Expanded Archaeal Genomes Shed New Light on the Evolution of Isoprenoid Biosynthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Dataset Construction
2.2. Sequences Acquisition
2.3. Phylogenetic Analysis
3. Results and Discussion
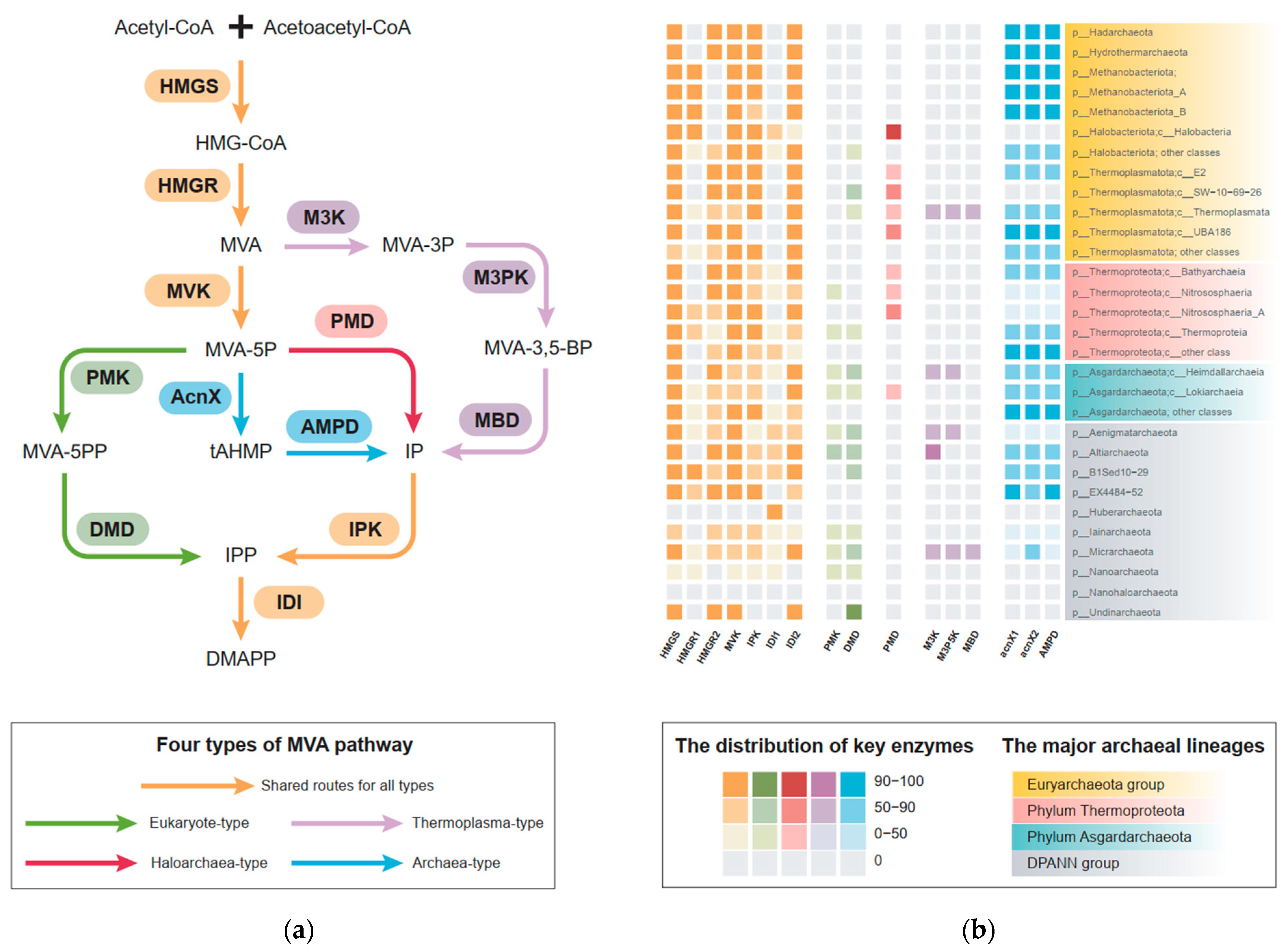
3.1. Distribution Pattern of MVA Pathway in the Domain Archaea
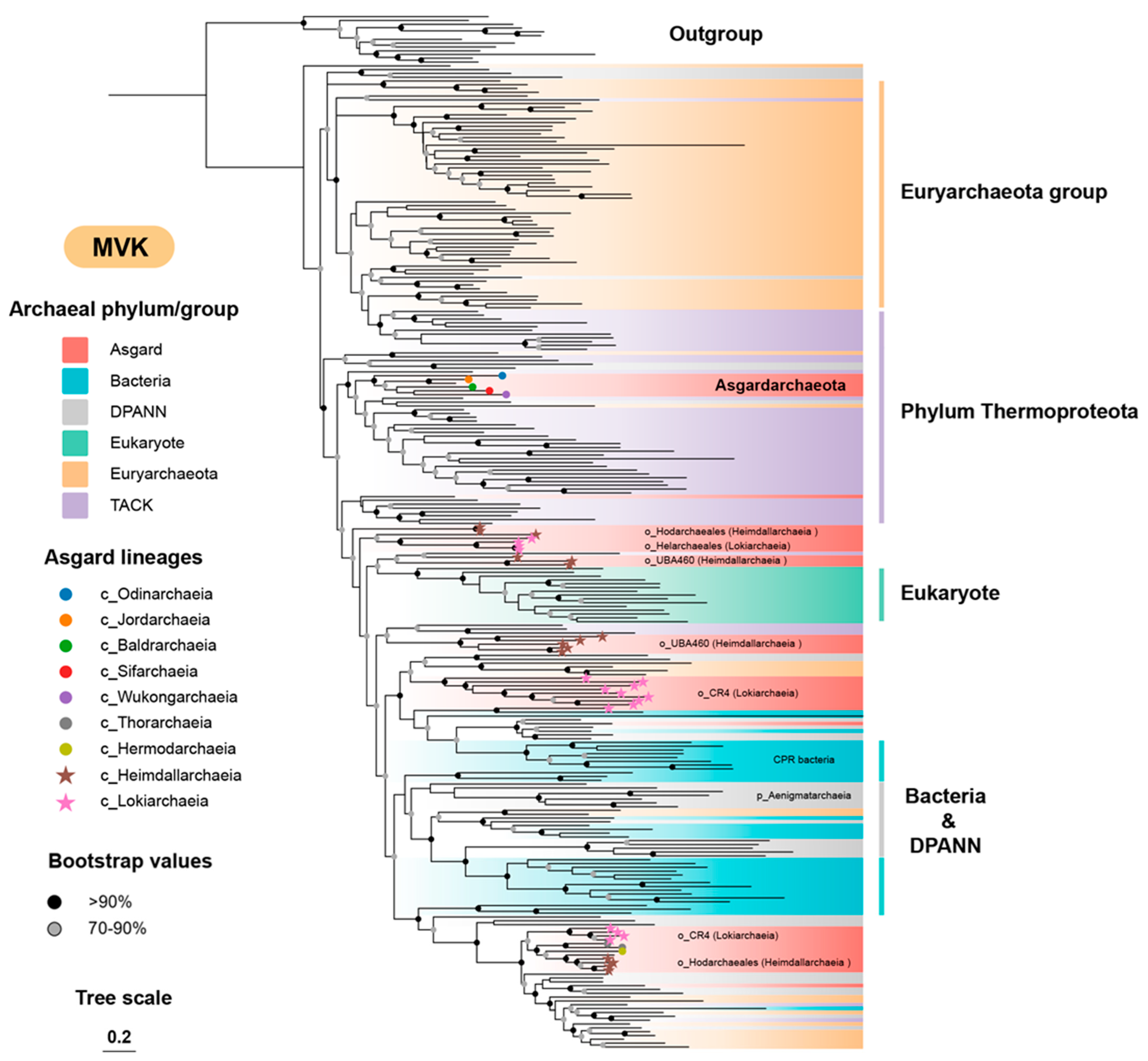
3.2. The Archaeal Origin of the MVA Pathway
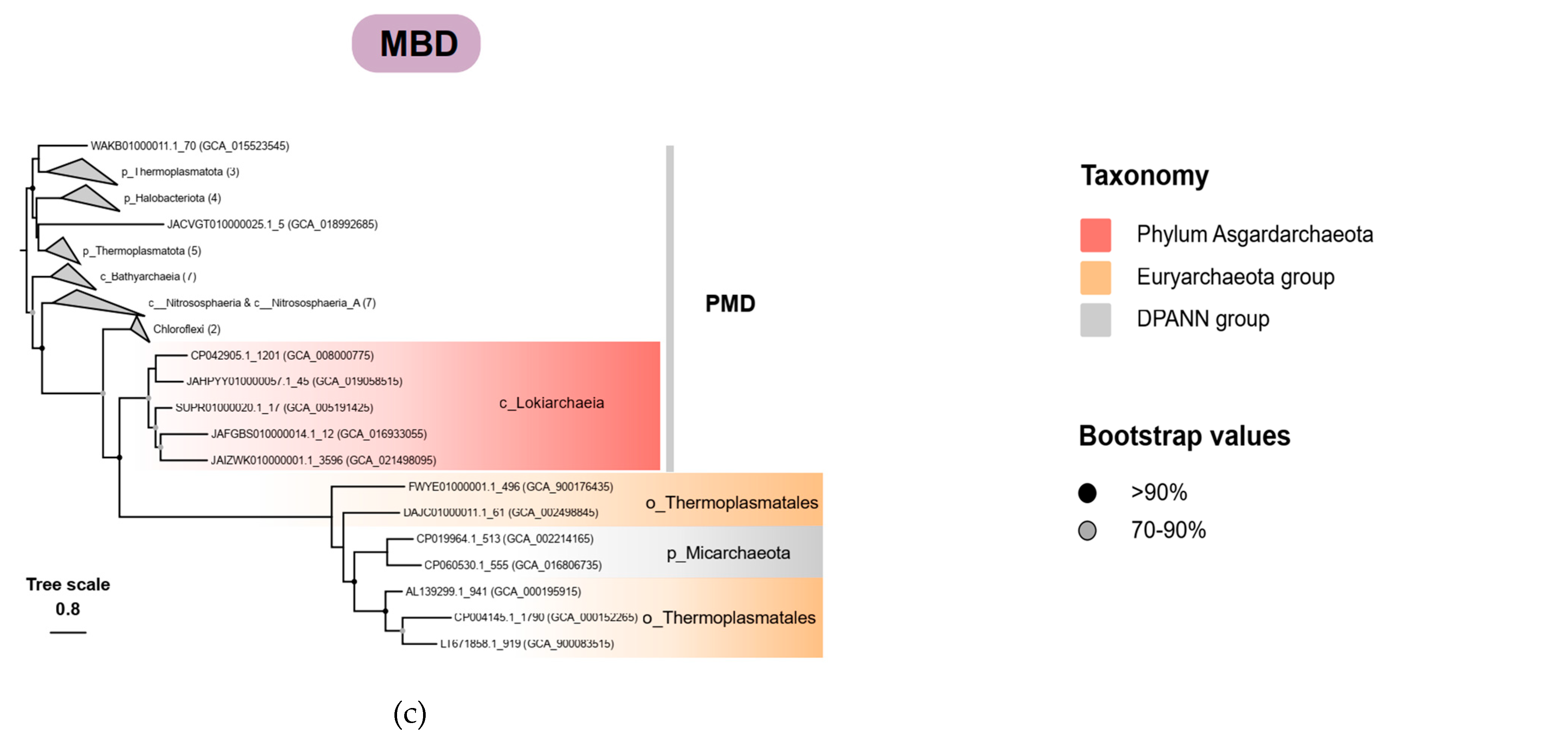
3.3. The Evolution of the Haloarchaea-Type and Thermoplasma-Type MVA Pathway
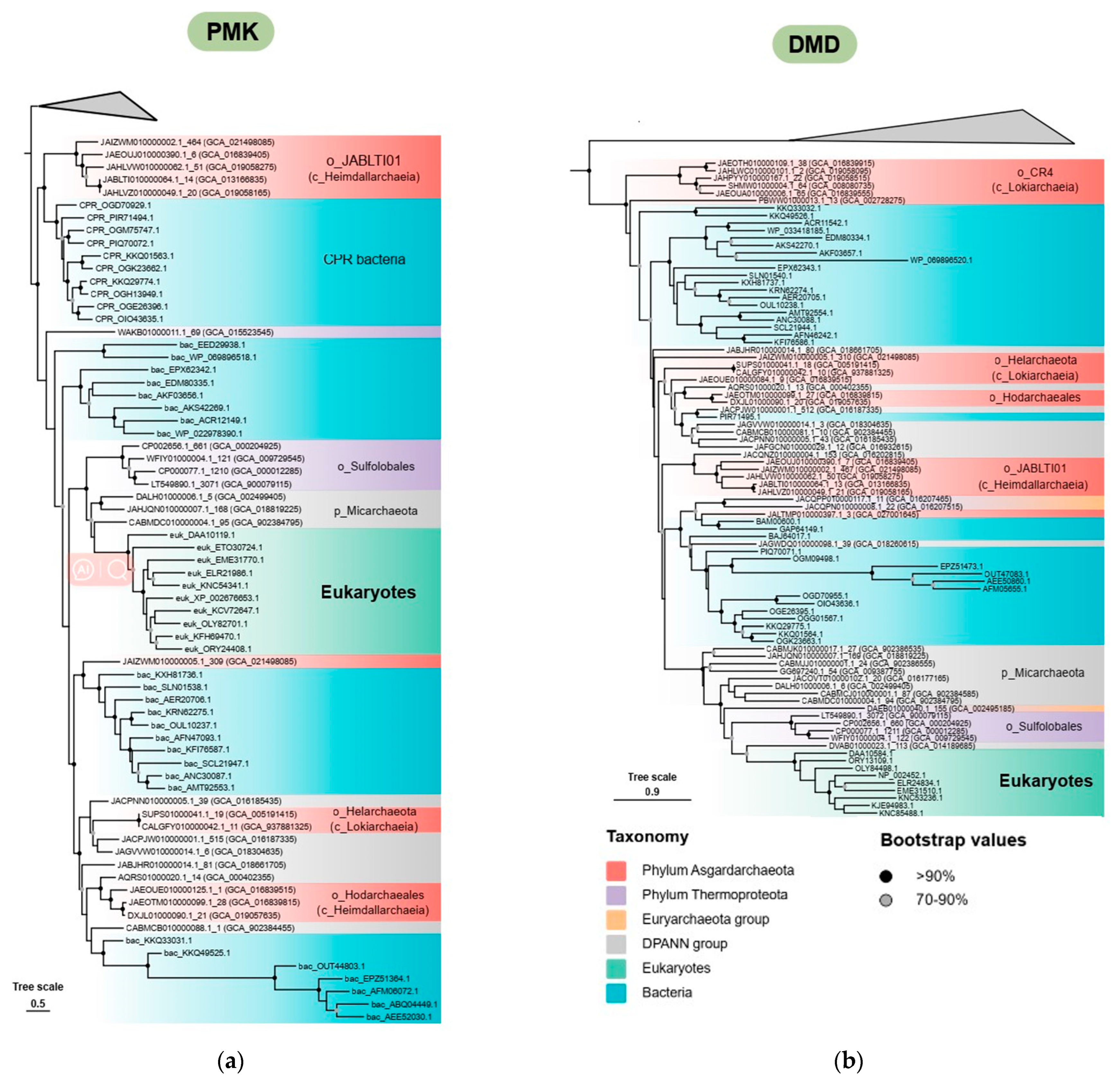
3.4. The Origin and Evolution of the Eukaryote-Type MVA Pathway
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boronat, A.; Rodriguez-Concepcion, M. Terpenoid biosynthesis in prokaryotes. Adv. Biochem. Eng. Biotechnol. 2015, 148, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Avalos, M.; Garbeva, P.; Vader, L.; van Wezel, G.P.; Dickschat, J.S.; Ulanova, D. Biosynthesis, evolution and ecology of microbial terpenoids. Nat. Prod. Rep. 2022, 39, 249–272. [Google Scholar] [CrossRef] [PubMed]
- Lange, B.M.; Rujan, T.; Martin, W.; Croteau, R. Isoprenoid biosynthesis: The evolution of two ancient and distinct pathways across genomes. Proc. Natl. Acad. Sci. USA 2000, 97, 13172–13177. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.P.; Dehesh, K. The eukaryotic MEP-pathway genes are evolutionarily conserved and originated from Chlaymidia and cyanobacteria. Bmc Genom. 2021, 22, 137. [Google Scholar] [CrossRef] [PubMed]
