then ground through a 1 mm sieve. The dry matter (method 925.45), organic matter (method 990.03), and ether extract (method 920.29) proportions were determined according to the Association of Official Analytical Chemists [13]. The Kjedahl method was applied to determine total nitrogen content of feed using a nitrogen analyzer, and the crude protein content was calculated as follows: total nitrogen × 6.25. The neutral detergent fiber and acid detergent fiber proportions were measured using an automatic fiber analyzer according to Robertson [14] and Van Soest et al. [15], respectively.
2.4. Fecal Short-Chain Fatty Acids Analysis
The SCFA concentrations in the stool samples were measured using gas chromatography (GC) via a capillary column (AT-FFAP: 30 m × 0.32 mm × 0.5 μm) using a Shimadzu GC-2010 plus system with a flame ionization detector (Shimadzu Corporation, Kyoto, Japan) according to the method reported by Liu et al. [3] with a minor revision. A 1.000 g fresh feces sample was vortex-mixed with 4 mL of ultrapure water, shaken (Promax 2020; Heidolph Instruments GmbH CO. KG, Shanghai, China) at 4 °C for 3 h, and then centrifuged (15,000 rpm; HT190R, Hunan Xiangyi Laboratory Instrument Development Co., Ltd., Changsha, China) at 4 °C for 15 min. Subsequently, the supernatant (1000 μL) was mixed with 200 μL of metaphosphoric acid solution (25%; Shanghai Macklin Biochemical Technology Co., Ltd., Shanghai, China) for 30 min, centrifuged (15,000 rpm) at 4 °C for 15 min, and finally filtered through a 0.22 μm filter membrane. Aliquots of the supernatants (800 μL) were pipetted into a glass gas chromatography vial to make preparations for the following GC instrument analysis. Briefly, the temperature of the flame ionization detector was set to 280 °C. The carrier gas was highly purified N2 (99.99%) with a flow rate of 0.8 mL/min. The SCFA concentrations in fecal samples were determined by comparing them with the standard curve.
2.5. DNA Extraction, 16S rRNA Gene Amplification, and Sequencing
The total genomic DNA of fecal bacteria was extracted from 1.00 g samples by using a commercial fecal DNA extraction kit (DP328, Tiangen Biotech, Beijing, China), which was used in accordance with the manufacturer’s instructions. The DNA concentrations and purity were determined using NanoDrop One (Thermo Fisher Scientific, Madison, WI, USA). The quality of the extracted DNA was tested using 1% agarose gel electrophoresis (Axygen Biosciences, Union City, CA, USA). The samples with a purity of 1.8 and above were used for further processing in PCR protocols.
The conventional polymerase chain reaction amplification and bioinformatics analysis of extracted DNA samples were conducted by Shanghai Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). The 16S rRNA gene hypervariable regions V3–V4 were used to identify bacteria and amplified using primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). The bacterial 16S amplification and the quality filtering, clustering, and analysis of the 16S rRNA sequencing data were conducted in accordance with Liu et al.’s approach [11]. The reaction conditions and procedures of the PCR amplification of the 16S rRNA gene were as follows: initial denaturation at 95 °C for 3 min, followed by 30 cycles, including denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 30 s, with a final extension at 72 °C for 10 min and holding at 10 °C. The PCR mixtures were prepared in triplicate in 20 μL volumes, which consisted of 4 μL of 5 × TransStart FastPfu buffer, 2 μL of 2.5 mM deoxyribonucleotides triphosphate (dNTPs), 0.8 μL of forward primer (5 mM), 0.8 μL of reverse primer (5 mM), 0.2 μL of bovine serum albumin, 0.4 μL of TransStart FastPfu DNA Polymerase, 10 ng of template DNA, and ddH2O added until 20 μL was reached. Agarose gel (2.0%) electrophoresis (Axygen Biosciences, Union City, CA, USA) was applied to assess the success of PCR reactions.
After amplification, purified amplicons were pooled equimolarly and paired-end sequenced (2 × 300 bp) using an Illumina MiSeq PE300 platform (Illumina, San Diego, CA, USA) by Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). Data were analyzed using the free online Majorbio Cloud Platform (www.Majorbio.com, accessed on 26 March 2024).
2.6. Statistical Analyses
The data on growth performance and short-chain fatty acids was obtained using SAS software (SAS 9.4, SAS institute Inc., Cary, NC, USA). Results are expressed as the means ± standard error (SEM). Multiple-comparison p-values were adjusted using the t-test. Statistical significance was set at p-values < 0.05.
