The Impact of Essential Amino Acids on the Gut Microbiota of Broiler Chickens
Abstract
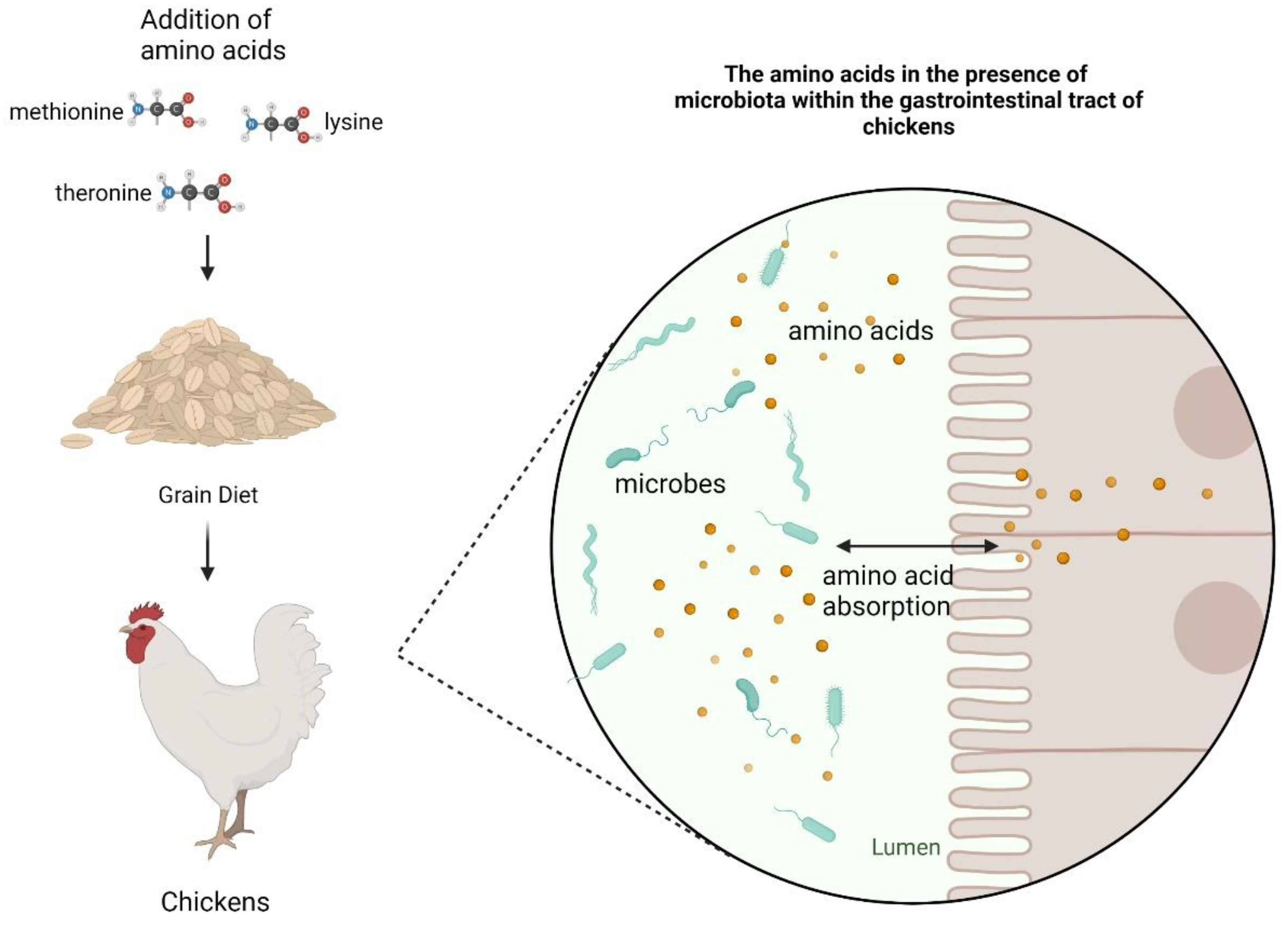
1. Introduction
2. Shaping the Gut Microbiota Population
3. Amino Acid Utilization in Bacteria
4. Recent Studies That Relate to Amino Acid Influence of Intestinal Microbiota
5. Future Studies
6. Discussion
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kidd, M.T. Nutritional modulation of immune function in broilers. Poult. Sci. 2004, 83, 650–657. [Google Scholar] [CrossRef]
- Bortoluzzi, C.; Rochell, S.J.; Applegate, T.J. Threonine, arginine, and glutamine: Influences on intestinal physiology, immunology, and microbiology in broilers. Poult. Sci. 2018, 97, 937–945. [Google Scholar] [CrossRef]
- Willems, O.W.; Miller, S.P.; Wood, B.J. Aspects of selection for feed efficiency in meat producing poultry. Worlds Poult. Sci. J. 2013, 69, 77–88. [Google Scholar] [CrossRef]
- Gottard, E.T.; Prokoski, K.; Horn, D.; Viott, A.D.; Santos, T.C.; Fernandes, J.I.M. Regeneration of the intestinal mucosa in Eimeria and E. Coli challenged broilers supplemented with amino acids. Poul. Sci. 2016, 95, 1056–1065. [Google Scholar] [CrossRef]
- Karauet, A.; Grayson, I. Amino acids in human and animal nutrition. Adv. Biochem. Eng. Biotechnol. 2014, 143, 189–228. [Google Scholar]
- Martínez, Y.; Li, X.; Liu, G.; Bin, P.; Yan, W.; Más, D.; Valdivié, M.; Hu, C.A.; Ren, W.; Yin, Y. The role of methionine on metabolism, oxidative stress, and diseases. Amino Acids. 2017, 49, 2091–2098. [Google Scholar] [CrossRef]
- Ahmed, M.E.; Abbas, T.E. Effects of Dietary Levels of Methionine on Broiler Performance and Carcass Characteristics. Int. J. Poult. Sci. 2011, 10, 147–151. [Google Scholar]
- Nasr, J.; Kheiri, F. Increasing Amino Acids Density Improves Broiler Live Weight. Int. J. Poult. Sci. 2011, 10, 523–526. [Google Scholar] [CrossRef][Green Version]
- Bunchasak, C. Role of dietary methionine in poultry production. Poult. Sci. 2009, 46, 169–179. [Google Scholar] [CrossRef]
- Tsiagbe, V.K.; Kraus, R.J.; Benevenga, N.J.; Harper, A.E.; Sunde, M.L. Identification of Volatile Sulfur Derivatives Released from Feathers of Chicks Fed Diets with Various Levels of Sulfur-Containing Amino Acids. J. Nutri. 1987, 117, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.-Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yin, Y.-L.; Li, D.S.; Kim, W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Levine, R.L. Methionine in proteins defends against oxidative stress. FASEB J. 2009, 23, 464–472. [Google Scholar] [CrossRef]