- Rohmer, M.; Knani, M.; Simonin, P.; Sutter, B.; Sahm, H. Isoprenoid Biosynthesis in Bacteria—A Novel Pathway for the Early Steps Leading to Isopentenyl Diphosphate. Biochem. J. 1993, 295, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu. Rev. Plant Phys. 1999, 50, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Smit, A.; Mushegian, A. Biosynthesis of isoprenoids via mevalonate in Archaea: The lost pathway. Genome Res. 2000, 10, 1468–1484. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Azami, Y.; Miyagawa, M.; Hashimoto, C.; Yoshimura, T.; Hemmi, H. Biochemical evidence supporting the presence of the classical mevalonate pathway in the thermoacidophilic archaeon Sulfolobus solfataricus. J. Biochem. 2013, 153, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Castelle, C.J.; Banfield, J.F. Major New Microbial Groups Expand Diversity and Alter our Understanding of the Tree of Life. Cell 2018, 172, 1181–1197. [Google Scholar] [CrossRef]
- Hoshino, Y.; Gaucher, E.A. On the Origin of Isoprenoid Biosynthesis. Mol. Biol. Evol. 2018, 35, 2185–2197. [Google Scholar] [CrossRef]
- Grochowski, L.L.; Xu, H.; White, R.H. Methanocaldococcus jannaschii uses a modified mevalonate pathway for biosynthesis of isopentenyl diphosphate. J. Bacteriol. 2006, 188, 3192–3198. [Google Scholar] [CrossRef] [PubMed]
- Dellas, N.; Thomas, S.T.; Manning, G.; Noel, J.P. Discovery of a metabolic alternative to the classical mevalonate pathway. Elife 2013, 2, e00672. [Google Scholar] [CrossRef] [PubMed]
- Vannice, J.C.; Skaff, D.A.; Keightley, A.; Addo, J.K.; Wyckoff, G.J.; Miziorko, H.M. Identification in Haloferax volcanii of phosphomevalonate decarboxylase and isopentenyl phosphate kinase as catalysts of the terminal enzyme reactions in an archaeal alternate mevalonate pathway. J. Bacteriol. 2014, 196, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Giani, M.; Miralles-Robledillo, J.M.; Peiró, G.; Pire, C.; Martínez-Espinosa, R.M. Deciphering Pathways for Carotenogenesis in Haloarchaea. Molecules 2020, 25, 1197. [Google Scholar] [CrossRef] [PubMed]