3. Results
3.1. Growth Performance
As designed, there were no differences (p > 0.05) in initial body weight between the CON and RPF groups (Table 2). The FBW and ADG were greater (p < 0.05) and the DMI: ADG was lower (p < 0.05) in the RPF group compared to those in the CON group. There were no differences in DMI between the CON and RPF groups.

Table 2.
Effect of dietary supplementation of rumen-protected fat on feed intake and growth performance of goats.
3.2. Fecal Short-Chain Fatty Acids
The concentrations of total SCFAs, acetate, propionate, and butyrate were lower (p < 0.05) in the RPF group than in the CON group (Table 3). There were no differences in iso-VFAs and the ratio of acetate to propionate (p > 0.05) between the RPF group and CON group.

Table 3.
Effect of dietary supplementation of rumen-protected fat on feces concentrations of short-chain fatty acids in goats.
3.3. Collective Sequencing Data Summary
A total of 1,007,445 raw reads were generated from the fecal samples, and 984,087 high-quality sequences remained after quality filtering and the removal of chimeric sequences. A total of 3275 OTUs were obtained based on the 97% nucleotide sequence identity among reads.
A total of 1843 OTUs were shared between the CON group and RPF group, accounting for 68.2% and 76.3% of the total OTUs in the CON and RPF groups, respectively (Figure 1). In addition, the number of OTUs specific to the CON and RPF groups were 860 and 572, respectively. There were no differences in ACE, Chao, Shannon, Simpson, and Sobs between the CON group and RPF group (Table 4).
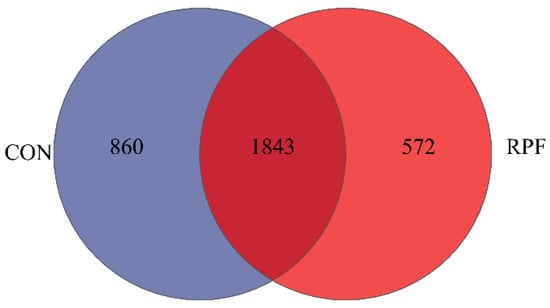
Figure 1.
Flower plot showing different and similar OTUs in goats offered rumen-protected fat. CON = control group; RPF = rumen-protected fat.

Table 4.
The alpha diversity in response to dietary rumen-protected fat in goat feces.
3.4. Microbial Community Composition in the Feces
A total of 15 bacteria phyla were identified in the feces of the CON group and RPF group. The dominant phylum was Firmicutes, accounting for 69.0% and 72.1%, and the second-most-dominant phylum was Bacteroidetes, accounting for 22.9% and 22.6% in the CON group and RPF group, respectively (Figure 2; Table 5). The relative abundances of Firmicutes and Verrucomicrobiota were greater (p < 0.05), whereas those of Spirochaetota, Fibrobacterota, and others were lower (p < 0.05) in the CON group and RPF group.
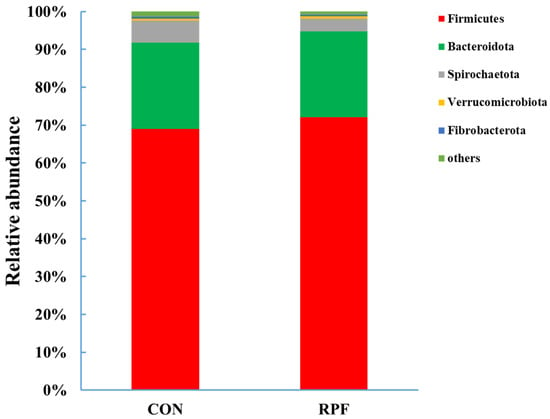
Figure 2.
Fecal bacterial relative abundances (at phylum level, >0.5% of total reads) in goats offered rumen-protected fat. CON = control group; RPF = rumen-protected fat.

Table 5.
Effect of dietary supplementation of rumen-protected fat on fecal bacteria (at phylum level, >1.0% of total reads) in goat.
A total of 216 bacterial genera were identified in the fecal samples of the two groups of goats. The dominant genus was unclassified_f_Lachnospiraceae, accounting for 12.8% and 12.1%, and the second-most-dominant genus was Oscillospiraceae-UCG-005, accounting for 7.84% and 7.55% in the CON and RPF groups, respectively (Figure 3; Table 6). The relative abundances of Ruminococcus, Rikenellaceae_RC9_gut_group, Treponema, norank_f__norank_o__RF39, Eubacterium_siraeum_group, and Ruminococcus_torques_group were lower (p < 0.05) in the RPF group than in the CON group. The relative abundances of Bacteroides, norank_f__norank_o__Clostridia_UCG-014, norank_f__Eubacterium_coprostanoligenes_group, Eubacterium_ruminantium_group, norank_f__Oscillospirale-UCG-010, Oscillospiraceae_UCG-002, and Family_XIII_AD3011_group were greater (p < 0.05) in the RPF group than in the CON group.