- Del Vesco, A.P.; Gasparino, E.; Neto, A.R.O.; Rossi, R.M.; Soares, M.A.M.; da Silva, S.C.C. Effect of methionine supplementation on mitochondrial genes expression in the breast muscle and liver of broilers. Livest. Sci. 2013, 151, 284–291. [Google Scholar] [CrossRef][Green Version]
- Daenner, E.; Bessei, W. Influence of supplementation with liquid DL-methionine hydroxy analogue-free acid (Alimet)or DL-methionine on performance of broilers. J. Appl. Poult. Res. 2003, 12, 101–105. [Google Scholar] [CrossRef]
- Motl, M.A.; Fritts, C.A.; Waldroup, P.W. Influence of Dietary Sodium Level on Utilization of Methionine from DL-Methionine and Liquid Methionine-Hydroxy Analogue1. J. Appl. Poult. Res. 2005, 14, 147–155. [Google Scholar] [CrossRef]
- Liu, Y.L.; Song, G.L.; Yi, G.F.; Hou, Y.Q.; Huang, J.W.; Vazquez-Anon, M.; Knight, C.D. Effect of supplementing 2-hydroxy-4-(methylthio) butanoic acid and DL-methionine in corn-soybean-cottonseed meal diets on growth performance and carcass quality of broilers. Asian-Australas. J. Anim. Sci. 2006, 19, 1197–1205. [Google Scholar] [CrossRef]
- Wallis, I.R. Dietary supplements of methionine increase breast meat yield and decrease abdominal fat in growing broiler chick-ens. Aust. J. Exp. Agric. 1999, 39, 131–141. [Google Scholar] [CrossRef]
- Mandal, A.B.; Elangovan, A.V.; Johri, T.S. Comparing Bio-efficacy of Liquid DL-methionine Hydroxy Analogue Free Acid with DL-methionine in Broiler Chickens. Asian-Australas. J. Anim. Sci. 2004, 17, 102–108. [Google Scholar] [CrossRef]
- Carew, L.B.; McMurtry, J.P.; Alster, F.A. Effects of methionine deficiencies on plasma levels of thyroid hormones, insulin-like growth factors-I and -II, liver and body weights, and feed intake in growing chickens. Poult. Sci. 2003, 82, 1932–1938. [Google Scholar] [CrossRef]
- Conde-Aguilera, J.A.; Cobo-Ortega, C.; Tesseraud, S.; Lessire, M.; Mercier, Y.; van Milgen, J. Changes in body compositionin broilers by a sulfur amino acid deficiency during growth. Poult. Sci. 2013, 92, 1266–1275. [Google Scholar] [CrossRef]
- Kidd, M.T.; Kerr, B.J.; Anthony, M.B. Dietary interactions between lysine and threonine in broilers. Poult. Sci. 1997, 76, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Struys, E.A.; Jakobs, C. Metabolism of lysine in α-aminoadipic semialdehyde dehydrogenase-deficient fibroblasts: Evidence for an alternative pathway of pipecolic acid formation. FEBS Lett. 2010, 584, 181–186. [Google Scholar] [CrossRef]
- Toride, Y. Lysine and Other Amino Acids for Feed: Production and Contribution to Protein Utilization in Animal Feeding. 2000 FAO. Available online: http://www.fao.org/3/y5019e/y5019e0a.htm (accessed on 20 December 2023).
