Urea Fertilization Significantly Promotes Nitrous Oxide Emissions from Agricultural Soils and Is Attributed to the Short-Term Suppression of Nitrite-Oxidizing Bacteria during Urea Hydrolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Collection
2.2. Experimental Setup
2.3. Chemical Determination and Analysis
2.4. DNA Extraction and qPCR Analysis
2.5. Statistical Analysis
3. Results
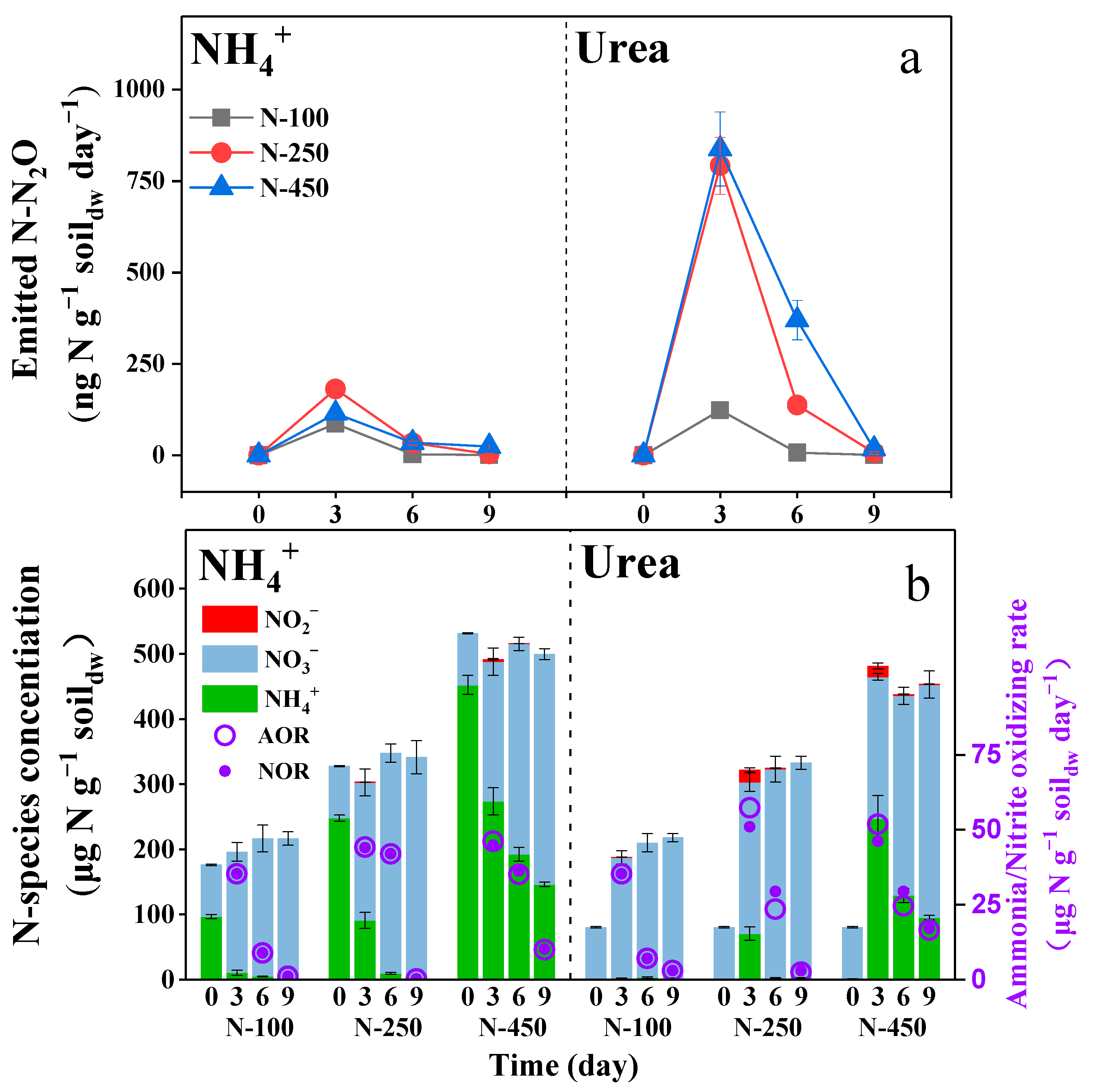
3.1. Urea as Nitrogen Fertilizer Short-Termly Suppressed the Soil Nitrite-Oxidizing Activity
3.2. Urea as Nitrogen Fertilizer Promoted the Soil N2O Emission
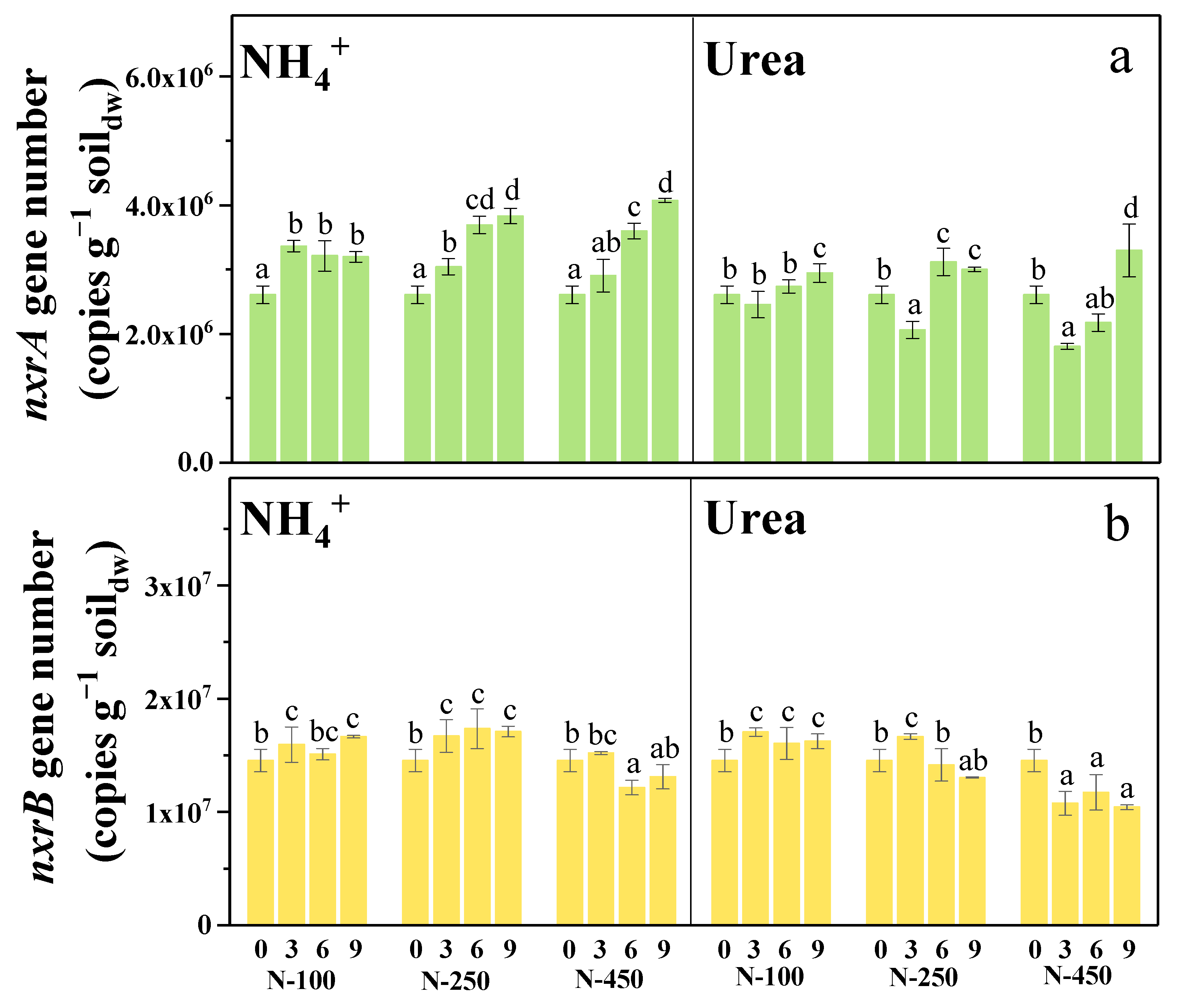
3.3. Urea as Nitrogen Fertilizer Short-Termly Suppressed the NOB Growth
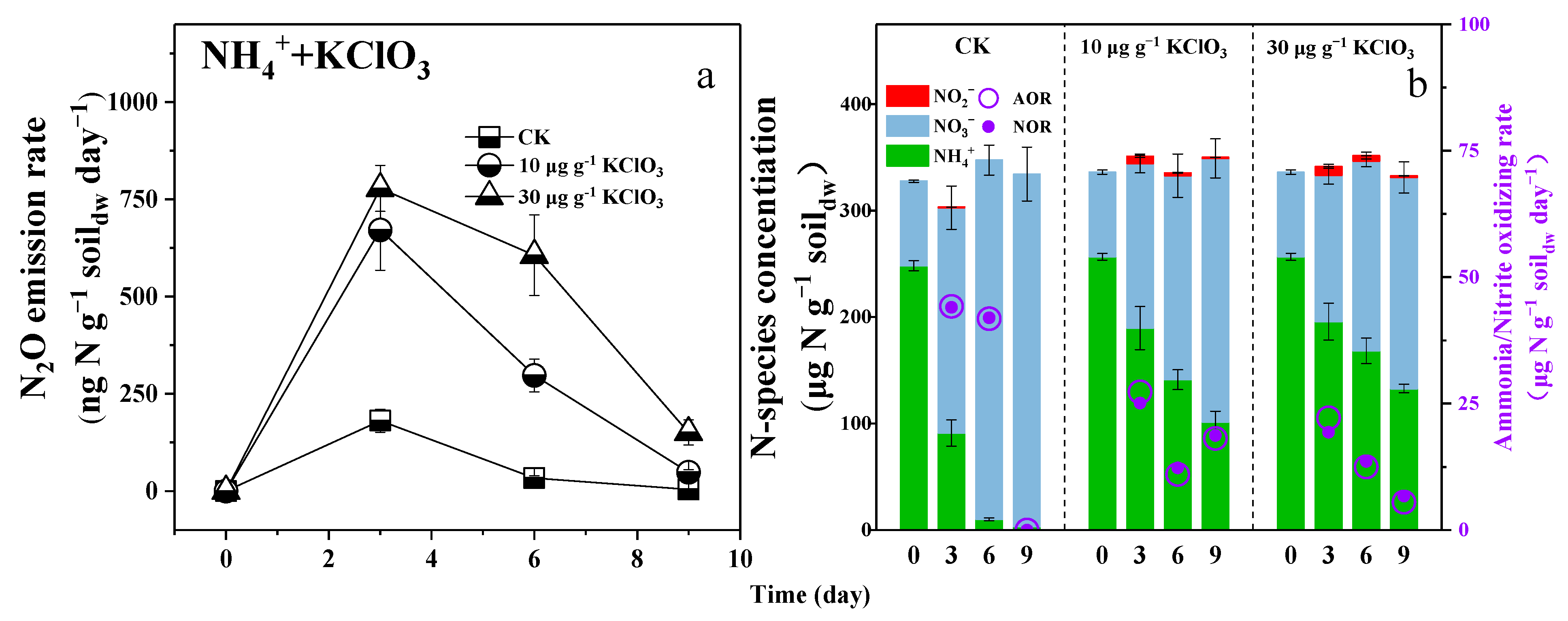
3.4. Retarding Urea Hydrolysis or Maintaining NOB Activity Could Reduce N2O Emission
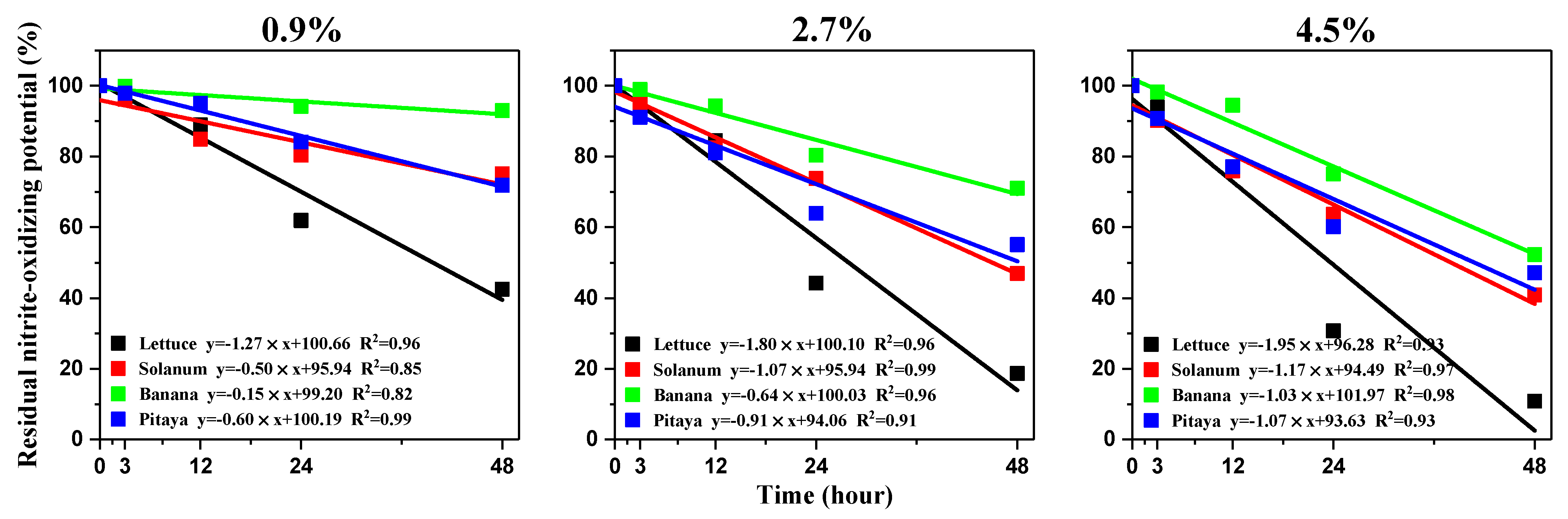
3.5. Free Ammonia Suppressed the NOB Activity in Soils
4. Discussion
4.1. The Suppression of NOB Disrupted the Equilibrium between Ammonia OXIDATION and Nitrite Oxidation, Ultimately Leading to an Elevation in Soil N2O Emission
4.2. NOB Suppression Only Occurred in a Short-Term Time during Urea Hydrolysis
4.3. Short-Term NOB Suppression Only Happened among the Genus Nitrobacter
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tian, H.; Xu, R.; Canadell, J.G.; Thompson, R.L.; Winiwarter, W.; Suntharalingam, P.; Davidson, E.A.; Ciais, P.; Jackson, R.B.; Janssens-Maenhout, G.; et al. A Comprehensive Quantification of Global Nitrous Oxide Sources and Sinks. Nature 2020, 586, 248–256. [Google Scholar] [CrossRef]
- Sovacool, B.K.; Griffiths, S.; Kim, J.; Bazilian, M. Climate Change and Industrial F-Gases: A Critical and Systematic Review of Developments, Sociotechnical Systems and Policy Options for Reducing Synthetic Greenhouse Gas Emissions. Renew. Sustain. Energ. Rev. 2021, 141, 110759. [Google Scholar] [CrossRef]
