Upper Respiratory Tract Disease in a Dog Infected by a Highly Pathogenic Avian A/H5N1 Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Description
2.2. Blood Analysis
2.3. Ultrasound and X-ray Examination
2.4. Viral and Serogical Tests
3. Results
3.1. Clinical Course of the Disease and Treatment
3.2. Blood Analysis
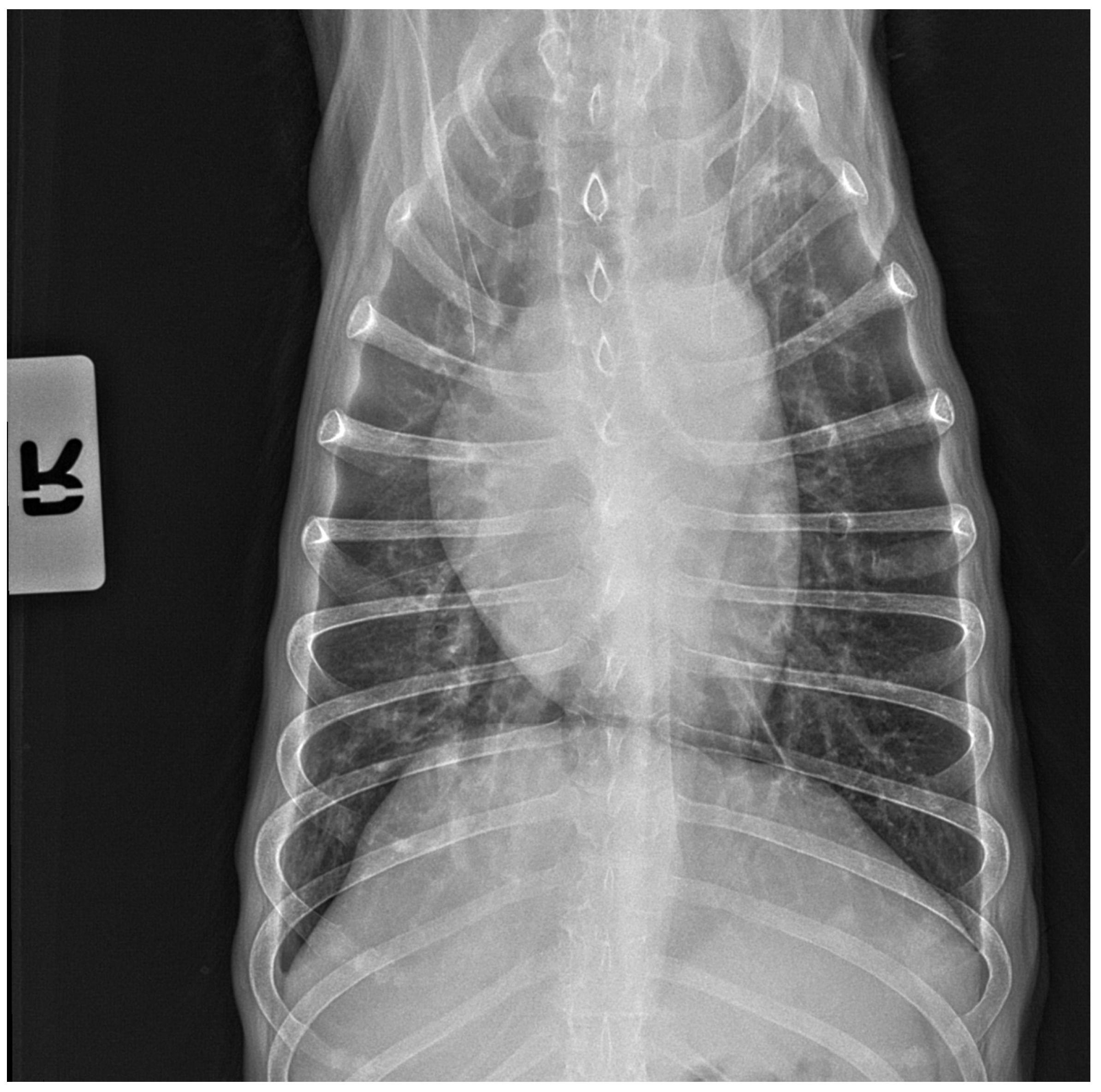
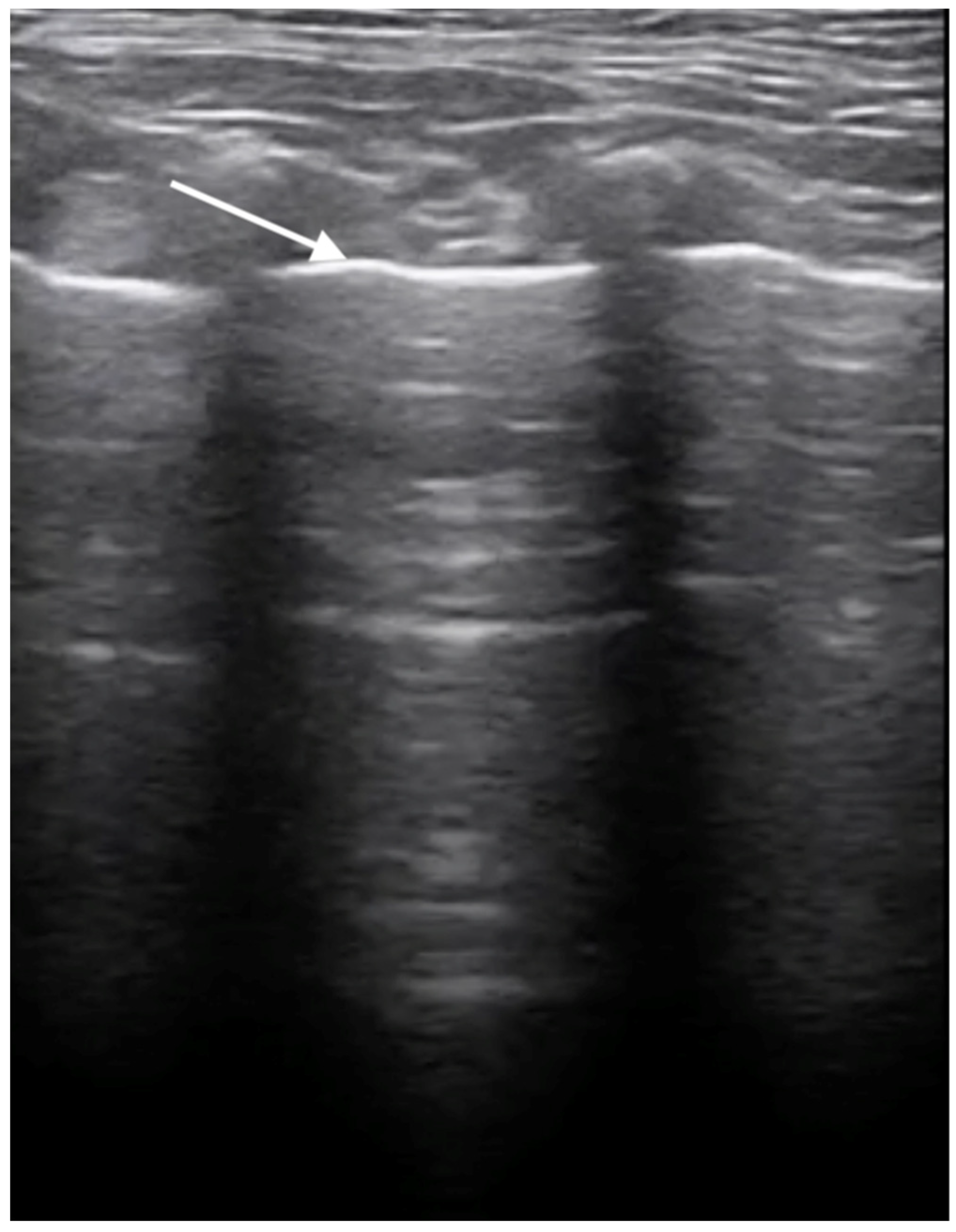
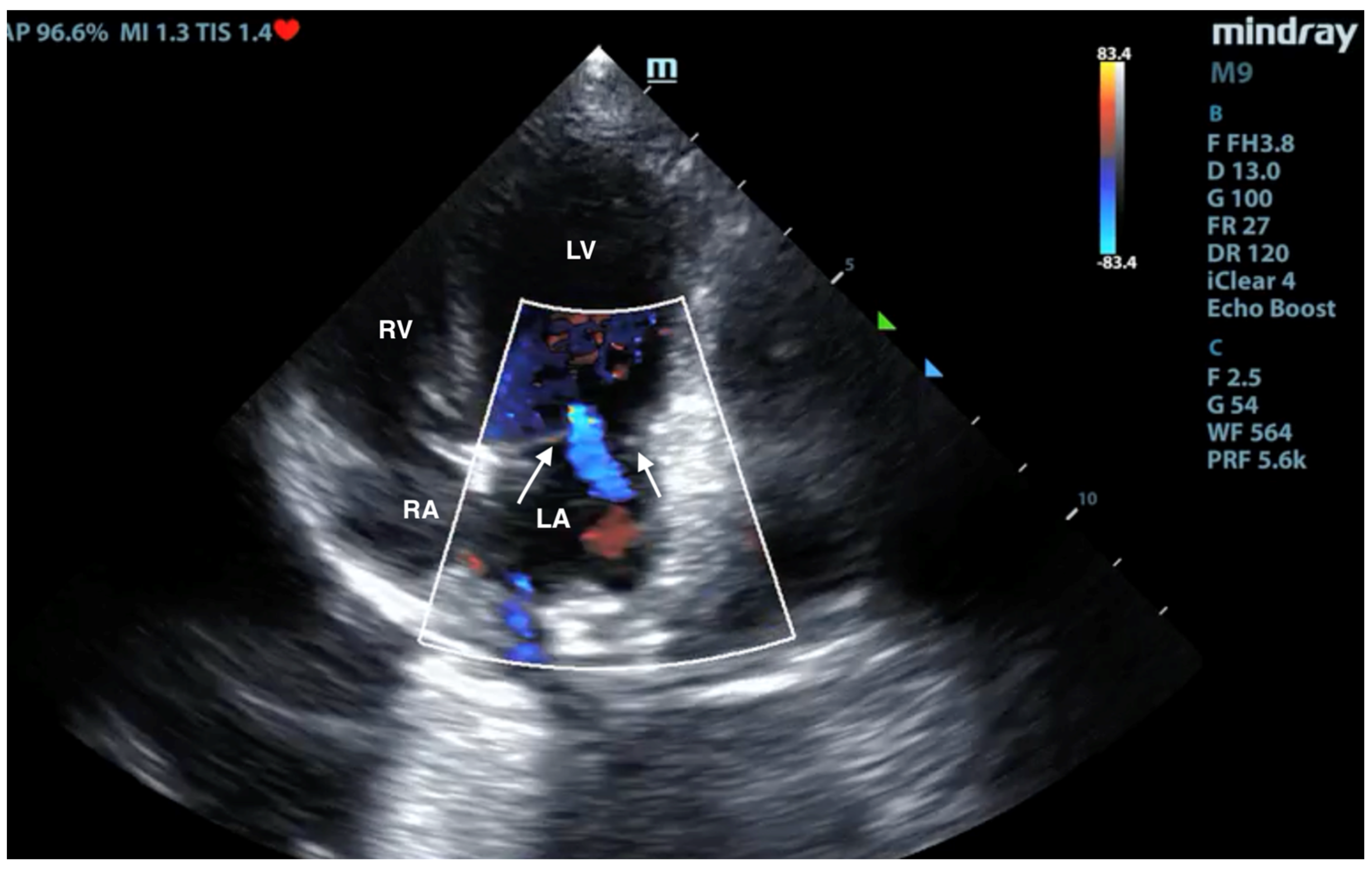
3.3. X-ray and Ultrasound Examination
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Crawford, P.C.; Dubovi, E.J.; Castleman, W.L.; Stephenson, I.; Gibbs, E.P.J.; Chen, L.; Smith, C.; Hill, R.C.; Ferro, P.; Pompey, J.; et al. Transmission of equine influenza virus to dogs. Science 2005, 21, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Rivailler, P.; Ijeoma Perry, I.P.; Jang, Y.; Davis, C.T.; Chen, L.; Dubovi, E.J.; Donis, R.O. Evolution of canine and equine influenza (H3N8) viruses co-circulating between 2005 and 2008. Virology 2010, 5, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Songserm, T.; Amonsin, A.; Jam-on, R.; Sae-Heng, N.; Meemak, N.; Pariyothorn, N.; Payungporn, S.; Theamboonlers, A.; Poovorawan, Y. Avian Influenza H5N1 in Naturally Infected Domestic Cat. Emerg. Infect. Dis. 2006, 12, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zeng, H.; He, J.; Zhu, Y.; Wang, P.; Guo, J.; Guo, J.; Zhou, H.; Qin, Y.; Ouyang, K.; et al. Reassortant H9N2 canine influenza viruses containing the pandemic H1N1/2009 ribonucleoprotein complex circulating in pigs acquired enhanced virulence in mice. Virology 2024, 589, 109927. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.; Li, H.; Feng, C.; Guo, W.; Feng, Y.; Zhu, D.; Chen, H.; Zhang, Y. Emergence of a novel reassortant H3N6 canine influenza virus. Front. Microbiol. 