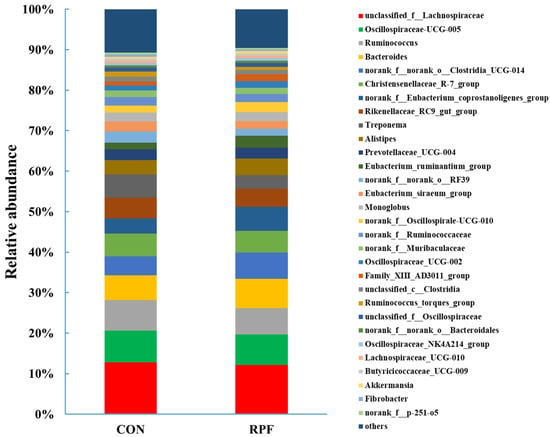
Figure 3.
Fecal bacterial relative abundances (at genus level, >0.5% total reads) in goats offered rumen-protected fat. CON = control group; RPF = rumen-protected fat.

Table 6.
Effect of the dietary supplementation of rumen-protected fat on fecal bacteria (at phylum level, >0.5% of total reads) in goats.
Differences in microbiota that varied with RPF supplementation were further identified using linear discriminant analysis effect size (LEfSe; Figure 4A,B). With a default LDA cutoff of ±2.0, 19 and 15 different taxa were found in the CON group and RPF group, respectively. The bacteria biomarkers in the CON group were norank_f_Bacteroidates_RF16_group, Campylobacter, Agathobacter, Peptococcus, norank_f_norank_o_Rhodospirllales, Elusimicrobium, Syntrophococcus, and Sphaerochaeta and in the RPF group were norank_f_Enbacterium_coprostanoligenes_group, norank_f_norank_o_Clostridia_ UCG-014, Odoribacter, Sanguibacteroides, Eubacterium_brachy_group, Lachnospiraceae_NK3A20_group, and Aeriscardovia.
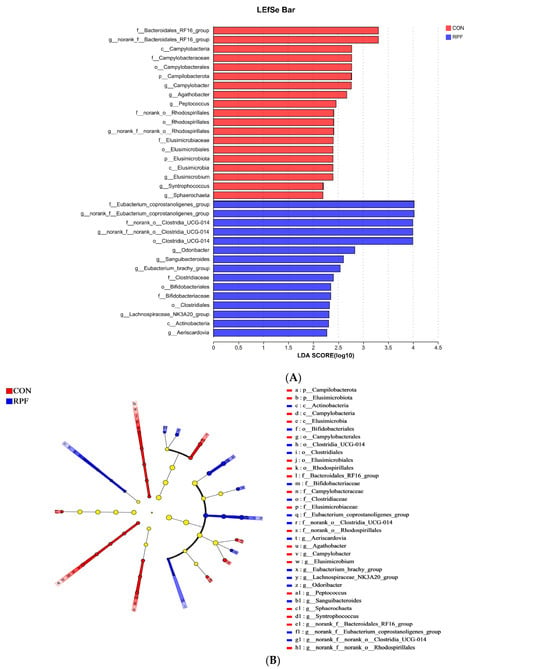
Figure 4.
Linear discriminant analysis effect size (LEfSe) results for fecal microbiota in goats consuming diets including rumen-protected fat. (A) Linear discriminant analysis. (B) Cladogram reported. Prefixes represent abbreviations for the taxonomic rank of each taxon: phylum (p_), class (c_), order (o_), family (f_) and genus (g_). CON = control group; RPF = rumen-protected fat.
3.5. Correlations between Fecal Bacteria and Short-Chain Fatty Acids
There were 16 positive (p < 0.05) and 19 negative (p < 0.05) correlations between the relative abundances of bacterial genera and the concentrations of SCFAs and minerals (Figure 5). Ruminococcus and Treponema were correlated positively with concentrations of fecal total SCFAs and butyrate. Norank_f_norank_o_Clostridia_UCG_014, Norank_f_Enbacterium_coprostan-oligenes_group, and Family_XIII_AD3011_group were negatively correlated with the concentrations of total SCFAs, acetate, propionate, and butyrate. Oscillospiraceae_NK4A214 and Butyricicoccaceae_UCG_009 were positively correlated with concentrations of iso-VFAs. Bacteroides, Eubacterium_ruminantium_group, and Akkermansia were negatively correlated with concentrations of butyrate.
