Unlocking the Uterine Code: Microbiota, Immune Cells, and Therapy for Recurrent Reproductive Failure
Abstract
1. Introduction
2. Materials and Methods
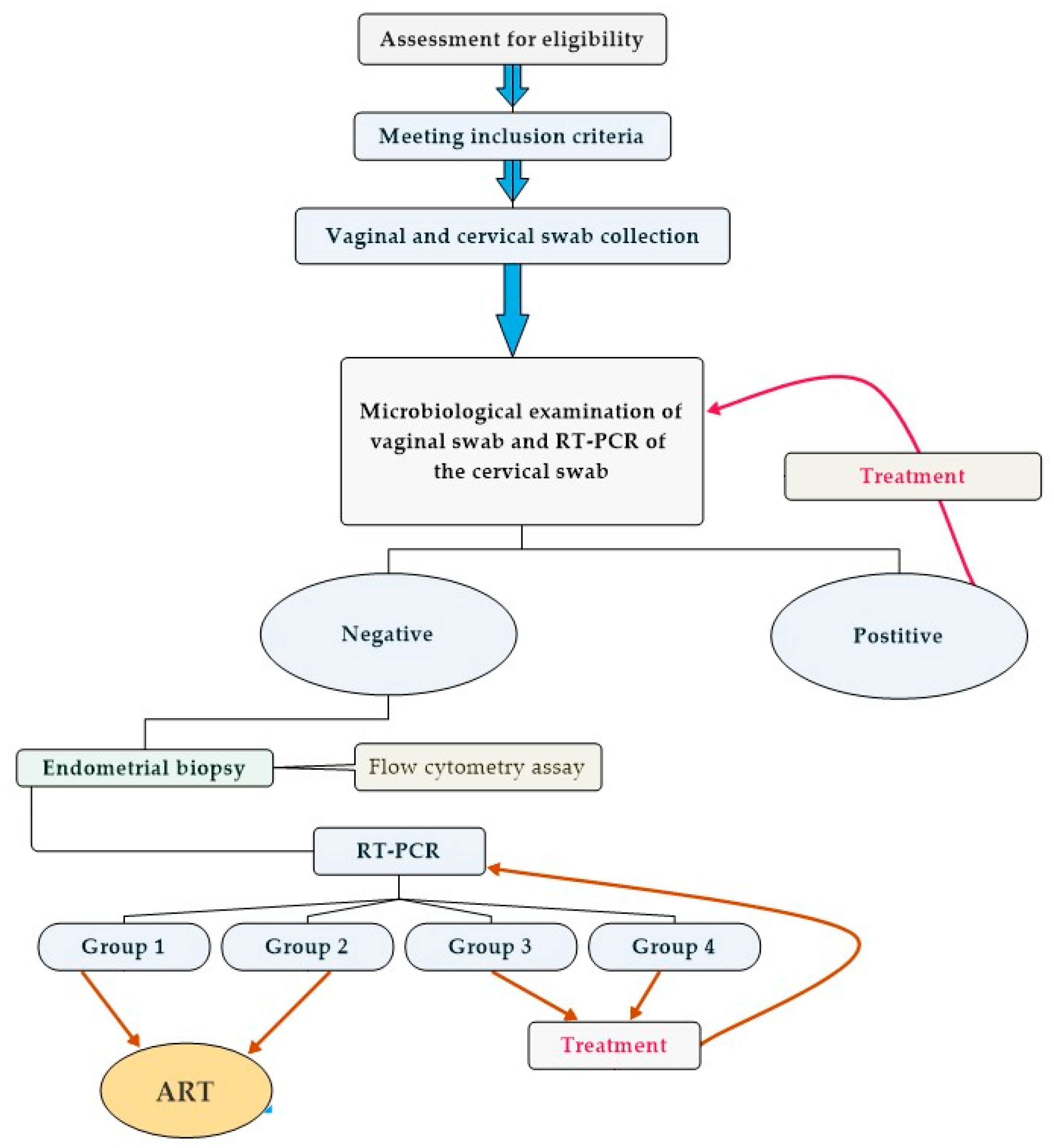
2.1. Study Design and Participant Recruitment
2.2. Ethical Approval
2.3. Sample Collection Procedure
2.4. Sample Storage Protocol
2.5. DNA Extraction
2.6. Sample Analysis
2.7. Analysis of Endometrial Immune Cells
- Tube 1: CD3 FITC/CD16+56 PE/CD45 Per CP
- Tube 2: CD34 FITC/CD56 PE/CD45 Per CP
- Tube 3: CD138 FITC/CD45 Per CP
- -
- Leucocytes (CD45+), presented as a percentage relative to all endometrial cells (leucocytes, stromal cells, epithelial cells). By targeting CD45, we aimed to identify and quantify the total leukocyte population within the endometrium, providing an overview of the overall immune cell infiltration and composition;
- -
- Lymphocytes, macrophages, and neutrophils (CD45+, CD16+), presented as a percentage by gate and relative to all leukocytes;
- -
- Uterine/decidual-type uterine natural killer cells (uNK, CD3-, CD16-, CD56 bright), progenitor natural killer cells (CD34+ uNK cells, CD34+, CD16-, CD56+), and T cells (CD3+, CD16-, CD56-), presented as a percentage relative to all lymphocytes. CD56 and CD16 are markers commonly found on natural killer (NK) cells. NK cells play a crucial role