- Reay, D.S.; Davidson, E.A.; Smith, K.A.; Smith, P.; Melillo, J.M.; Dentener, F.; Crutzen, P.J. Global Agriculture and Nitrous Oxide Emissions. Nat. Clim. Chang. 2012, 2, 410–416. [Google Scholar] [CrossRef]
- Winiwarter, W.; Höglund-Isaksson, L.; Klimont, Z.; Schöpp, W.; Amann, M. Technical Opportunities to Reduce Global Anthropogenic Emissions of Nitrous Oxide. Environ. Res. Lett. 2018, 13, 014011. [Google Scholar] [CrossRef]
- Tian, H.; Yang, J.; Xu, R.; Lu, C.; Canadell, J.G.; Davidson, E.A.; Jackson, R.B.; Arneth, A.; Chang, J.; Ciais, P.; et al. Global Soil Nitrous Oxide Emissions since the Preindustrial Era Estimated by an Ensemble of Terrestrial Biosphere Models: Magnitude, Attribution, and Uncertainty. Glob. Chang. Biol. 2019, 25, 640–659. [Google Scholar] [CrossRef]
- Aliyu, G.; Luo, J.; Di, H.J.; Lindsey, S.; Liu, D.; Yuan, J.; Chen, Z.; Lin, Y.; He, T.; Zaman, M.; et al. Nitrous Oxide Emissions from China’s Croplands Based on Regional and Crop-Specific Emission Factors Deviate from IPCC 2006 Estimates. Sci. Total Environ. 2019, 669, 547–558. [Google Scholar] [CrossRef]
- Hergoualc’h, K.; Mueller, N.; Bernoux, M.; Kasimir, Ä.; Weerden, T.J.; Ogle, S.M. Improved Accuracy and Reduced Uncertainty in Greenhouse Gas Inventories by Refining the IPCC Emission Factor for Direct N2O Emissions from Nitrogen Inputs to Managed Soils. Glob. Chang. Biol. 2021, 27, 24. [Google Scholar] [CrossRef]
- Shcherbak, I.; Millar, N.; Robertson, G.P. Global Metaanalysis of the Nonlinear Response of Soil Nitrous Oxide (N2O) Emissions to Fertilizer Nitrogen. Proc. Natl. Acad. Sci. USA 2014, 111, 9199–9204. [Google Scholar] [CrossRef]