- Morris, T.R.; Al-Azzawi, K.; Gous, R.M.; Simpson, G.L. Effects of protein concentration on responses to dietary lysine by chicks. Br. Poult. Sci. 1987, 28, 185–195. [Google Scholar] [CrossRef]
- Abebe, S.; Morris, T.R. Note on the effect of protein concentration of responses to dietary lysine by chicks. Br. Poult. Sci. 1990, 31, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Maksoud, A.; Yan, F.; Cerrate, S.; Coto, C.; Wang, Z.; Waldroup, P.W. Effect of dietary crude protein, lysine level and amino acid balance on performance of broilers 0 to 18 days of age. Int. J. Poult. Sci. 2010, 9, 21–27. [Google Scholar] [CrossRef][Green Version]
- Eits, R.M.; Kwakkel, R.P.; Verstegen, M.W.A.; Emmans, G.C. Responses of broiler chickens to dietary protein. Effects of early life protein nutrition on later responses. Br. Poult. Sci. 2003, 44, 398–409. [Google Scholar] [CrossRef]
- Dozier, W.A., III; Kidd, M.T.; Corzo, A. Dietary amino acid responses of broiler chickens. J. Appl. Poult. Res. 2008, 17, 157–167. [Google Scholar] [CrossRef]
- Bhogoju, S.; Nahashon, S.N.; Donkor, J.; Kimathi, B.; Johnson, D.; Khwatenge, C.; Bowden-Taylor, T. Effect of varying dietary concentrations of lysine on growth performance of the Pearl Grey guinea fowl. Poult Sci. 2017, 9, 1306–1315. [Google Scholar] [CrossRef]
- Rezaei, M.; Nassiri, H.; Moghaddam, H.; Pour, R.J.; Kermanshahi, H. The Effects of Dietary Protein and Lysine Levels on Broiler Performance, Carcass Characteristics and N Excretion. Int. J. Poul. Sci. 2004, 3, 148–152. [Google Scholar]
- Khwatenge, C.; Kimathi, B.; Taylor-Bowden, T.; Nahashon, S. Expression of Lysine-Mediated Neuropeptide Hormones Controlling Satiety and Appetite in Broiler Chickens. Poult Sci. 2020, 99, 1409–1420. [Google Scholar] [CrossRef]
- Min, Y.N.; Liu, S.G.; Qu, Z.X.; Meng, G.H.; Gao, Y.P. Effects of dietary threonine levels on growth performance, serum biochemical indexes, antioxidant capacities, and gut morphology in broiler chickens. Poult. Sci. 2017, 96, 1290–1297. [Google Scholar] [CrossRef]
- Rezaeipour, V.; Fononi, H.; Irani, M. Effects of dietary L-threonine and Saccharomyces cerevisiae on performance, intestinal morphology and immune response of broiler chickens. S. Afr. J. Anim. Sci. 2012, 42, 266–273. [Google Scholar] [CrossRef]
- Fernandez, S.R.; Aoyagi, S.; Han, Y.; Parsons, C.M.; Baker, D.H. Limiting order of amino acids in corn and soybean meal for growth of the chick. Poul. Sci. 1994, 73, 1887–1896. [Google Scholar] [CrossRef]
- Han, Y.; Suzuki, H.; Parsons, C.M.; Baker, D.H. Amino acid fortification of a low-protein corn and soybean meal diet for chicks. Poult Sci. 1992, 71, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Kidd, M.T. Nutritional considerations concerning threonine in broilers. World’s Poult. Sci. J. 2000, 56, 139–151. [Google Scholar] [CrossRef]
- Stoll, B.; Henry, J.; Reeds, P.J.; Yu, H.; Jahoor, F.; Burrin, D.G. Catabolism dominates the first-pass intestinal metabolism of dietary essential amino acids in milk protein-fed piglets. J. Nutr. 1998, 128, 606–614. [Google Scholar] [CrossRef]
- Van der Schoor, S.R.D.; Wattimena, D.L.; Huijmans, J.; Vermes, A.; van Goudoever, J.B. The gut takes nearly all: Threonine kinetics in infants. Am. J. Clin. Nutr. 2007, 86, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Corzo, A.; Moran, E.T.; Hoehler, D.; Zinner, B. Tryptophan need of broiler males from 42 to 56 days. Poult. Sci. 2005, 84, 226–231. [Google Scholar] [CrossRef]
- Smith, J.E.; Hingtgen, J.N.; Lane, J.D.; Aprison, M.H. Neurochemical correlates of behavior. Content of tryptophan, 5-hydroxytryptophan, serotonin, 5-hyroxyindolacetic acid, tyrosine, dopamine and norepinephrine in a nerve ending fraction isolated from three brain areas of the pigeon during the period of behavioral depression following an injection of tryptophan. J. Neurochem. 1976, 27, 747–751. [Google Scholar]
- Lee, S.R.; Britton, W.M. Effect of elevated dietary tryptophan on avian hypothalamic serotonin, 5-hydroxyindole acetic acid and norepinephrine. Poult. Sci. 1982, 61, 1500. [Google Scholar]