in immune surveillance and regulation at the maternal–fetal interface. By targeting CD3, we aimed to quantify the population of T cells infiltrating the endometrium, as aberrations in T-cell subsets have been implicated in the pathogenesis of RIF and RPL;
- -
- Plasma cells (CD45+, CD138+), expressed as a percentage of total leukocytes and relative to total cells (leukocytes and stromal cells). CD138, also known as syndecan-1, is a cell marker specific for plasma cells, which are often associated with chronic inflammatory processes.
2.8. Statistical Analysis
3. Results
3.1. Pre-Treatment Pathogen Analysis Results
3.2. Group Classification Based on Molecular Genetic Testing
- Group 1 includes women (n = 41) with a normal endometrial microbiota characterized by more than 90% Lactobacillus spp. presence and the absence of dysbiotic bacteria;
- Group 2 includes women (n = 57) whose biopsies exhibited a low bacterial mass, with the absence of both Lactobacillus spp. and dysbiotic bacteria;
- Group 3 includes women (n = 25) with a disturbed endometrial microbiota, marked by less than 90% Lactobacillus spp. presence and more than 10% dysbiotic bacteria.
- Group 4 includes women (n = 77) with severely disturbed microbiota, characterized by the absence of Lactobacillus spp. and more than 10% dysbiotic bacteria.