- Shepherd, A.; Yan, X.; Nayak, D.; Newbold, J.; Moran, D.; Dhanoa, M.S.; Goulding, K.; Smith, P.; Cardenas, L.M. Disaggregated N2O Emission Factors in China Based on Cropping Parameters Create a Robust Approach to the IPCC Tier 2 Methodology. Atmos. Environ. 2015, 122, 272–281. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, M.; Bai, M.; Tian, Z.; Wang, S.; Wang, Z. Nitrogen Removal Improvement by Denitrifying Ammonium Oxidation in Anoxic/Oxic-Sequence Batch Biofilm Reactor System. J. Environ. Chem. Eng. 2022, 10, 10722. [Google Scholar] [CrossRef]
- Tian, X.; Cong, J.; Wang, H.; Zheng, H.; Wang, Z.; Chu, Y.; Wang, Y.; Xue, Y.; Yin, Y.; Cui, Z. Cropland Nitrous Oxide Emissions Exceed the Emissions of RCP 2.6: A Global Spatial Analysis. Sci. Total Environ. 2023, 858, 159738. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, D.; Liu, S.; Liao, Y.; Han, J. Can Controlled-Release Urea Replace the Split Application of Normal Urea in China? A Meta-Analysis Based on Crop Grain Yield and Nitrogen Use Efficiency. Field Crops Res. 2022, 275, 108343. [Google Scholar] [CrossRef]
- Wu, H.; MacDonald, G.K.; Galloway, J.N.; Zhang, L.; Gao, L.; Yang, L.; Yang, J.; Li, X.; Li, H.; Yang, T. The Influence of Crop and Chemical Fertilizer Combinations on Greenhouse Gas Emissions: A Partial Life-Cycle Assessment of Fertilizer Production and Use in China. Resour. Conserv. Recycl. 2021, 168, 105303. [Google Scholar] [CrossRef]
- Zhu, X.; Burger, M.; Doane, T.A.; Horwath, W.R. Ammonia Oxidation Pathways and Nitrifier Denitrification Are Significant Sources of N2O and NO under Low Oxygen Availability. Proc. Natl. Acad. Sci. USA 2013, 110, 6328–6333. [Google Scholar] [CrossRef]