- Harrison, L.; D’Mello, J.F.P. Large neutral amino acids in the diet and neurotransmitter concentration in the chick brain. Proceeding Nutr. Soc. 1986, 45, 72. [Google Scholar]
- Shea, M.M.; Mench, J.A.; Thomas, O.P. The effect of dietary tryptophan on aggressive behavior in developing and mature broiler breeder males. Poult. Sci. 1990, 69, 1664–1669. [Google Scholar] [CrossRef]
- Shea, M.M.; Douglas, L.W.; Mench, J.A. The interaction of dominance status and supplemental tryptophan on aggression in Gallus domesticus males. Pharmacol. Biochem. Behav. 1991, 38, 587–591. [Google Scholar] [CrossRef]
- Denbow, D.M.; Hobbs, F.C.; Hulet, R.M.; Graham, P.P.; Potter, L.M. Supplemental dietary L-tryptophan effects on growth, meat quality, and brain catecholamine and idolamine concentrations in turkeys. Br. Poult. Sci. 1993, 34, 715–724. [Google Scholar] [CrossRef]
- Shea-Moore, M.M.; Thomas, O.P.; Mench, J.A. Decreases in aggression in tryptophan supplemented broiler breeder males are not due to increases in blood niacin levels. Poult. Sci. 1996, 75, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Hamm, H.E.; Menaker, M. Retinal rhythms in chicks: Circadian variation in melatonin and serotonin N-acetyltransferase activity. Proc. Natl. Acad. Sci. USA 1980, 77, 4998–5002. [Google Scholar] [CrossRef] [PubMed]
- Lacy, M.P.; Van Kery, H.P.; Skewes, P.A.; Denbow, D.M. Tryptophan’s influence on feed and body temperature in the fowl. Poult. Sci. 1986, 65, 1193–1196. [Google Scholar] [CrossRef]
- Newberry, R.C.; Blair, R. Behavioral responses of broiler chickens to handling: Effects of dietary tryptophan and the two lighting regimens. Poult. Sci. 1983, 72, 1237–1244. [Google Scholar] [CrossRef]
- Mund, M.D.; Riaz, M.; Mirza, M.A. Effect of dietary tryptophan supplementation on growth performance, immune response and anti-oxidant status of broiler chickens from 7 to 21 days. Vet. Med. Sci. 2020, 6, 48–53. [Google Scholar] [CrossRef]
- Linh, N.T.; Guntoro, B.; Hoang Qui, N. Immunomodulatory, behavioral, and nutritional response of tryptophan application on poultry. Vet. World. 2021, 14, 2244–2250. [Google Scholar] [CrossRef]
- Dethlefsen, L.; McFall-Ngai, M.; Relman, D.A. An ecological and evolutionary presprctive on human-microbe mutualism and disease. Nature 2007, 449, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Yatao, X.; Tiantian, Z.; Qian, R.; Chao, S. 16S ribosomal RNA sequencing reveals a modulation of intestinal microbiome and immune response by dietary L-theanine supplementation in broiler chickens. Poult. Sci. 2019, 98, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Apajalahti, J.; Vienola, K. Interaction between chicken intestinal microbiota and protein digestion. Anim. Feed Sci. Technol. 2016, 221, 323–330. [Google Scholar] [CrossRef]
- Qu, A.; Brulc, J.M.; Wilson, M.K.; Law, B.F.; Theoret, J.R.; Joens, L.A.; Konkel, M.E.; Angly, F.; Dinsdale, E.A.; Edwards, R.A.; et al. Comparative metagenomics reveals host specific metavirulomes and horizontal gene transfer elements in the chicken cecum microbiome. PLoS ONE 2008, 3, e2945. [Google Scholar] [CrossRef] [PubMed]
- Danzeisen, J.L.; Kim, H.B.; Isaacson, R.E.; Tu, Z.J.; Johnson, T.J. Modulations of the chicken cecal microbiome and metagenome in response to anticoccidial and growth promoter treatment. PLoS ONE 2011, 6, e27949. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Si, W.; Forster, R.J.; Huang, R.; Yu, H.; Yin, Y.; Yang, C.; Han, Y. 16S rRNA gene-based analysis of mucosaassociated bacterial community and phylogeny in the chicken gastrointestinal tracts: From crops to ceca. FEMS Microbiol. Ecol. 2007, 59, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Rehman, H.U.; Vahjen, W.; Awad, W.A.; Zentek, J. Indigenous bacteria and bacterial metabolic products in the gastrointestinal tract of broiler chickens. Arch. Anim. Nutr. 2007, 61, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Morrison, M.; Yu, Z. Bacterial census of poultry intestinal microbiome. Poult. Sci. 2013, 3, 671–683. [Google Scholar] [CrossRef]