- Azami, Y.; Hattori, A.; Nishimura, H.; Kawaide, H.; Yoshimura, T.; Hemmi, H. (R)-Mevalonate 3-Phosphate Is an Intermediate of the Mevalonate Pathway in Thermoplasma acidophilum. J. Biol. Chem. 2014, 289, 15957–15967. [Google Scholar] [CrossRef] [PubMed]
- Vinokur, J.M.; Korman, T.P.; Cao, Z.; Bowie, J.U. Evidence of a Novel Mevalonate Pathway in Archaea. Biochemistry 2014, 53, 4161–4168. [Google Scholar] [CrossRef] [PubMed]
- Vinokur, J.M.; Cummins, M.C.; Korman, T.P.; Bowie, J.U. An Adaptation To Life In Acid Through A Novel Mevalonate Pathway. Sci. Rep. 2016, 6, 39737. [Google Scholar] [CrossRef]
- Krause, S.; Gfrerer, S.; von Kugelgen, A.; Reuse, C.; Dombrowski, N.; Villanueva, L.; Bunk, B.; Sproer, C.; Neu, T.R.; Kuhlicke, U.; et al. The importance of biofilm formation for cultivation of a Micrarchaeon and its interactions with its Thermoplasmatales host. Nat. Commun. 2022, 13, 1735. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, H.; Motoyama, K.; Sobue, F.; Ito, T.; Kawaide, H.; Yoshimura, T.; Hemmi, H. Modified mevalonate pathway of the archaeon Aeropyrum pernix proceeds via trans-anhydromevalonate 5-phosphate. Proc. Natl. Acad. Sci. USA 2018, 115, 10034–10039. [Google Scholar] [CrossRef]
- Boucher, Y.; Doolittle, W.F. The role of lateral gene transfer in the evolution of isoprenoid biosynthesis pathways. Mol. Microbiol. 2000, 37, 703–716. [Google Scholar] [CrossRef]
- Boucher, Y.; Kamekura, M.; Doolittle, W.F. Origins and evolution of isoprenoid lipid biosynthesis in archaea. Mol. Microbiol. 