- Volpi, I.; Laville, P.; Bonari, E.; o di Nasso, N.N.; Bosco, S. Improving the Management of Mineral Fertilizers for Nitrous Oxide Mitigation: The Effect of Nitrogen Fertilizer Type, Urease and Nitrification Inhibitors in Two Different Textured Soils. Geoderma 2017, 307, 181–188. [Google Scholar] [CrossRef]
- Tierling, J.; Kuhlmann, H. Emissions of Nitrous Oxide (N2O) Affected by PH-Related Nitrite Accumulation during Nitrification of N Fertilizers. Geoderma 2018, 310, 12–21. [Google Scholar] [CrossRef]
- Venterea, R.T. Nitrite-Driven Nitrous Oxide Production under Aerobic Soil Conditions: Kinetics and Biochemical Controls. Glob. Chang. Biol. 2007, 13, 1798–1809. [Google Scholar] [CrossRef]
- Breuillin-Sessoms, F.; Venterea, R.T.; Sadowsky, M.J.; Coulter, J.A.; Clough, T.J.; Wang, P. Nitrification Gene Ratio and Free Ammonia Explain Nitrite and Nitrous Oxide Production in Urea-Amended Soils. Soil Biol. Biochem. 2017, 111, 143–153. [Google Scholar] [CrossRef]
- Hink, L.; Nicol, G.W.; Prosser, J.I. Archaea Produce Lower Yields of N2O than Bacteria during Aerobic Ammonia Oxidation in Soil. Environ. Microbiol. 2017, 19, 4829–4837. [Google Scholar] [CrossRef]
- Wertz, S.; Degrange, V.; Prosser, J.I.; Poly, F.; Commeaux, C.; Guillaumaud, N.; Le Roux, X. Decline of Soil Microbial Diversity Does Not Influence the Resistance and Resilience of Key Soil Microbial Functional Groups Following a Model Disturbance. Environ. Microbiol. 2007, 9, 2211–2219. [Google Scholar] [CrossRef]
- Han, S.; Zeng, L.; Luo, X.; Xiong, X.; Wen, S.; Wang, B.; Chen, W.; Huang, Q. Shifts in Nitrobacter- and Nitrospira-like Nitrite-Oxidizing Bacterial Communities under Long-Term Fertilization Practices. Soil Biol. Biochem. 2018, 124, 118–125. [Google Scholar] [CrossRef]