3.3. Results of Endometrial Microbiota Examination
3.4. Results of Endometrial Immune Cells
3.5. Results of Follow-Up of the Treatment Effect
4. Discussion
5. Limitations
6. Implication for Clinical Practice
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the human microbiome. Nutr. Rev. 2012, 70 (Suppl. S1), S38–S44. [Google Scholar] [CrossRef]
- Group, N.H.W.; Peterson, J.; Garges, S.; Giovanni, M.; McInnes, P.; Wang, L.; Schloss, J.A.; Bonazzi, V.; McEwen, J.E.; Wetterstrand, K.A.; et al. The NIH Human Microbiome Project. Genome Res. 2009, 19, 2317–2323. [Google Scholar] [CrossRef]
- Baker, J.M.; Chase, D.M.; Herbst-Kralovetz, M.M. Uterine Microbiota: Residents, Tourists, or Invaders? Front. Immunol. 2018, 9, 208. [Google Scholar] [CrossRef]
- Franasiak, J.; Werner, M.; Juneau, C.; Tao, X.; Landis, J.; Zhan, Y.; Treff, N.; Scott, R. Endometrial microbiome at the time of embryo transfer: Next-generation sequencing of the 16S ribosomal subunit. J. Assist. Reprod. Genet. 2016, 33, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Benner, M.; Ferwerda, G.; Joosten, I.; Van der Molen, R.G. How uterine microbiota might be responsible for a receptive, fertile endometrium. Hum. Reprod. Update 2018, 24, 393–415. [Google Scholar] [CrossRef] [PubMed]
- Svenstrup, H.F.; Fedder, J.; Abraham-Peskir, J.; Birkelund, S.; Christiansen, G. Mycoplasma genitalium attaches to human spermatozoa. Hum. Reprod. 2003, 18, 2103–2109. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.; Hutchinson, A.P.; Lekovich, J.P.; Hobeika, E.; Elias, R.T. Antibiotic Prophylaxis for Gynecologic Procedures prior to and during the Utilization of Assisted Reproductive Technologies: A Systematic Review. J. Pathog. 2016, 2016, 4698314. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.M.; Haick, A.; Nkwopara, E.; Garcia, R.; Rendi, M.; Agnew, K.; Fredricks, D.N.; Eschenbach, D. Colonization of the upper genital tract by vaginal bacterial species in nonpregnant women. Am. J. Obs. Gynecol. 2015, 212, 611.e1–611.e9. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Codoñer, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; Jimenez-Almazán, J.; Alonso, R.; Alamá, P.; Remohí, J.; Pellicer, A. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703. [Google Scholar] [CrossRef] [PubMed]
- Verstraelen, H.; Vilchez-Vargas, R.; Desimpel, F.; Jauregui, R.; Vankeirsbilck, N.; Weyers, S.; Verhelst, R.; De Sutter, P.; Pieper, D.H.; Van De Wiele, T. Characterisation of the human uterine microbiome in non-pregnant women through deep sequencing of the V1-2 region of the 16S rRNA gene. PeerJ 2016, 4, e1602. [Google Scholar] [CrossRef]
- Tao, X.; Franasiak, J.M.; Zhan, Y.; Scott III, R.T.; Rajchel, J.; Bedard, J.; Newby, R., Jr.; Scott, R.T.; Treff, N.R.; Chu, T. Characterizing the endometrial microbiome by analyzing the ultra-low bacteria from embryo transfer catheter tips in IVF cycles: Next generation sequencing (NGS) analysis of the 16S ribosomal gene. Hum. Microbiome J. 2017, 3, 15–21. [Google Scholar] [CrossRef]
- Kyono, K.; Hashimoto, T.; Nagai, Y.; Sakuraba, Y. Analysis of endometrial microbiota by 16S ribosomal RNA gene sequencing among infertile patients: A single-center pilot study. Reprod. Med. Biol. 2018, 17, 297–306. [Google Scholar] [CrossRef]