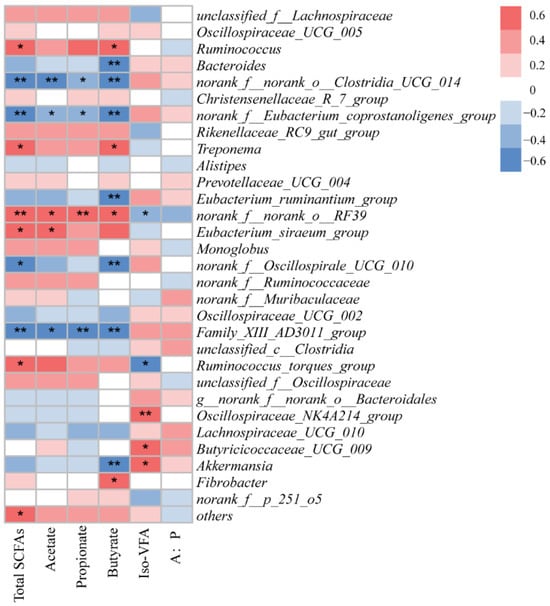
Figure 5.
Correlation of fecal bacterial relative abundance at genus level with feces SCFA concentrations. * p < 0.05, and ** p < 0.01 according to correlation coefficient.
4. Discussion
4.1. Effect of Dietary Rumen-Protected Fat on Growth Performance
RPFs are an important ingredient in the diets of ruminants, especially dairy cows [16]. It is well accepted that RPF benefits herd reproductive performance because they can increase energy density and reduce the risk of metabolic disorders [6,17]. Numerous studies have reported that RPF could improve the milk yield of dairy cows [6] but did not influence the ADG in Dorper sheep [10] and beef cattle [18]. However, in the present study, we found that the ADG was greater in the RPF group than in the CON group, which is in agreement with a previous study concerning finishing goats [19]. This could explain how the supplementation of RPF increased the total tract digestibility of crude protein, lipids, or crude fiber in steers [20], ewes [21], and sheep [10]. Our results showed that the final body weight was greater in the RPF group than in the CON group, which is in agreement with a previous study that reported that body weights increased in finishing beef steers [7].
4.2. Effects of Dietary Rumen-Protected Fat on Fecal Short-Chain Fatty Acid Concentrations
Short chain fatty acids, a type of fatty acid with fewer than six carbon atoms, are produced by gut bacteria when they ferment fiber, and they exert several effects on the host’s metabolism and immune system [22]. In the present study, we found that the concentrations of total SCFAs, acetate, propionate, and butyrate were lower in the RPF group than in the CON group, which is in agreement with a previous study on mice [23]. Acetate and butyrate are mainly generated by fiber. A previous study reported that RPF could enhance the total tract digestibility of fiber in ruminants [10]. In the present study, we found no differences in the DMI between the CON and RPF groups; that is, there was a lower fecal fiber concentration in the RPF group than in the CON group. Hence, the concentrations of acetate and butyrate were greater in the CON group than in the RPF group.
4.3. Microbial Community Composition in the Feces
Firmicutes and Bacteroidetes were the dominant bacterial phyla in feces, which is in agreement with previous studies on goats [24], sheep [25], dairy cows [26], and cattle [27]. Spirochaetota, including Treponema, is a phylum of double-membrane Gram-negative anaerobic bacteria regarded as a pathogenic bacterium. A previous study reported that RPF could improve starch digestibility, resulting in a lower starch content in the RPF group [19]. Furthermore, it was found that the Spirochaetota phylum and Treponema genus existed in starch-rich materials. Moreover, Treponema was associated with pectin and xylan degradation in the gastrointestinal tract [28,29]. Hence, the RAs of the Spirochaetota phylum and Treponema genus were lower in the RPF group than in the CON group. Furthermore, we observed that Treponema positively correlated with total SCFAs and butyrate. Verrucomicrobiota are widely distributed in various mammals and encode numerous carbohydrate-degrading enzymes, peptidases, and sulfatases, and their ecological functions are still poorly understood [30]. In the present study, the RA of Verrucomicrobiota was greater in the CON group than in the RPF group, and the reason behind this needs to be clarified in the future. In addition, the levels of Fibrobacterota, an important cellulose-degrading phylum, were lower in the RPF group than in the CON group, which could explain why the fecal fiber content was lower in the RPF group than in the CON group [31].
At the genus level, the most-dominant fecal bacterium was unclassified_f__Lachnospiraceae, followed by Oscillospiraceae-UCG-005 and Ruminococcus. However, previous studies reported that the most-abundant genera in feces were bacteriodes for Hainan black goats and Saanen goats [32], Escherichia for sheep [33], and Prevotella for cattle [27].