- Bhogoju, S.; Nahashon, S.; Wang, X.; Darris, C.; Kilonzo-Nthenge, A. A comparative analysis of microbial profile of Guinea fowl and chicken using metagenomic approach. PLoS ONE. 2018, 13, e0191029. [Google Scholar] [CrossRef]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R. Human genetics shape the gut microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Toivonen, R.K.; Emani, R.; Munukka, E.; Rintala, A.; Laiho, A.; Pietilä, S. Fermentable fibres condition colon microbiota and promote diabetogenesis in NOD mice. Diabetologia 2014, 57, 2183–2192. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Blouin, J.-M.; Santacruz, A.; Lan, A.; Andriamihaja, M.; Wilkanowicz, S.; Benetti, P.H.; Tomé, D.; Sanz, Y.; Blachier, F. High-protein diet modifies colonic microbiota and luminal environment but not colonocyte metabolism in the rat model: The increased luminal bulk connection. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Yang, Y.; Luo, Z.; Guan, L.; Zhu, W. The colonic microbiome and epithelial transcriptome are altered in rats fed a high-protein diet compared with a normal-protein diet. J. Nutr. 2016, 146, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.A.; Ragab, S.H.; El Baky, A.A.; Shoeib, A.R.; Alhosary, Y.; Fekry, D. Frequency of Firmicutes and Bacteroidetes in gut microbiota in obese and normal weight Egyptian children and adults. Arch. Med. Sci. 2010, 7, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Jami, E.; White, B.A.; Mizrahi, I. Potential Role of the Bovine Rumen Microbiome in Modulating Milk Composition and Feed Efficiency. PLoS ONE 2014, 9, e85423. [Google Scholar] [CrossRef] [PubMed]
- Myer, P.R.; Smith, T.P.; Wells, J.E.; Kuehn, L.A.; Freetly, H.C. Rumen microbiome from steers differing in feed efficiency. PLoS ONE 2015, 10, e0129174. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.L.; Wu, G.; Zhu, W.Y. Amino acid metabolism in intestinal bacteria: Links between gut ecology and host health. Front. Biosci. 2011, 16, 1768–1786. [Google Scholar] [CrossRef]
- Apajalahti, J.; Kettunen, A.; Graham, H. Characteristics of the gastrointestinal microbial communities, with special reference to the chicken. World’s Poult. Sci. J. 2004, 60, 223–232. [Google Scholar] [CrossRef]
- Portune, K.J.; Beaumont, M.; Davila, A.M.; Tomé, D.; Blachier, F.; Sanz, Y. Gut microbiota role in dietary protein metabolism and health-related outcomes: The two sides of the coin. Trends Food Sci. Technol. 2016, 57, 213–232. [Google Scholar] [CrossRef]
- Tork, V.A.; Hughes, R.J.; Mikkelsen, L.L.; Perez-Maldonado, R.; Balding, K.; MacApline, R.; Percy, N.J.; Opel-Keller, K. Identification and characheristics of potential performance-related gut microbiome in broiler chicken across various feeding trials. Appl. Environ. Microbiol. 2011, 77, 5868–5878. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Denman, S.E.; Hughes, R.J.; Geier, M.S.; Crowley, T.M.; Chen, H.; Haring, V.R.; Moore, R.J. Intestinal microbiota associated with differential feed conversion efficiency in chickens. Appl. Microbiol. Biotechnol. 2012, 96, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Geier, M.S.; Denman, S.E.; Haring, V.R.; Crowley, T.M.; Hughes, R.J.; Moore, R.J. Identification of chicken intestinal microbiota correlated with the efficiency of energy extraction from feed. Vet. Microbiol. 2013, 164, 85–92. [Google Scholar] [CrossRef]
- Chambers, J.R.; Gong, J. The intestinal microbiota and its modulation for Salomella control in chickens. Food Res. Int. 2011, 44, 3149–3159. [Google Scholar] [CrossRef]
- Chen, Y.P.; Cheng, Y.F.; Li, X.H.; Yang, W.L.; Wen, C.; Zhuang, S.; Zhou, Y.M. Effects of threonine supplementation on the growth performance, immunity, oxidative status, intestinal integrity, and barrier function of broilers at the early age. Poult. Sci. 2017, 6, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.Y.; Azzam, M.M.M.; Zou, X.T. Effects of dietary threonine supplementation on intestinal barrier function and gut microbiota of laying hens. Poult. Sci. 2017, 96, 3654–3663. [Google Scholar] [CrossRef]