2004, 52, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Lombard, J.; Moreira, D. Origins and early evolution of the mevalonate pathway of isoprenoid biosynthesis in the three domains of life. Mol. Biol. Evol. 2011, 28, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Moody, E.R.R.; Mahendrarajah, T.A.; Dombrowski, N.; Clark, J.W.; Petitjean, C.; Offre, P.; Szollosi, G.J.; Spang, A.; Williams, T.A. An estimate of the deepest branches of the tree of life from ancient vertically evolving genes. Elife 2022, 11, e66695. [Google Scholar] [CrossRef] [PubMed]
- Coleman, G.A.; Davin, A.A.; Mahendrarajah, T.A.; Szantho, L.L.; Spang, A.; Hugenholtz, P.; Szollosi, G.J.; Williams, T.A. A rooted phylogeny resolves early bacterial evolution. Science 2021, 372, 6542. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Vinokur, J.; Motoyama, K.; Ishikawa, R.; Collazo, M.; Cascio, D.; Sawaya, M.R.; Ito, T.; Bowie, J.U.; Hemmi, H. Crystal structure of mevalonate 3,5-bisphosphate decarboxylase reveals insight into the evolution of decarboxylases in the mevalonate metabolic pathways. J. Biol. Chem. 2022, 298, 102111. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Makarova, K.S.; Huang, W.C.; Wolf, Y.I.; Nikolskaya, A.N.; Zhang, X.X.; Cai, M.W.; Zhang, C.J.; Xu, W.; Luo, Z.H.; et al. Expanded diversity of Asgard archaea and their relationships with eukaryotes. Nature 2021, 593, 553. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Wang, Y.; Huang, D.; Hou, J.; Li, L.; Hu, H.; Zhao, X.; Wang, F. Expanding Asgard members in the domain of Archaea sheds new light on the origin of eukaryotes. Sci. China Life Sci. 2022, 65, 818–829. [Google Scholar] [CrossRef]
- Spang, A.; Saw, J.H.; Jorgensen, S.L.; Zaremba-Niedzwiedzka, K.; Martijn, J.; Lind, A.E.; van Eijk, R.; Schleper, C.; Guy, L.; Ettema, T.J.G. Complex archaea that bridge the gap between prokaryotes and eukaryotes. Nature 2015, 521, 173–179. [Google Scholar] [CrossRef]