This difference could be explained by the differences in diets and animal species between these studies. As for fibrolytic bacteria, such as Ruminococcus [34] and Rikenellaceae_RC9_gut_group [35], the RA of these bacteria was greater in the CON group than in the RPF group. This could be explained by the fact that the fecal fiber content was lower in the RPF group than in the CON group [30]. It was reported that Ruminococcus was positively correlated with cecal acetate concentrations in broilers [4]. However, we found that Ruminococcus strains were not correlated with acetate. This difference could be related to the types of diets or animal species. Bacteroides is regarded as a predictive biomarker for weight change [36]. In the present study, we found that the RA of Bacteroides was greater in the RPF group than in the CON group, which could be explained by the fact that a greater RA of Bacteroides indicates a greater body weight [37]. The abundance of Norank_f_norank_o_Clostridia_UCG-014, a beneficial bacterium, was greater in the RPF group than in the CON group, which is in agreement with a previous study on yaks [38]. In addition, we found Norank_f_norank_o_Clostridia_UCG-014 was negatively correlated with total SCFAs, acetate, propionate, and butyrate. Eubacterium_coprostanoligenes could generate beneficial SCFAs and then play an anti-inflammatory role for the host. Our results showed a greater RA of Eubacterium_coprostanoligenes in the RPF group than in the CON group, which is in agreement with a previous study in which it was reported that a high-fat diet led to a sharp increase in the RA of norank_f__Eubacterium_coprostanoligenes_group [39]. Moreover, we found norank_f__Eubacterium_coprostanoligenes _group was negatively correlated with total SCFAs, acetate, propionate, and butyrate.
4.4. Correlations between Fecal Bacteria and Short-Chain Fatty Acids
Eubacterium_siraeum_group is a validly published species of the genus Eubacterium, but it may belong to a new genus based on phylogenomic analysis. A previous study reported that Eubacterium_siraeum_group is associated with cellulose degradation in Eospalax cansus [40]. Hence, in the present study, we found that Eubacterium_siraeum_group was positively correlated with fecal acetate. Akkermansia is often reported to facilitate butyrate, which is associated with positive health effects in the gut. Interestingly, we found that Akkermansia was negative correlated with butyrate. Ruminococcus_torques_group is a Gram-stain-positive, obligately anaerobic bacterium known to be a bile-acid-converting bacterium. Our results showed the Ruminococcus_torques_group was positively correlated with total SCFAs, which is in agreement with a previous study conducted using a colitis mouse model [41].
5. Conclusions
In the RPF group, the ADG was greater and the DMI-to-ADG ratio was lower than those in the CON group. Additionally, fecal bacteria were altered when providing supplementary RPF to the goats. The relative abundances of Ruminococcus, Rikenellaceae_RC9_gut_group, Treponema, nor-ank_f__norank_o__RF39, Eubacterium_siraeum_group, and Ruminococcus_torques_group were lower in the RPF group than in the CON group. The relative abundances of Bacteroides, norank_f__norank_o__Clostridia_UCG-014, norank_f__Eubacterium_coprostanoligenes_group, Eubacterium_ruminantium_group, norank_f__Oscillospirale-UCG-010, Oscillospiraceae_UCG-002, and Family_XIII_AD3011_group were greater in the RPF group than in the CON group. We conclude that the supplementation of 2.4% RPF to Leizhou goats could improve their growth performance and alter their fecal bacteria. Future studies need to investigate the nutrient digestibility and absorption effects of RPF on the rumen and hindgut.
Author Contributions
Conceptualization, H.L.; Methodology, W.P., Q.W., K.W., M.Z. and X.H.; Software, H.L.; Validation, H.L. and H.Z.; Formal Analysis, H.L.; Investigation, K.M.; Resources, J.H.; Data Curation, H.L.; Writing—Original Draft Preparation, H.L.; Writing—Review and Editing, H.L. and H.Z.; Visualization, H.L.; Supervision, J.H.; Project Administration, H.L.; Funding Acquisition, H.L., Y.Y. and H.Z. All authors reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The present work was jointly supported by the Hainan Provincial Natural Science Foundation of China (grant numbers 324QN301 and 324QN300), the Central Public-interest Scientific Institution Basal Research Fund (grant number 1630102024006), and the Special Fund for Agricultural Product Quality and Safety of Ministry of Agriculture and Rural Affairs of China: “Evaluation and Analysis of Quality and Safety of Tropical and Subtropical New Feed Resources” (grant number 16230077).