- Khattak, F.; Helmbrecht, A. Effect of different levels tryptophan on productive performance egg, quality, blood biochemistry, and caecal microbiota of hens housed in enriched colony cages under commercial stocking density. Poult. Sci. 2018, 98, 2094–2104. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Adhikari, P.; Oakley, B.; Kim, M.K. Changes in cecum microbial community in response to total sulfur amino acid (TSAA:DL-methionine) in antibiotic-free and supplemented poultry birds. Poult. Sci. 2019, 98, 5809–5819. [Google Scholar] [CrossRef]
- Yin, J.; Li, Y.; Han, H.; Zheng, J.; Wang, L.; Ren, W.; Chen, S.; Wu, F.; Fang, R.; Huang, X.; et al. Effects of Lysine deficiency and Lys-Lys dipeptide on cellular apoptosis and amino acids metabolism. Mol. Nutr. Food Res. 2017, 61, 1600754. [Google Scholar] [CrossRef]
- Metges, C.C. Contribution of Microbial Amino Acids to Amino Acid Homeostasis of the Host. J. Nutr. 2000, 130, 1857S–1864S. [Google Scholar] [CrossRef] [PubMed]
- Bergen, W.G.; Wu, G. Intestinal nitrogen recycling and utilization in health and disease. J. Nutr. 2009, 139, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Morowitz, M.J.; Carlisle, E.; Alverdy, J.C. Contributions of intestinal bacteria to nutrition and metabolism in the critically ill. Surg. Clin. N. Am. 2011, 91, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Halvorson, H. Utilization of single L-amino acids as sole source of carbon and nitrogen by bacteria. Can J. Microbiol. 1972, 18, 1647–1650. [Google Scholar] [CrossRef] [PubMed]
- Dave, R.I.; Shah, N.P. Effect of cysteine on the viability of yoghurt and probiotic bacteria in yoghurts made with commercial starter cultures. Int. Diary J. 1997, 8, 537–545. [Google Scholar] [CrossRef]
- Tammie, A.Y.; Robinson, R.K. Yoghurt: Science and technology; Woodhead Publishing Ltd.: Cambridge, UK, 1999. [Google Scholar]
- Azari, N.; Ezzatpanah, H.; Tajabadi-Ebrahimi, M.; Mizani, M. Effect of Cysteine and Lysine on Some Technical Properties of Yoghurt Starter Culture Bacteria. Nutr. Food Sci. Res. 2018, 5, 43–48. [Google Scholar] [CrossRef][Green Version]
- Flannery, W.L.; Kennedy, D.M. The nutrition of Virbrio costicolus. I. A simplified synthetic medium. Can. J. Microbiol. 1962, 8, 923–928. [Google Scholar] [CrossRef]
- Dundas, I.D.; Srinivasan, V.R.; Halvorson, H.O. A chemically defined medium for Halobacterium salinarium strain 1. Can. Microbil. 1963, 9, 619–624. [Google Scholar] [CrossRef]
- Pfefferle, W.; Möckel, B.; Bathe, B.; Marx, A. Biotechnological manufacture of lysine. Adv. Biochem. Eng. Biotechnol. 2003, 79, 59–112. [Google Scholar]
- Yang, Y.; Pollard, A.M.; Höfler, C.; Poschet, G.; Rüdiger, M.W.; Hell Victor Sourjik, H.V. Relation between chemotaxis and consumption of amino acids in bacteria. Mol. Microbiol. 2015, 96, 1272–1282. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Allison, C.; Gibson, S.A.W.; Cummings, J.H. Contribution of the microflora to proteolysis in the human large intestine. J. Appl. Bacteriol. 1988, 64, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Cummings, J.H.; Macfarlane, S.; Gibson, G.R. Influence of retention time on degradation of pancreatic enzymes by human colonic bacteria grown in a 3-stage continuous culture system. J. Appl. Bacteriol. 1989, 67, 520–527. [Google Scholar] [CrossRef]
- Vispo, C.; Karasov, W.H. The interaction of avian gut microbes and their host: An elusive symbiosis. In Gastrointestinal Microbiology: Volume 1 Gastrointestinal Ecosystems and Fermentations; Springer: Boston, MA, USA, 1997; pp. 116–155. [Google Scholar]