- Carosso, A.; Revelli, A.; Gennarelli, G.; Canosa, S.; Cosma, S.; Borella, F.; Tancredi, A.; Paschero, C.; Boatti, L.; Zanotto, E.; et al. Controlled ovarian stimulation and progesterone supplementation affect vaginal and endometrial microbiota in IVF cycles: A pilot study. J. Assist. Reprod. Genet. 2020, 37, 2315–2326. [Google Scholar] [CrossRef]
- Moreno, I.; Garcia-Grau, I.; Perez-Villaroya, D.; Gonzalez-Monfort, M.; Bau, D.; Gomez, C.; Valbuena, D.; Vilella, F.; Simon, C. O-126 Endometrial microbiota composition is associated with reproductive outcome in infertile patients. Hum. Reprod. 2021, 36, deab126.051. [Google Scholar] [CrossRef]
- Chen, C.; Song, X.; Wei, W.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017, 8, 875. [Google Scholar] [CrossRef]
- Li, F.; Chen, C.; Wei, W.; Wang, Z.; Dai, J.; Hao, L.; Song, L.; Zhang, X.; Zeng, L.; Du, H.; et al. The metagenome of the female upper reproductive tract. Gigascience 2018, 7, giy107. [Google Scholar] [CrossRef]
- Winters, A.D.; Romero, R.; Gervasi, M.T.; Gomez-Lopez, N.; Tran, M.R.; Garcia-Flores, V.; Pacora, P.; Jung, E.; Hassan, S.S.; Hsu, C.-D.; et al. Does the endometrial cavity have a molecular microbial signature? Sci. Rep. 2019, 9, 9905. [Google Scholar] [CrossRef] [PubMed]
- Leoni, C.; Ceci, O.; Manzari, C.; Fosso, B.; Volpicella, M.; Ferrari, A.; Fiorella, P.; Pesole, G.; Cicinelli, E.; Ceci, L.R. Human Endometrial Microbiota at Term of Normal Pregnancies. Genes 2019, 10, 971. [Google Scholar] [CrossRef] [PubMed]
- Sola-Leyva, A.; Andres-Leon, E.; Molina, N.M.; Terron-Camero, L.C.; Plaza-Diaz, J.; Saez-Lara, M.J.; Gonzalvo, M.C.; Sanchez, R.; Ruiz, S.; Martinez, L.; et al. Mapping the entire functionally active endometrial microbiota. Hum. Reprod. 2021, 36, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Riganelli, L.; Iebba, V.; Piccioni, M.; Illuminati, I.; Bonfiglio, G.; Neroni, B.; Calvo, L.; Gagliardi, A.; Levrero, M.; Merlino, L.; et al. Structural Variations of Vaginal and Endometrial Microbiota: Hints on Female Infertility. Front. Cell. Infect. Microbiol. 2020, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.; Moreno, I.; Simon, C. Bacterial vaginosis and its association with infertility, endometritis, and pelvic inflammatory disease. Am. J. Obs. Gynecol. 2021, 224, 251–257. [Google Scholar] [CrossRef]
- Hernandes, C.; Silveira, P.; Rodrigues Sereia, A.F.; Christoff, A.P.; Mendes, H.; Valter de Oliveira, L.F.; Podgaec, S. Microbiome Profile of Deep Endometriosis Patients: Comparison of Vaginal Fluid, Endometrium and Lesion. Diagnostics 2020, 10, 163. [Google Scholar] [CrossRef]
- Lozano, F.M.; Bernabeu, A.; Lledo, B.; Morales, R.; Diaz, M.; Aranda, F.I.; Llacer, J.; Bernabeu, R. Characterization of the vaginal and endometrial microbiome in patients with chronic endometritis. Eur. J. Obs. Gynecol. Reprod. Biol. 2021, 263, 25–32. [Google Scholar] [CrossRef]
- Kitaya, K.; Nagai, Y.; Arai, W.; Sakuraba, Y.; Ishikawa, T. Characterization of Microbiota in Endometrial Fluid and Vaginal Secretions in Infertile Women with Repeated Implantation Failure. Mediat. Inflamm. 2019, 2019, 4893437. [Google Scholar] [CrossRef]