Data Availability Statement
Data are contained within the article.
Acknowledgments
We thank the South Subtropical Crops Research Institute, part of the Chinese Academy of Tropical Agricultural Sciences Public Technology Center, for their assistance with acquiring short-chain fatty acids measurements.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- China National Commission of Animal Genetic Resources. Animal Genetic Resources in China: Sheep and Goats; China Agriculture Press: Beijing, China, 2011. [Google Scholar]
- Feng, H.; Shi, H.; Yang, F.; Yun, Y.; Wang, X. Impact of anthocyanins derived from Dioscorea alata L. on growth performance, carcass characteristics, antioxidant capacity, and immune function of Hainan black goats. Front. Vet. Sci. 2023, 10, 1283947. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, G.; Degen, A.; Ji, K.; Jiao, D.; Liang, Y.; Xiao, L.; Long, R.; Zhou, J. Effect of feed level and supplementary rumen protected lysine and methionine on growth performance, rumen fermentation, blood metabolites and nitrogen balance in growing Tan lambs fed low protein diets. Anim. Feed Sci. Technol. 2021, 279, 115024. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Liu, X.; Liu, R.; Wang, Y.; Huang, X.; Li, Y.; Liu, R.; Yang, X. Dietary folic acid addition reduces abdominal fat deposition mediated by alterations in gut microbiota and SCFA production in broilers. Anim. Nutr. 2023, 12, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Pormalekshahi, A.; Fatahnia, F.; Jafari, H.; Azarfar, A.; Varmaghany, S.; Taasoli, G. Interaction effect of ruminal undegradable protein level and rumen-protected conjugated linoleic acid (CLA) inclusion in the diet of growing goat kids on meat CLA content and quality traits. Br. J. Nutr. 2019, 122, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Manriquez, D.; Chen, L.; Melendez, P.; Pinedo, P. The effect of an organic rumen-protected fat supplement on performance, metabolic status, and health of dairy cows. BMC Vet. Res. 2019, 15, 450. [Google Scholar] [CrossRef] [PubMed]
- Bakker, C.E.; Blair, A.D.; Grubbs, J.K.; Taylor, A.R.; Brake, D.W.; Long, N.M.; Underwood, K.R. Effects of rumen-protected long-chain fatty acid supplementation during the finishing phase of beef steers on live performance, carcass characteristics, beef quality, and serum fatty acid profile. Transl. Anim. Sci. 2019, 3, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- de Lima, J.A.M.; Bezerra, L.R.; Feitosa, T.J.O.; Oliveira, J.R.; de Oliveira, D.L.V.; Mazzetto, S.E.; Cavalcanti, M.T.; Pereira Filho, J.M.; Oliveira, R.L.; de Oliveira, J.P.F.; et al. Production, characterization, and dietary supplementation effect of rumen-protected fat on ruminal function and blood parameters of sheep. Trop. Anim. Health Prod. 2023, 55, 142. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hao, L.Z.; Cao, X.L.; Yang, G.; Degen, A.A.; Xiao, L.; Liu, S.J.; Zhou, J.W. Effects of supplementary concentrate and/or rumen-protected lysine plus methionine on productive performance, milk composition, rumen fermentation, and bacterial population in grazing, lactating yaks (Bos grunniens), and average daily gain of their calves. Anim. Feed Sci. Technol. 2023, 297, 115591. [Google Scholar] [CrossRef]
- Behan, A.A.; Loh, T.C.; Fakurazi, S.; Kaka, U.; Kaka, A.; Samsudin, A.A. Effects of supplementation of rumen protected fats on rumen ecology and digestibility of nutrients in sheep. Animals 2019, 9, 400. [Google Scholar] [CrossRef]
- Liu, H.; Ran, T.; Zhang, C.F.; Yang, W.Z.; Wu, X.K.; Degen, A.; Long, R.J.; Shi, Z.J.; Zhou, J.W. Comparison of rumen bacterial communities between yaks (Bos grunniens) and Qaidam cattle (Bos taurus) fed a low protein diet with different energy levels. Front. Microbiol. 2022, 13, 982338. [Google Scholar] [CrossRef]