- Liu, B.; Lin, W.; Huang, S.; Sun, Q.; Yin, H.; Luo, J. Removal of Mg2+ Inhibition Benefited the Growth and Isolation of Ammonia-Oxidizing Bacteria: An Inspiration from Bacterial Interaction. Sci. Total Environ. 2022, 838, 155923. [Google Scholar] [CrossRef]
- Xu, G.; Xu, X.; Yang, F.; Liu, S. Selective Inhibition of Nitrite Oxidation by Chlorate Dosing in Aerobic Granules. J. Hazard. Mater. 2011, 185, 249–254. [Google Scholar] [CrossRef]
- Cantarella, H.; Otto, R.; Soares, J.R.; de Brito Silva, A.G. Agronomic Efficiency of NBPT as a Urease Inhibitor: A Review. J. Adv. Res. 2018, 13, 19–27. [Google Scholar] [CrossRef]
- Duan, P.; Fan, C.; Zhang, Q.; Xiong, Z. Overdose Fertilization Induced Ammonia-Oxidizing Archaea Producing Nitrous Oxide in Intensive Vegetable Fields. Sci. Total Environ. 2019, 650, 1787–1794. [Google Scholar] [CrossRef]
- Yang, Y.; Xiao, Y.; Li, C.; Wang, B.; Gao, Y.; Zhou, G. Nitrogen Addition, rather than Altered Precipitation, Stimulates Nitrous Oxide Emissions in an Alpine Steppe. Ecol. Evol. 2021, 11, 15153–15163. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.M.; Shen, J.P.; Du, S.; Han, L.L.; He, J.Z. Nitrogen Fertiliser-Induced Changes in N2O Emissions Are Attributed More to Ammonia-Oxidising Bacteria Rather than Archaea as Revealed Using 1-Octyne and Acetylene Inhibitors in Two Arable Soils. Biol. Fertil. Soils 2016, 52, 1163–1171. [Google Scholar] [CrossRef]
- Hink, L.; Gubry-Rangin, C.; Nicol, G.W.; Prosser, J.I. The Consequences of Niche and Physiological Differentiation of Archaeal and Bacterial Ammonia Oxidisers for Nitrous Oxide Emissions. ISME J. 2018, 12, 1084–1093. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, X.; Ju, X.; Wu, D. Oxygen-Depletion by Rapid Ammonia Oxidation Regulates Kinetics of N2O, NO and N2 Production in an Ammonium Fertilised Agricultural Soil. Soil Biol. Biochem. 2021, 163, 108460. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, Y.; Dong, G.; Du, Z.; Wu, W.; Chadwick, D.; Bol, R. The Importance of Ammonia Volatilization in Estimating the Efficacy of Nitrification Inhibitors to Reduce N2O Emissions: A Global Meta-Analysis. Environ. Pollut. 2021, 271, 116365. [Google Scholar] [CrossRef]
- Sha, Z.; Ma, X.; Loick, N.; Lv, T.; Cardenas, L.M.; Ma, Y.; Liu, X.; Misselbrook, T. Nitrogen stabilizers mitigate reactive N and greenhouse gas emissions from an arable soil in North China Plain: Field and laboratory investigation. J. Clean. Prod. 2020, 258, 121025. [Google Scholar] [CrossRef]
- Krol, D.J.; Forrestal, P.J.; Wall, D.; Lanigan, G.J.; Sanz-Gomez, J.; Richards, K.G. Nitrogen fertilisers with urease inhibitors reduce nitrous oxide and ammonia losses, while retaining yield in temperate grassland. Sci. Total Environ. 2020, 725, 138329. [Google Scholar] [CrossRef]
- Singh, J.; Kunhikrishnan, A.; Bolan, N.S.; Saggar, S. Impact of urease inhibitor on ammonia and nitrous oxide emissions from temperate pasture soil cores receiving urea fertilizer and cattle urine. Sci. Total Environ. 2013, 465, 56–63. [Google Scholar] [CrossRef]
- Daims, H.; Lücker, S.; Wagner, M. A New Perspective on Microbes Formerly Known as Nitrite-Oxidizing Bacteria. Trends Microbiol. 2016, 24, 699–712. [Google Scholar] [CrossRef]
- Taylor, A.E.; Myrold, D.D.; Bottomley, P.J. Temperature Affects the Kinetics of Nitrite Oxidation and Nitrification Coupling in Four Agricultural Soils. Soil Biol. Biochem. 2019, 136, 107523. [Google Scholar] [CrossRef]