- Diaz-Martínez, M.d.C.; Bernabeu, A.; Lledó, B.; Carratalá-Munuera, C.; Quesada, J.A.; Lozano, F.M.; Ruiz, V.; Morales, R.; Llácer, J.; Ten, J. Impact of the vaginal and endometrial microbiome pattern on assisted reproduction outcomes. J. Clin. Med. 2021, 10, 4063. [Google Scholar] [CrossRef]
- Amabebe, E.; Anumba, D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef] [PubMed]
- Carson, D.D.; DeSouza, M.M.; Kardon, R.; Zhou, X.; Lagow, E.; Julian, J. Mucin expression and function in the female reproductive tract. Hum. Reprod. Update 1998, 4, 459–464. [Google Scholar] [CrossRef]
- Kitaya, K.; Tada, Y.; Hayashi, T.; Taguchi, S.; Funabiki, M.; Nakamura, Y. Comprehensive endometrial immunoglobulin subclass analysis in infertile women suffering from repeated implantation failure with or without chronic endometritis. Am. J. Reprod. Immunol. 2014, 72, 386–391. [Google Scholar] [CrossRef]
- Randis, T.M.; Gelber, S.E.; Hooven, T.A.; Abellar, R.G.; Akabas, L.H.; Lewis, E.L.; Walker, L.B.; Byland, L.M.; Nizet, V.; Ratner, A.J. Group B Streptococcus β-hemolysin/cytolysin breaches maternal-fetal barriers to cause preterm birth and intrauterine fetal demise in vivo. J. Infect. Dis. 2014, 210, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Pruski, P.; Correia, G.D.; Lewis, H.V.; Capuccini, K.; Inglese, P.; Chan, D.; Brown, R.G.; Kindinger, L.; Lee, Y.S.; Smith, A. Direct on-swab metabolic profiling of vaginal microbiome host interactions during pregnancy and preterm birth. Nat. Commun. 2021, 12, 5967. [Google Scholar] [CrossRef] [PubMed]
- Suff, N.; Karda, R.; Diaz, J.A.; Ng, J.; Baruteau, J.; Perocheau, D.; Tangney, M.; Taylor, P.W.; Peebles, D.; Buckley, S.M. Ascending vaginal infection using bioluminescent bacteria evokes intrauterine inflammation, preterm birth, and neonatal brain injury in pregnant mice. Am. J. Pathol. 2018, 188, 2164–2176. [Google Scholar] [CrossRef]
- Giusto, K.; Patki, M.; Koya, J.; Ashby, C.R., Jr.; Munnangi, S.; Patel, K.; Reznik, S.E. A vaginal nanoformulation of a SphK inhibitor attenuates lipopolysaccharide-induced preterm birth in mice. Nanomedicine 2019, 14, 2835–2851. [Google Scholar] [CrossRef]
- Yudin, M.H.; Landers, D.V.; Meyn, L.; Hillier, S.L. Clinical and cervical cytokine response to treatment with oral or vaginal metronidazole for bacterial vaginosis during pregnancy: A randomized trial. Obstet. Gynecol. 2003, 102, 527–534. [Google Scholar]
- Armstrong, E.; Hemmerling, A.; Miller, S.; Burke, K.E.; Newmann, S.J.; Morris, S.R.; Reno, H.; Huibner, S.; Kulikova, M.; Liu, R. Metronidazole treatment rapidly reduces genital inflammation through effects on bacterial vaginosis–associated bacteria rather than lactobacilli. J. Clin. Investig. 2022, 132, e152930. [Google Scholar] [CrossRef]
- Byrne, E.H.; Doherty, K.E.; Bowman, B.A.; Yamamoto, H.S.; Soumillon, M.; Padavattan, N.; Ismail, N.; Moodley, A.; Sabatini, M.E.; Ghebremichael, M.S. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 2015, 42, 965–976. [Google Scholar]
- Campisciano, G.; Gheit, T.; De Seta, F.; Cason, C.; Zanotta, N.; Delbue, S.; Ricci, G.; Ferrante, P.; Tommasino, M.; Comar, M. Oncogenic virome benefits from the different vaginal microbiome-immune axes. Microorganisms 2019, 7, 414. [Google Scholar] [CrossRef]