2023, 11, 1186869. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.J.; Ling, Z.S.; Zhu, Y.L.; Jiang, S.J.; Xie, Z.J. Genetic characterization of a novel influenza A virus H5N2 isolated from a dog in China. Vet. Microbiol. 2012, 23, 409–416. [Google Scholar] [CrossRef]
- Su, S.; Qi, W.; Zhou, P.; Xiao, C.; Yan, Z.; Cui, J.; Jia, K.; Zhang, G.; Gray, G.C.; Liao, M.; et al. First evidence of H10N8 Avian influenza virus infections among feral dogs in live poultry markets in Guangdong province, China. Clin. Infect. Dis. 2014, 1, 748–750. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.C.; Bromfield, C.R.; Crawford, P.C.; Dodds, W.J.; Gibbs, E.P.; Hernandez, J.A. Serological evidence of H3N8 canine influenza-like virus circulation in USA dogs prior to 2004. Vet. J. 2012, 191, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Wasik, B.R.; Rothschild, E.; Voorhees, I.E.H.; Reedy, S.E.; Murcia, P.R.; Pusterla, N.; Chambers, T.M.; Goodman, L.B.; Holmes, E.C.; Kile, J.C.; et al. Understanding the divergent evolution and epidemiology of H3N8 influenza viruses in dogs and horses. Virus Evol. 2023, 18, vead052. [Google Scholar] [CrossRef]
- Crispe, E.; Finlaison, D.S.; Hurt, A.C.; Kirkland, P.D. Infection of dogs with equine influenza virus: Evidence for transmission from horses during the Australian outbreak. Aust. Vet. J. 2011, 89, 27–28. [Google Scholar] [CrossRef]
- Zhu, H.; Hughes, J.; Murcia, P.R. Origins and evolutionary dynamics of H3N2 canine influenza virus. J. Virol. 2015, 89, 5406–5418. [Google Scholar] [CrossRef] [PubMed]
- Voorhees, I.E.H.; Glaser, A.L.; Toohey-Kurth, K.; Newbury, S.; Dalziel, B.D.; Dubovi, E.J.; Poulsen, K.; Leutenegger, C.; Willgert, K.J.E.; Brisbane-Cohen, L.; et al. Spread of canine influenza A(H3N2) virus, United States. Emerg. Infect. Dis. 2017, 23, 1950–1957. [Google Scholar] [CrossRef] [PubMed]