- Li, X.; Wan, W.; Zheng, L.; Wang, A.; Luo, X.; Huang, Q.; Chen, W. Community Assembly Mechanisms and Co-Occurrence Patterns of Nitrite-Oxidizing Bacteria Communities in Saline Soils. Sci. Total Environ. 2021, 772, 145472. [Google Scholar] [CrossRef]
- Luo, X.; Wang, A.; Hou, C.; Han, S.; Huang, Q.; Chen, W. The Limited Effects of Carbonaceous Material Amendments on Nitrite-Oxidizing Bacteria in an Al Fisol. Sci. Total Environ. 2020, 734, 139398. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Zhu, Y.; Lin, W.; Luo, J. Urea Fertilization Significantly Promotes Nitrous Oxide Emissions from Agricultural Soils and Is Attributed to the Short-Term Suppression of Nitrite-Oxidizing Bacteria during Urea Hydrolysis. Microorganisms 2024, 12, 685. https://doi.org/10.3390/microorganisms12040685
Jiang Y, Zhu Y, Lin W, Luo J. Urea Fertilization Significantly Promotes Nitrous Oxide Emissions from Agricultural Soils and Is Attributed to the Short-Term Suppression of Nitrite-Oxidizing Bacteria during Urea Hydrolysis. Microorganisms. 2024; 12(4):685. https://doi.org/10.3390/microorganisms12040685
Chicago/Turabian StyleJiang, Yiming, Yueyue Zhu, Weitie Lin, and Jianfei Luo. 2024. "Urea Fertilization Significantly Promotes Nitrous Oxide Emissions from Agricultural Soils and Is Attributed to the Short-Term Suppression of Nitrite-Oxidizing Bacteria during Urea Hydrolysis" Microorganisms 12, no. 4: 685. https://doi.org/10.3390/microorganisms12040685
APA StyleJiang, Y., Zhu, Y., Lin, W., & Luo, J. (2024). Urea Fertilization Significantly Promotes Nitrous Oxide Emissions from Agricultural Soils and Is Attributed to the Short-Term Suppression of Nitrite-Oxidizing Bacteria during Urea Hydrolysis. Microorganisms, 12(4), 685. https://doi.org/10.3390/microorganisms12040685