- Inversetti, A.; Zambella, E.; Guarano, A.; Dell’Avanzo, M.; Di Simone, N. Endometrial Microbiota and Immune Tolerance in Pregnancy. Int. J. Mol. Sci. 2023, 24, 2995. [Google Scholar] [CrossRef]
- Kuon, R.J.; Weber, M.; Heger, J.; Santillan, I.; Vomstein, K.; Bar, C.; Strowitzki, T.; Markert, U.R.; Toth, B. Uterine natural killer cells in patients with idiopathic recurrent miscarriage. Am. J. Reprod. Immunol. 2017, 78, e12721. [Google Scholar] [CrossRef] [PubMed]
- Gellersen, B.; Brosens, I.A.; Brosens, J.J. Decidualization of the human endometrium: Mechanisms, functions, and clinical perspectives. Semin. Reprod. Med. 2007, 25, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Diao, L.; Huang, C.; Li, Y.; Zeng, Y.; Kwak-Kim, J.Y.H. The role of decidual immune cells on human pregnancy. J. Reprod. Immunol. 2017, 124, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kim, C.J.; Kim, D.J.; Kang, J.H. Immune cells in the female reproductive tract. Immune Netw. 2015, 15, 16–26. [Google Scholar] [CrossRef]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- La Rocca, C.; Carbone, F.; Longobardi, S.; Matarese, G. The immunology of pregnancy: Regulatory T cells control maternal immune tolerance toward the fetus. Immunol. Lett. 2014, 162, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Marron, K.; Walsh, D.; Harrity, C. Detailed endometrial immune assessment of both normal and adverse reproductive outcome populations. J. Assist. Reprod. Genet. 2019, 36, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.W.; Norwitz, G.A.; Pavlicev, M.; Tilburgs, T.; Simon, C.; Norwitz, E.R. Endometrial Decidualization: The Primary Driver of Pregnancy Health. Int. J. Mol. Sci. 2020, 21, 4092. [Google Scholar] [CrossRef] [PubMed]
- Cicinelli, E.; Matteo, M.; Trojano, G.; Mitola, P.C.; Tinelli, R.; Vitagliano, A.; Crupano, F.M.; Lepera, A.; Miragliotta, G.; Resta, L. Chronic endometritis in patients with unexplained infertility: Prevalence and effects of antibiotic treatment on spontaneous conception. Am. J. Reprod. Immunol. 2018, 79, e12782. [Google Scholar] [CrossRef] [PubMed]
- Cicinelli, E.; De Ziegler, D.; Nicoletti, R.; Colafiglio, G.; Saliani, N.; Resta, L.; Rizzi, D.; De Vito, D. Chronic endometritis: Correlation among hysteroscopic, histologic, and bacteriologic findings in a prospective trial with 2190 consecutive office hysteroscopies. Fertil. Steril. 2008, 89, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Cicinelli, E.; Vitagliano, A.; Kumar, A.; Lasmar, R.B.; Bettocchi, S.; Haimovich, S.; Kitaya, K.; de Ziegler, D.; Simon, C.; Moreno, I. Unified diagnostic criteria for chronic endometritis at fluid hysteroscopy: Proposal and reliability evaluation through an international randomized-controlled observer study. Fertil. Steril. 2019, 112, 162–173.e2. [Google Scholar] [CrossRef] [PubMed]
- Bayer-Garner, I.B.; Korourian, S. Plasma cells in chronic endometritis are easily identified when stained with syndecan-1. Mod. Pathol. 2001, 14, 877–879. [Google Scholar] [CrossRef]
- DNA-Technology, LLC. Femoflor16_en: Product Manual. Available online: https://dna-technology.com/sites/default/files/femoflor16_en.pdf (accessed on 12 February 2024).