- Lei, F.; Yin, Y.; Wang, Y.; Deng, B.; Yu, H.D.; Li, L.; Xiang, C.; Wang, S.; Zhu, B.; Wang, X. Higher-level production of volatile fatty acids in vitro by chicken gut microbiotas than by human gut microbiotas as determined by functional analyses. Appl. Environ. Microbiol. 2012, 16, 5763–5772. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 2014, 5, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Torok, V.A.; Ophel-Keller, K.; Loo, M.; Hughes, R.J. Application of methods for identifying broiler chicken gut bacterial species linked with increased energy metabolism. Appl. Environ. Microbiol. 2008, 74, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Shakouri, M.D.; Iji, P.A.; Mikkelsen, L.L.; Cowieson, A.J. Intestinal function and gut microflora of broiler chickens as influenced by cereal grains and microbial enzyme supplementation. J. Anim. Physiol. Anim. Nutr. 2009, 93, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Hammons, S.; Oh, P.L.; Martínez, I.; Clark, K.; Schlegel, V.L.; Sitorius, E.; Scheideler, S.E.; Walter, J. A small variation in diet influences the Lactobacillus strain composition in the crop of broiler chickens. Syst. Appl. Microbiol. 2010, 33, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Tang, J.W.; Yao, X.H.; Wu, Y.F.; Wang, X.; Feng, J. Effects of dietary inclusion of fermented cottonseed meal on growth, cecal microbial population, small intestinal morphology, and digestive enzyme activity of broilers. Trop. Anim. Health Prod. 2013, 45, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, A.; de Leeuw, M.; Penaud-Frézet, S.; Dimova, D.; Murphy, R.A. Phylogenetic and functional alterations in bacterial community compositions in broiler ceca as a result of mannan oligosaccharide supplementation. Appl. Environ. Microbiol. 2015, 81, 3460–3470. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Dong, J.; Cheng, X.; Hu, J.; Liu, Y.; He, G.; Zhang, J.; Yu, H.; Jia, L.; Zhou, D. Biological characteristics and whole-genome analysis of the potential probiotic, Lactobacillus reuteri S5. Lett. Appl. Microbiol. 2022, 74, 593–603. [Google Scholar] [CrossRef]
- Bhogoju, S.; Nahashon, S. Recent Review advances in Probiotic Application in Animal Health and Nutrition: A review. Agriculture 2022, 12, 304. [Google Scholar] [CrossRef]
- Ruan, D.; Fouad, A.M.; Fan, Q.L.; Huo, X.H.; Kuang, Z.X.; Wang, H.; Guo, C.Y.; Deng, Y.F.; Zhang, C.; Zhang, J.H.; et al. Dietary L-arginine supplementation enhances growth performance, intestinal antioxidative capacity, immunity and modulates gut microbiota in yellow-feathered chickens. Poult. Sci. 2020, 99, 6935–6945. [Google Scholar] [CrossRef] [PubMed]
- Ruan, D.; Fan, Q.L.; Zhang, S.; Ei-Senousey, H.K.; Fouad, A.M.; Lin, X.J.; Dong, X.L.; Deng, Y.F.; Yan, S.J.; Zheng, C.T.; et al. Dietary isoleucine supplementation enhances growth performance, modulates the expression of genes related to amino acid transporters and protein metabolism, and gut microbiota in yellow-feathered chickens. Poult. Sci. 2023, 102, 102774. [Google Scholar] [CrossRef]
- Ramírez-Pérez, O.; Cruz-Ramón, V.; Chinchilla-Lópe, P.; Méndez-Sánchez, N. The role of the gut microbiota in bile acid metabolism. Ann. Hepatol. 2017, 16, 15–20. [Google Scholar] [CrossRef]
- Gao, K.; Mu, C.L.; Farzi, A.; Zhu, W.Y. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Dasi-Montoro, L.; Villagra, A.; de Toro, M.; Garcia-Perez, T.; Vega, S.; Marin, C. Assessment of microbiota modulation in poultry to combat infectious diseases. Animals 2021, 11, 615. [Google Scholar] [CrossRef]
- Glendinning, L.; Stewart, R.D.; Pallen, M.J. Assembly of hundreds of novel bacterial genomes from the chicken caecum. Genome Biol. 2020, 21, 34. [Google Scholar] [CrossRef]
- Bomba, A.; Jonecová, Z.; Gancarˇcíková, S.; Nemcová, R. The gastrointestinal microbiota of farm animals. Gastrointest. Microbiol. 2006, 381–400. Available online: https://books.google.com.hk/books?hl=en&lr=&id=Gl9YHHY978sC&oi=fnd&pg=PA381&dq=The+gastrointestinal+microbiota+of+farm+animals&ots=oHgO9NjYdC&sig=bk61m6zh95XpACNQrzvZJ0p_C80&redir_esc=y#v=onepage&q=The%20gastrointestinal%20microbiota%20of%20farm%20animals&f=false (accessed on 20 December 2023).