- Williams, T.A.; Cox, C.J.; Foster, P.G.; Szollosi, G.J.; Embley, T.M. Phylogenomics provides robust support for a two-domains tree of life. Nat. Ecol. Evol. 2020, 4, 138–147. [Google Scholar] [CrossRef]
- Hoshino, Y.; Gaucher, E.A. Evolution of bacterial steroid biosynthesis and its impact on eukaryogenesis. Proc. Natl. Acad. Sci. USA 2021, 118, e2101276118. [Google Scholar] [CrossRef]
- Wu, F.; Speth, D.R.; Philosof, A.; Cremiere, A.; Narayanan, A.; Barco, R.A.; Connon, S.A.; Amend, J.P.; Antoshechkin, I.A.; Orphan, V.J. Unique mobile elements and scalable gene flow at the prokaryote-eukaryote boundary revealed by circularized Asgard archaea genomes. Nat. Microbiol. 2022, 7, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; St John, E.; Anantharaman, K.; Reysenbach, A.L. Global patterns of diversity and metabolism of microbial communities in deep-sea hydrothermal vent deposits. Microbiome 2022, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Chaumeil, P.A.; Mussig, A.J.; Hugenholtz, P.; Parks, D.H. GTDB-Tk v2: Memory friendly classification with the genome taxonomy database. Bioinformatics 2022, 38, 5315–5316. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, D.; Chen, G.L.; Locascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 2010, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2--approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Daugherty, M.; Grishin, N.V.; Osterman, A.L.; Zhang, H. Structure and mechanism of homoserine kinase: Prototype for the GHMP kinase superfamily. Structure 2000, 8, 1247–1257. [Google Scholar] [CrossRef]
- Thomas, S.T.; Louie, G.V.; Lubin, J.W.; Lundblad, V.; Noel, J.P. Substrate Specificity and Engineering of Mevalonate 5-Phosphate Decarboxylase. Acs Chem. Biol. 2019, 14, 1767–1779. [Google Scholar] [CrossRef]