- Wasik, B.R.; Voorhees, I.E.H.; Parrish, C.R. Canine and Feline Influenza. Cold Spring Harb. Perspect. Med. 2021, 11, a038562. [Google Scholar] [CrossRef]
- Webster, R.G.; Guan, Y.; Poon, L.; Krauss, S.; Webby, R.; Govorkovai, E.; Peiris, M. The spread of the H5N1 bird flu epidemic in Asia in 2004. Arch. Virol. Suppl. 2005, 19, 17–29. [Google Scholar] [CrossRef]
- Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Mirinavičiūtė, G.; Niqueux, É.; Ståhl, K.; Staubach, C.; Terregino, C.; Willgert, K.; et al. European Food Safety Authority; European Centre for Disease Prevention and Control; European Union Reference Laboratory for Avian Influenza; Avian influenza overview September-December 2023. EFSA J. 2023, 21, e8539. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cumulative Number of Confirmed Human Cases for Avian Influenza A(H5N1). Available online: https://cdn.who.int/media/docs/default-source/influenza/h5n1-human-casecumulative-table/2023_may_tableh5n1.pdf?sfvrsn=934b4b02_6&download=true (accessed on 11 January 2024).
- Kuiken, T.; Rimmelzwaan, G.; van Riel, D.; van Amerongen, G.; Baars, M.; Fouchier, R.; Osterhaus, A. Avian H5N1 Influenza in Cats. Science 2004, 306, 241. [Google Scholar] [CrossRef] [PubMed]
- Thiry, E.; Zicola, A.; Addie, D.; Egberink, H.; Hartmann, K.; Lutz, H.; Poulet, H.; Horzinek, M. Highly pathogenic avian influenza H5N1 virus in cats and other carnivores. Vet. Microbiol. 2007, 122, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Keawcharoen, J.; Oraveerakul, K.; Kuiken, T.; Fouchier, R.A.M.; Amonsin, A.; Payungporn, S.; Noppornpanth, S.; Wattanodorn, S.; Theambooniers, A.; Tantilertcharoen, R.; et al. Avian Influenza H5N1 in Tigers and Leopards. Emerg. Infect. Dis. 2004, 10, 2189–2191. [Google Scholar] [CrossRef] [PubMed]