- Zhu, N.; Yang, X.; Liu, Q.; Chen, Y.; Wang, X.; Li, H.; Gao, H. “Iron triangle” of regulating the uterine microecology: Endometrial microbiota, immunity and endometrium. Front. Immunol. 2022, 13, 928475. [Google Scholar] [CrossRef]
- Agostinis, C.; Mangogna, A.; Bossi, F.; Ricci, G.; Kishore, U.; Bulla, R. Uterine Immunity and Microbiota: A Shifting Paradigm. Front. Immunol. 2019, 10, 2387. [Google Scholar] [CrossRef]
- Skafte-Holm, A.; Humaidan, P.; Bernabeu, A.; Lledo, B.; Jensen, J.S.; Haahr, T. The Association between Vaginal Dysbiosis and Reproductive Outcomes in Sub-Fertile Women Undergoing IVF-Treatment: A Systematic PRISMA Review and Meta-Analysis. Pathogens 2021, 10, 295. [Google Scholar] [CrossRef]
- Espinos, J.J.; Fabregues, F.; Fontes, J.; Garcia-Velasco, J.A.; Llacer, J.; Requena, A.; Checa, M.A.; Bellver, J.; Spanish Infertility, S.G. Impact of chronic endometritis in infertility: A SWOT analysis. Reprod. Biomed. Online 2021, 42, 939–951. [Google Scholar] [CrossRef]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef]
- Rautava, S.; Collado, M.C.; Salminen, S.; Isolauri, E. Probiotics modulate host-microbe interaction in the placenta and fetal gut: A randomized, double-blind, placebo-controlled trial. Neonatology 2012, 102, 178–184. [Google Scholar] [CrossRef]
- Delgado-Diaz, D.J.; Tyssen, D.; Hayward, J.A.; Gugasyan, R.; Hearps, A.C.; Tachedjian, G. Distinct Immune Responses Elicited from Cervicovaginal Epithelial Cells by Lactic Acid and Short Chain Fatty Acids Associated with Optimal and Non-Optimal Vaginal Microbiota. Front. Cell. Infect. Microbiol. 2020, 9, 446. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, Z.E.; Łaniewski, P.; Thomas, N.; Roe, D.J.; Chase, D.M.; Herbst-Kralovetz, M.M. Deciphering the complex interplay between microbiota, HPV, inflammation and cancer through cervicovaginal metabolic profiling. eBioMedicine 2019, 44, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Fuhler, G.M. The immune system and microbiome in pregnancy. Best Pract. Res. Clin. Gastroenterol. 2020, 44–45, 101671. [Google Scholar] [CrossRef]
- Silvia Ventimiglia, M.; Jimena Valeff, N.; Pozo Alban, M.; Manuel Paturlanne, J.; Juriol, L.; Quadrana, F.; Cecotti, M.; Malamud, M.; Javier Dibo, M.; de Los Angeles Serradell, M.; et al. Probiotic Lactobacillus kefiri prevents endotoxin-induced preterm birth and stillbirth in mice. Reproduction 2021, 161, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Vacca, P.; Cantoni, C.; Vitale, M.; Prato, C.; Canegallo, F.; Fenoglio, D.; Ragni, N.; Moretta, L.; Mingari, M.C. Crosstalk between decidual NK and CD14+ myelomonocytic cells results in induction of Tregs and immunosuppression. Proc. Natl. Acad. Sci. USA 2010, 107, 11918–11923. [Google Scholar] [CrossRef]
- D’Ippolito, S.; Di Nicuolo, F.; Pontecorvi, A.; Gratta, M.; Scambia, G.; Di Simone, N. Endometrial microbes and microbiome: Recent insights on the inflammatory and immune “players” of the human endometrium. Am. J. Reprod. Immunol. 2018, 80, e13065. [Google Scholar] [CrossRef] [PubMed]
- Annunziato, F. Go with the immunological flow—Guidelines for flow cytometry. Eur. J. Immunol. 2017, 47, 1578. [Google Scholar] [CrossRef]