- Crooks, G.E.; Hon, G.; Chandonia, J.-M.; Brenner, S.E. WebLogo: A Sequence Logo Generator: Figure 1. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Capella-Gutierrez, S.; Silla-Martinez, J.M.; Gabaldon, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Xie, J.; Chen, Y.; Cai, G.; Cai, R.; Hu, Z.; Wang, H. Tree Visualization By One Table (tvBOT): A web application for visualizing, modifying and annotating phylogenetic trees. Nucleic Acids Res. 2023, 51, W587–W592. [Google Scholar] [CrossRef]
- Eme, L.; Tamarit, D.; Caceres, E.F.; Stairs, C.W.; De Anda, V.; Schon, M.E.; Seitz, K.W.; Dombrowski, N.; Lewis, W.H.; Homa, F.; et al. Inference and reconstruction of the heimdallarchaeial ancestry of eukaryotes. Nature 2023, 618, 992–999. [Google Scholar] [CrossRef]
- Baker, B.J.; De Anda, V.; Seitz, K.W.; Dombrowski, N.; Santoro, A.E.; Lloyd, K.G. Diversity, ecology and evolution of Archaea. Nat. Microbiol. 2020, 5, 887–900. [Google Scholar] [CrossRef]
- Ghaly, T.M.; Tetu, S.G.; Penesyan, A.; Qi, Q.; Rajabal, V.; Gillings, M.R. Discovery of integrons in Archaea: Platforms for cross-domain gene transfer. Sci. Adv. 2022, 8, eabq6376. [Google Scholar] [CrossRef]
- Koga, Y. Early Evolution of Membrane Lipids: How did the Lipid Divide Occur? J. Mol. Evol. 2011, 72, 274–282. [Google Scholar] [CrossRef]
- Lombard, J.; Lopez-Garcia, P.; Moreira, D. The early evolution of lipid membranes and the three domains of life. Nat. Rev. Microbiol. 2012, 10, 507–515. [Google Scholar] [CrossRef]
- Matsumi, R.; Atomi, H.; Driessen, A.J.; van der Oost, J. Isoprenoid biosynthesis in Archaea--biochemical and evolutionary implications. Res. Microbiol. 2011, 162, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.; Amritkar, K.; Wolfe, M.; Kaçar, B.; Landick, R. Evolutionary flexibility and rigidity in the bacterial methylerythritol phosphate (MEP) pathway. Front. Microbiol. 