- Songserm, T.; Amonsin, A.; Jam-on, R.; Sae-Heng, N.; Pariyothorn, N.; Payungporn, S.; Theamboonlers, A.; Chutinimitkul, S.; Thanawongnuwech, R.; Poovorawan, Y. Fatal avian influenza A H5N1 in a dog. Emerg. Infect. Dis. 2006, 12, 1744–1747. [Google Scholar] [CrossRef]
- Hiono, T.; Kobayashi, D.; Kobayashi, A.; Suzuki, T.; Satake, Y.; Harada, R.; Matsuno, K.; Sashika, M.; Ban, H.; Kobayashi, M.; et al. Virological, pathological, and glycovirological investigations of an Ezo red fox and a tanuki naturally infected with H5N1 high pathogenicity avian influenza viruses in Hokkaido, Japan. Virology 2023, 578, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Fournié, G.; Métras, R.; Song, D.; Donnelly, C.A.; Pfeiffer, D.U.; Nouvellet, P. Lessons for cross-species viral transmission surveillance from highly pathogenic avian influenza Korean cat shelter outbreaks. Nat. Commun. 2023, 31, 6958. [Google Scholar] [CrossRef] [PubMed]
- Szaluś-Jordanow, O.; Golke, A.; Dzieciątkowski, T.; Chrobak-Chmiel, D.; Rzewuska, M.; Czopowicz, M.; Sapierzyński, R.; Kardas, M.; Biernacka, K.; Mickiewicz, M.; et al. A Fatal A/H5N1 Avian Influenza Virus Infection in a Cat in Poland. Microorganisms 2023, 9, 2263. [Google Scholar] [CrossRef] [PubMed]
- Domańska-Blicharz, K.; Świętoń, E.; Świątalska, A.; Monne, I.; Fusaro, A.; Tarasiuk, K.; Wyrostek, K.; Styś-Fijoł, N.; Giza, A.; Pietruk, M.; et al. Outbreak of Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus in Cats, Poland, June to July 2023. Euro Surveill. 2023, 28, 2300366. [Google Scholar] [CrossRef] [PubMed]
- Rabalski, L.; Milewska, A.; Pohlmann, A.; Gackowska, K.; Lepionka, T.; Szczepaniak, K.; Swiatalska, A.; Sieminska, I.; Arent, Z.; Beer, M.; et al. Emergence and Potential Transmission Route of Avian Influenza A (H5N1) Virus in Domestic Cats in Poland, June 2023. Euro Surveill. 2023, 28, 2300390. [Google Scholar] [CrossRef] [PubMed]