- Sanots, J.G.D.L.; Storch, O.; Gil-Turnes, C. Bacillus cereus var. toyii and Saccharomyces boulardii increased feed efficiency in broilers infected with Salmonella enteridis. Br. Poul. Sci. 2005, 46, 494–497. [Google Scholar]
- Manor, O.; Dai, C.L.; Kornilov, S.A.; Smith, B.; Price, N.D.; Lovejoy, J.C. Health and disease markers correlate with gut microbiome composition across thousands of people. Nat. Commun. 2020, 11, 1–2. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Kumar, S.; Oakley, B.; Kim, W.K. Chicken gut microbiota: Importance and detection technology. Front. Vet. Sci. 2018, 5, 254. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Hughes, R.J.; Moore, R.J. Microbiota of the chicken gastrointestinal tract: Influence on health, productivity and disease. Appl. Microbiol. Biotechnol. 2014, 98, 4301–4310. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, C.; Salvini, E.; Viri, M.; Deidda, F.; Amoruso, A.; Visciglia, A.; Drago, L.; Calgaro, M.; Vitulo, N.; Pane, M.; et al. Randomized Double-Blind Crossover Study for Evaluating a Probiotic Mixture on Gastrointestinal and Behavioral Symptoms of Autistic Children. J. Clin. Med. 2022, 11, 5263. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh, P.J.; Ramey, R.R.; Bircher, J.S. Evolution of mammals and their gut microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Kers, J.G.; Velkers, F.C.; Fischer, E.A.; Hermes, G.D.; Stegeman, J.A.; Smidt, H. Host and environmental factors affecting the intestinal microbiota in chickens. Front. Microbiol. 2018, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Akerele, G.; Al Hakeem, W.G.; Lourenco, J.; Selvaraj, R.K. The Effect of Necrotic Enteritis Challenge on Production Performance, Cecal Microbiome, and Cecal Tonsil Transcriptome in Broilers. Pathogens 2022, 11, 839. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Sun, Y.; Fan, Y.; Zhang, H.; Yang, J.; Chi, R.; Gao, Y.; Liu, J.; Li, K.; Li, W.; et al. Microbial Diversity and Community Composition of Duodenum Microbiota of High and Low Egg-Yielding Taihang Chickens Identified Using 16S rRNA Amplicon Sequencing. Life 2022, 12, 1262. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Han, C.; Li, S.; Geng, Y.; Wei, Y.; Shi, W.; Bao, Y. High-throughput sequencing-based analysis of the intestinal microbiota of broiler chickens fed with compound small peptides of Chinese medicine. Poult. Sci. 2021, 100, 100897. [Google Scholar] [CrossRef]
- Jozefiak, D.; Sip, A.; Rawski, M.; Rutkowski, A.; Kaczmarek, S.; Hojberg, O.; Jensen, B.B.; Engberg, R.M. Dietary divercin modifies gastrointestinal microbiota and improves growth performance in broiler chickens. Br. Poult. Sci. 2011, 52, 492–499. [Google Scholar] [CrossRef]
- Agboola, A.; Omidiwura, B.; Iyayi, E. No Feed, No Animal—The Parable of the Twelve Baskets. Acad. Lett. 2021, Article 740. [Google Scholar] [CrossRef]
- Zou, A.; Nadeau, K.; Xiong, X.; Wang, P.W.; Copeland, J.K.; Lee, J.Y.; Pierre, J.S.; Ty, M.; Taj, B.; Brumell, J.H.; et al. Systematic profiling of the chicken gut microbiome reveals dietary supplementation with antibiotics alters expression of multiple microbial pathways with minimal impact on community structure. Microbiome 2022, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Jebessa, E.; Guo, L.; Chen, X.; Bello, S.F.; Cai, B.; Girma, M.; Hanotte, O.; Nie, Q. Influence of Eimeria maxima coccidia infection on gut microbiome diversity and composition of the jejunum and cecum of indigenous chicken. Front. Immunol. 2022, 13, 994224. [Google Scholar] [CrossRef] [PubMed]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Gordon Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef] [PubMed]

| Amino Acid of Interest | Published Work | Findings |
|---|---|---|
| Threonine | [77,78] | Threonine supplementation fluctuates the microbial balance in the intestine. Threonine supplemented with a low CP diet brought back bacterial diversity and abundance of beneficial bacteria in laying hens. |
| Tryptophan | [79] | Tryptophan (0.22%) supplemented in diets of hens produced a microbial shift toward beneficial bacteria. |
| Theanine | [54] | Theanine supplemented in broiler chicken diets significantly increased lactobacillus compared to the control group in the ileum and jejunum. |
| Methionine | [80] | Clear separation of microbial communities based on age of birds and between low and medium/ high levels of TSAA (DL-methionine). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor-Bowden, T.; Bhogoju, S.; Khwatenge, C.N.; Nahashon, S.N. The Impact of Essential Amino Acids on the Gut Microbiota of Broiler Chickens. Microorganisms 2024, 12, 693. https://doi.org/10.3390/microorganisms12040693
Taylor-Bowden T, Bhogoju S, Khwatenge CN, Nahashon SN. The Impact of Essential Amino Acids on the Gut Microbiota of Broiler Chickens. Microorganisms. 2024; 12(4):693. https://doi.org/10.3390/microorganisms12040693
Chicago/Turabian StyleTaylor-Bowden, Thyneice, Sarayu Bhogoju, Collins N. Khwatenge, and Samuel N. Nahashon. 2024. "The Impact of Essential Amino Acids on the Gut Microbiota of Broiler Chickens" Microorganisms 12, no. 4: 693. https://doi.org/10.3390/microorganisms12040693
APA StyleTaylor-Bowden, T., Bhogoju, S., Khwatenge, C. N., & Nahashon, S. N. (2024). The Impact of Essential Amino Acids on the Gut Microbiota of Broiler Chickens. Microorganisms, 12(4), 693. https://doi.org/10.3390/microorganisms12040693