2023, 14, 1286626. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V. Comparative genomics, minimal gene-sets and the last universal common ancestor. Nat. Rev. Microbiol. 2003, 1, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, M. The phylogenetic distribution of the cell division system would not imply a cellular LUCA but a progenotic LUCA. Biosystems 2021, 210, 104563. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, M. The Last Universal Common Ancestor (LUCA) and the Ancestors of Archaea and Bacteria were Progenotes. J. Mol. Evol. 2010, 72, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Holler, S.; Bartlett, S.; Loffler, R.J.G.; Casiraghi, F.; Diaz, C.I.S.; Cartwright, J.H.E.; Hanczyc, M.M. Hybrid organic-inorganic structures trigger the formation of primitive cell-like compartments. Proc. Natl. Acad. Sci. USA 2023, 120, e2300491120. [Google Scholar] [CrossRef] [PubMed]
- Black, R.A.; Blosser, M.C. A Self-Assembled Aggregate Composed of a Fatty Acid Membrane and the Building Blocks of Biological Polymers Provides a First Step in the Emergence of Protocells. Life 2016, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.F.; Nee, E.; Lane, N. Isoprenoids enhance the stability of fatty acid membranes at the emergence of life potentially leading to an early lipid divide. Interface Focus. 2019, 9, 20190067. [Google Scholar] [CrossRef]
- Imachi, H.; Nobu, M.K.; Nakahara, N.; Morono, Y.; Ogawara, M.; Takaki, Y.; Takano, Y.; Uematsu, K.; Ikuta, T.; Ito, M.; et al. Isolation of an archaeon at the prokaryote-eukaryote interface. Nature 2020, 577, 519–525. [Google Scholar] [CrossRef]