- Golke, A.; Dzieciątkowski, T.; Szaluś-Jordanow, O.; Jańczak, D.; Sapierzyński, R.; Moroz-Fik, A.; Frymus, T. Highly pathogenic A/H5N1 influenza virus infections in cats, ferrets and dogs in Poland and other countries. Mag. Weter. 2023, 4, 1–5. (In Polish) [Google Scholar]
- Amonsin, A.; Songserm, T.; Chutinimitkul, S.; Jam-On, R.; Sae-Heng, N.; Pariyothorn, N.; Payungporn, S.; Theamboonlers, A.; Poovorawan, Y. Genetic analysis of influenza A virus (H5N1) derived from domestic cat and dog in Thailand. Arch. Virol. 2007, 152, 1925–1933. [Google Scholar] [CrossRef] [PubMed]
- Stefańska, I.; Dzieciatkowski, T.; Brydak, L.B.; Romanowska, M. Application of three duplex real-time PCR assays for simultaneous detection of human seasonal and avian influenza viruses. Arch. Virol. 2013, 158, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Briand, F.X.; Souchaud, F.; Pierre, I.; Beven, V.; Hirchaud, E.; Hérault, F.; Planel, R.; Rigaudeau, A.; Bernard-Stoecklin, S.; Van der Werf, S.; et al. Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus in Domestic Cat, France, 2022. Emerg. Infect. Dis. 2023, 29, 1696. [Google Scholar] [CrossRef] [PubMed]
- Rijks, J.M.; Hesselink, H.; Lollinga, P.; Wesselman, R.; Prins, P.; Weesendorp, E.; Engelsma, M.; Heutink, R.; Harders, F.; Kik, M.; et al. Highly pathogenic avian influenza A(H5N1) virus in wild red foxes, The Netherlands, 2021. Emerg. Infect. Dis. 2021, 27, 2960. [Google Scholar] [CrossRef] [PubMed]
- Tammiranta, N.; Isomursu, M.; Fusaro, A.; Nylund, M.; Nokireki, T.; Giussani, E.; Zecchin, B.; Terregino, C.; Gadd, T. Highly pathogenic avian influenza A (H5N1) virus infections in wild carnivores connected to mass mortalities of pheasants in Finland. Infect. Genet. Evol. 2023, 111, 105423. [Google Scholar] [CrossRef] [PubMed]