- Liu, H.Y.; Yan, C.R.; Hao, C.Y.; Wang, D.Q.; Liu, Y.Z.; Luo, Z.B.; Han, S.Z.; Wang, J.X.; Li, D.X.; Zhu, J.; et al. Dynamic changes in intestinal microbiota and metabolite composition of pre-weaned beef calves. Microb. Pathog. 2023, 175, 105991. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC: Arlington, VA, USA, 2006. [Google Scholar]
- Robertson, J.B. The Detergent System of Fiber Analysis. In Topics in Dietary Fiber Research; Spiller, G.A., Ed.; Springer: Boston, MA, USA, 1978; pp. 1–42. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Pappenheim, S.; Yener, S.; Nichols, K.; Dijkstra, J.; Hettinga, K.; van Valenberg, H.J.F. Feeding hydrogenated palm fatty acids and rumen-protected protein to lactating Holstein-Friesian dairy cows modifies milk fat triacylglycerol composition and structure, and solid fat content. J. Dairy Sci. 2022, 105, 2828–2839. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.; Simões, P.; Bexiga, R.; Silva, E.; Mateus, L.; Fernandes, T.; Alves, S.P.; Bessa, R.J.B.; Lopes-da-Costa, L. Effects of feeding rumen-protected linseed fat to postpartum dairy cows on plasma n-3 polyunsaturated fatty acid concentrations and metabolic and reproductive parameters. J. Dairy Sci. 2022, 105, 361–374. [Google Scholar] [CrossRef] [PubMed]
- da Rosa E Silva, P.I.J.L.; Zervoudakis, J.T.; da Silva Cabral, L.; Hatamoto-Zervoudakis, L.K.; da Freiria, L.B.; E Silva, Y.R.V.B.; Paulino, P.V.R.; Tsuneda, P.P.; Possamai, A.J. Effects of rumen-protected oil supplementation on finishing grazing beef cattle. Trop. Anim. Health Prod. 2020, 52, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Zhang, Z.Y.; Zhang, X.L.; Lu, C.M.; Yang, W.Z.; Xie, X.L.; Xin, H.S.; Lu, X.T.; Ni, M.B.; Yang, X.Y.; et al. Effects of dietary Clostridium butyricum and rumen protected fat on meat quality, oxidative stability, and chemical composition of finishing goats. J. Anim. Sci. Biotechnol. 2024, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Ngidi, M.E.; Loerch, S.C.; Fluharty, F.L.; Palmquist, D.L. Effects of calcium soaps of long-chain fatty acids on feedlot performance, carcass characteristics and ruminal metabolism of steers. J. Anim. Sci. 1990, 68, 2555–2565. [Google Scholar] [CrossRef]
- Bhatt, R.S.; Sahoo, A.; Shinde, A.K.; Karim, S.A. Change in body condition and carcass characteristics of cull ewes fed diets supplemented with rumen bypass fat. Livest. Sci. 2013, 157, 132–140. [Google Scholar] [CrossRef]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD? EBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef]
- Li, Q.; Wang, M.; Zhang, Y.; Li, Y.; Zhang, X.; Li, H.; Peng, Y.; Zhu, C.; Zheng, P.; Yang, S.; et al. Pectin-derived oligogalacturonic acids ameliorate high-fat diet-induced obesity in mice by regulating gut microbiota and inflammation. J. Funct. Foods 2024, 112, 105928. [Google Scholar] [CrossRef]
- Jin, B.; Wang, R.; Hu, J.; Wang, Y.; Cheng, P.; Zhang, J.; Zhang, J.; Xue, G.; Zhu, Y.; Zhang, Y.; et al. Analysis of fecal microbiome and metabolome changes in goats with pregnant toxemia. BMC Vet. Res. 2024, 20, 2. [Google Scholar] [CrossRef] [PubMed]
- Tanca, A.; Fraumene, C.; Manghina, V.; Palomba, A.; Abbondio, M.; Deligios, M.; Pagnozzi, D.; Addis, M.F.; Uzzau, S. Diversity and functions of the sheep faecal microbiota: A multi-omic characterization. Microb. Biotechnol. 2017, 10, 541–554. [Google Scholar] [CrossRef]
- Huang, S.; Ji, S.; Wang, F.; Huang, J.; Alugongo, G.M.; Li, S. Dynamic changes of the fecal bacterial community in dairy cows during early lactation. AMB Express 2020, 10, 167. [Google Scholar] [CrossRef] [PubMed]
- Ran, T.; Jiao, P.; AlZahal, O.; Xie, X.; Beauchemin, K.A.; Niu, D.; Yang, W. Fecal bacterial community of finishing beef steers fed ruminally protected and non-protected active dried yeast. J. Anim. Sci. 2020, 98, skaa058. [Google Scholar] [CrossRef]