- Cai, M.; Liu, Y.; Yin, X.; Zhou, Z.; Friedrich, M.W.; Richter-Heitmann, T.; Nimzyk, R.; Kulkarni, A.; Wang, X.; Li, W.; et al. Diverse Asgard archaea including the novel phylum Gerdarchaeota participate in organic matter degradation. Sci. China Life Sci. 2020, 63, 886–897. [Google Scholar] [CrossRef]
- Manoharan, L.; Kozlowski, J.A.; Murdoch, R.W.; Loffler, F.E.; Sousa, F.L.; Schleper, C. Metagenomes from Coastal Marine Sediments Give Insights into the Ecological Role and Cellular Features of Loki- and Thorarchaeota. mBio 2019, 10, e02039-19. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Zhang, H.; Cao, X.; Kong, S.; Zhu, B.; Lin, X.; Zhou, Y.J. Construction and Optimization of Nonclassical Isoprenoid Biosynthetic Pathways in Yeast Peroxisomes for (+)-Valencene Production. J. Agric. Food Chem. 2023, 71, 11124–11130. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, P.; Hou, J.; Xiong, Y.; Xie, R.; Wang, Y.; Wang, F. Expanded Archaeal Genomes Shed New Light on the Evolution of Isoprenoid Biosynthesis. Microorganisms 2024, 12, 707. https://doi.org/10.3390/microorganisms12040707
Zhu P, Hou J, Xiong Y, Xie R, Wang Y, Wang F. Expanded Archaeal Genomes Shed New Light on the Evolution of Isoprenoid Biosynthesis. Microorganisms. 2024; 12(4):707. https://doi.org/10.3390/microorganisms12040707
Chicago/Turabian StyleZhu, Pengfei, Jialin Hou, Yixuan Xiong, Ruize Xie, Yinzhao Wang, and Fengping Wang. 2024. "Expanded Archaeal Genomes Shed New Light on the Evolution of Isoprenoid Biosynthesis" Microorganisms 12, no. 4: 707. https://doi.org/10.3390/microorganisms12040707
APA StyleZhu, P., Hou, J., Xiong, Y., Xie, R., Wang, Y., & Wang, F. (2024). Expanded Archaeal Genomes Shed New Light on the Evolution of Isoprenoid Biosynthesis. Microorganisms, 12(4), 707. https://doi.org/10.3390/microorganisms12040707