- Lindh, E.; Lounela, H.; Ikonen, N.; Kantala, T.; Savolainen-Kopra, C.; Kauppinen, A.; Osterlund, P.; Kareinen, L.; Katz, A.; Nokireki, T.; et al. Highly pathogenic avian influenza A(H5N1) virus infection on multiple fur farms in the South and Central Ostrobothnia regions of Finland, July 2023. Euro Surveill. 2023, 28, 2300400. [Google Scholar] [CrossRef] [PubMed]
- Agüero, M.; Monne, I.; Sánchez, A.; Zecchin, B.; Fusaro, A.; Ruano, M.J.; Del Valle Arrojo, M.; Fernández-Antonio, R.; Souto, A.M.; Tordable, P.; et al. Highly pathogenic avian influenza A(H5N1) virus infection in farmed minks, Spain, October 2022. Euro Surveill. 2023, 28, 2300001. [Google Scholar] [CrossRef] [PubMed]
- Giese, M.; Harder, T.C.; Teifke, J.P.; Klopfleisch, R.; Breithaupt, A.; Mettenleiter, T.C.; Vahlenkamp, T.W. Experimental infection and natural contact exposure of dogs with avian influenza virus (H5N1). Emerg. Infect. Dis. 2008, 14, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Maas, R.; Tacken, M.; Ruuls, L.; Koch, G.; van Rooij, E.; Stockhofe-Zurwieden, N. Avian influenza (H5N1) susceptibility and receptors in dogs. Emerg. Infect. Dis. 2007, 13, 1219–1221. [Google Scholar] [CrossRef] [PubMed]
- Reperant, L.A.; van Amerongen, G.; van de Bildt, M.W.; Rimmelzwaan, G.F.; Dobson, A.P.; Osterhaus, A.D.; Kuiken, T. Highly pathogenic avian influenza virus (H5N1) infection in red foxes fed infected bird carcasses. Emerg. Infect. Dis. 2008, 14, 1835–1841. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhong, G.; Wang, G.; Deng, G.; Li, Y.; Shi, J.; Zhang, Z.; Guan, Y.; Jiang, Y.; Bu, Z.; et al. Dogs are highly susceptible to H5N1 avian influenza virus. Virology 2010, 15, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Bonfante, F.; Bortolami, A.; Cassaniti, I.; Caruana, A.; Cottini, V.; Cereda, D.; Farioli, M.; Fusaro, A.; Lavazza, A.; et al. Asymptomatic infection with clade 2.3.4.4b highly pathogenic avian influenza A(H5N1) in carnivore pets, Italy, April 2023. Euro Surveill. 2023, 28, 2300441. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szaluś-Jordanow, O.; Golke, A.; Dzieciątkowski, T.; Czopowicz, M.; Kardas, M.; Mickiewicz, M.; Moroz-Fik, A.; Łobaczewski, A.; Markowska-Daniel, I.; Frymus, T. Upper Respiratory Tract Disease in a Dog Infected by a Highly Pathogenic Avian A/H5N1 Virus. Microorganisms 2024, 12, 689. https://doi.org/10.3390/microorganisms12040689
Szaluś-Jordanow O, Golke A, Dzieciątkowski T, Czopowicz M, Kardas M, Mickiewicz M, Moroz-Fik A, Łobaczewski A, Markowska-Daniel I, Frymus T. Upper Respiratory Tract Disease in a Dog Infected by a Highly Pathogenic Avian A/H5N1 Virus. Microorganisms. 2024; 12(4):689. https://doi.org/10.3390/microorganisms12040689
Chicago/Turabian StyleSzaluś-Jordanow, Olga, Anna Golke, Tomasz Dzieciątkowski, Michał Czopowicz, Michał Kardas, Marcin Mickiewicz, Agata Moroz-Fik, Andrzej Łobaczewski, Iwona Markowska-Daniel, and Tadeusz Frymus. 2024. "Upper Respiratory Tract Disease in a Dog Infected by a Highly Pathogenic Avian A/H5N1 Virus" Microorganisms 12, no. 4: 689. https://doi.org/10.3390/microorganisms12040689
APA StyleSzaluś-Jordanow, O., Golke, A., Dzieciątkowski, T., Czopowicz, M., Kardas, M., Mickiewicz, M., Moroz-Fik, A., Łobaczewski, A., Markowska-Daniel, I., & Frymus, T. (2024). Upper Respiratory Tract Disease in a Dog Infected by a Highly Pathogenic Avian A/H5N1 Virus. Microorganisms, 12(4), 689. https://doi.org/10.3390/microorganisms12040689