- Liu, J.; Pu, Y.Y.; Xie, Q.; Wang, J.K.; Liu, J.X. Pectin induces an in vitro rumen microbial population shift attributed to the pectinolytic Treponema group. Curr. Microbiol. 2015, 70, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, G.; Mikaelyan, A.; Fukui, C.; Matsuura, Y.; Watanabe, H.; Fujishima, M.; Brune, A. Fiber-associated spirochetes are major agents of hemicellulose degradation in the hindgut of wood-feeding higher termites. Proc. Natl. Acad. Sci. USA 2018, 115, E11996–E12004. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, M.; Brazel, D.M.; Swan, B.K.; Arnosti, C.; Chain, P.S.G.; Reitenga, K.G.; Xie, G.; Poulton, N.J.; Gomez, M.L.; Masland, D.E.D.; et al. Capturing single cell genomes of active polysaccharide degraders: An unexpected contribution of Verrucomicrobia. PLoS ONE 2012, 7, e35314. [Google Scholar] [CrossRef] [PubMed]
- Ransom-Jones, E.; Jones, D.L.; McCarthy, A.J.; McDonald, J.E. The Fibrobacteres: An important phylum of cellulose-degrading bacteria. Microb. Ecol. 2012, 63, 267–281. [Google Scholar] [CrossRef]
- Jiang, S.; Huo, D.; You, Z.; Peng, Q.; Ma, C.; Chang, H.; Lin, X.; Wang, L.; Zhang, J. The distal intestinal microbiome of hybrids of Hainan black goats and Saanen goats. PLoS ONE 2020, 15, e0228496. [Google Scholar] [CrossRef]
- Shabana, I.I.; Albakri, N.N.; Bouqellah, N.A. Metagenomic investigation of faecal microbiota in sheep and goats of the same ages. J. Taibah Univ. Sci. 2021, 15, 1–9. [Google Scholar] [CrossRef]
- Karri, S.; Vadela, M.B.; Gundi, V.A.K.B. Chapter 15—Fiber degradation strategies of bacteria in rumen ecosystem. In Recent Developments in Applied Microbiology and Biochemistry; Viswanath, B., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 153–159. [Google Scholar]
- Liu, H.; Li, Z.; Pei, C.; Degen, A.; Hao, L.; Cao, X.; Liu, H.; Zhou, J.; Long, R. A comparison between yaks and Qaidam cattle in in vitro rumen fermentation, methane emission, and bacterial community composition with poor quality substrate. Anim. Feed Sci. Technol. 2022, 291, 115395. [Google Scholar] [CrossRef]
- Christensen, L.; Sørensen, C.V.; Wøhlk, F.U.; Kjølbæk, L.; Astrup, A.; Sanz, Y.; Hjorth, M.F.; Benítez-Páez, A. Microbial enterotypes beyond genus level: Bacteroides species as a predictive biomarker for weight change upon controlled intervention with arabinoxylan oligosaccharides in overweight subjects. Gut Microbes 2020, 12, 1847627. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, M.F.; Blædel, T.; Bendtsen, L.Q.; Lorenzen, J.K.; Holm, J.B.; Kiilerich, P.; Roager, H.M.; Kristiansen, K.; Larsen, L.H.; Astrup, A. Prevotella-to-Bacteroides ratio predicts body weight and fat loss success on 24-week diets varying in macronutrient composition and dietary fiber: Results from a post-hoc analysis. Int. J. Obes. 2019, 43, 149–157. [Google Scholar] [CrossRef]
- Yi, S.; Dai, D.; Wu, H.; Chai, S.; Liu, S.; Meng, Q.; Zhou, Z. Dietary concentrate-to-forage ratio affects rumen bacterial community composition and metabolome of yaks. Front. Nutr. 2022, 9, 927206. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liang, T.; Zhang, Y.; Huang, K.; Yang, S.; Lv, H.; Chen, Y.; Zhang, C.; Guan, X. Vitexin alleviates high-fat diet induced brain oxidative stress and inflammation via anti-oxidant, anti-inflammatory and gut microbiota modulating properties. Free Radic. Biol. Med. 2021, 171, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Liang, N.; Zhang, X.; Han, C.; Nan, X. Functional differentiation related to decomposing complex carbohydrates of intestinal microbes between two wild zokor species based on 16SrRNA sequences. BMC Vet. Res. 2021, 17, 216. [Google Scholar] [CrossRef]
- Wan, F.; Wang, M.; Zhong, R.; Chen, L.; Han, H.; Liu, L.; Zhao, Y.; Lv, H.; Hou, F.; Yi, B.; et al. Supplementation with chinese medicinal plant extracts from lonicera hypoglauca and scutellaria baicalensis mitigates colonic inflammation by regulating oxidative stress and gut microbiota in a colitis mouse model. Front. Cell. Infect. Microbiol. 2021, 11, 798052. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).