- Vacca, P.; Vitale, C.; Montaldo, E.; Conte, R.; Cantoni, C.; Fulcheri, E.; Darretta, V.; Moretta, L.; Mingari, M.C. CD34+ hematopoietic precursors are present in human decidua and differentiate into natural killer cells upon interaction with stromal cells. Proc. Natl. Acad. Sci. USA 2011, 108, 2402–2407. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.J.; Chakraborty, D.; Kubota, K.; Renaud, S.J.; Rumi, M.A. Adaptive mechanisms controlling uterine spiral artery remodeling during the establishment of pregnancy. Int. J. Dev. Biol. 2014, 58, 247–259. [Google Scholar] [CrossRef]
- Matteo, M.; Cicinelli, E.; Greco, P.; Massenzio, F.; Baldini, D.; Falagario, T.; Rosenberg, P.; Castellana, L.; Specchia, G.; Liso, A. Abnormal pattern of lymphocyte subpopulations in the endometrium of infertile women with chronic endometritis. Am. J. Reprod. Immunol. 2009, 61, 322–329. [Google Scholar] [CrossRef]
- Kitaya, K.; Takeuchi, T.; Mizuta, S.; Matsubayashi, H.; Ishikawa, T. Endometritis: New time, new concepts. Fertil. Steril. 2018, 110, 344–350. [Google Scholar] [CrossRef]
- Gunther, V.; von Otte, S.; Maass, N.; Alkatout, I. Endometrial “Scratching” An update and overview of current research. J. Turk. Ger. Gynecol. Assoc. 2020, 21, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Gnainsky, Y.; Granot, I.; Aldo, P.; Barash, A.; Or, Y.; Mor, G.; Dekel, N. Biopsy-induced inflammatory conditions improve endometrial receptivity: The mechanism of action. Reproduction 2015, 149, 75–85. [Google Scholar] [CrossRef] [PubMed]
| Endometrial Microbiota Type Number of Biopsies (%) | |||||
|---|---|---|---|---|---|
| Microbiota Profile | Normal EM | Low Biomass | Disturbed EM | Severely Disturbed EM | Total |
| 41 (20.5) | 57 (28.5) | 25 (12.5) | 77 (38.5) | 200 (100) | |
| Aerobic microorganisms | - | - | 3 (1.5) | 3 (1.5) | 6 (3) |
| Anaerobic microorganisms | - | - | 3 (1.5) | 15 (6.5) | 18 (9.0) |
| Aerobic and anaerobic microorganisms | - | - | 2 (1.0) | 2 (1.0) | 4 (2.0) |
| Candida spp. | 4 (2.0) | 4 (2.0) | 1 (0.5) | 6 (3.0) | 15 (7.5) |
| Aerobic microorganisms and Candida spp. | - | - | 1 (0.5) | 11 (5.5) | 12 (6.0) |
| Anaerobic and Candida spp. | - | - | 7 (3.5) | 26 (13.0) | 33 (16.5) |
| Aerobic, anaerobic microorganisms, and Candida spp. | - | - | 10 (3.7) | 16 (8.0) | 26 (13.0) |
| Aerobic Microorganisms (n) | Anaerobic Microorganisms (n) | Aerobic and anaerobic Microorganisms (n) | Candida spp. (n) | Aerobic Microorganisms and Candida spp. (n) | Anaerobic and Candida spp. (n) | Aerobic, Anaerobic Microorganisms, and Candida spp. (n) | Delivery (n) | Ongoing Pregnancy (n) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| RIF (n = 107) | Normal EM (n = 22) | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 9 | 2 |
| Low biomass (n = 29) | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 4 | 3 | |
| Disturbed EM (n = 15) | 1 | 3 | 0 | 1 | 1 | 5 | 4 | 5 | 1 | |
| Severely disturbed EM (n = 41) | 2 | 7 | 2 | 1 | 5 | 17 | 7 | 9 | 7 | |
| Total RIF | 3 | 10 | 2 | 10 | 6 | 22 | 11 | 27 | 13 | |
| RPL (n = 93) | Normal EM (n = 19) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 1 |
| Low biomass (n = 28) | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 8 | 5 | |
| Disturbed EM (n = 10) | 2 | 0 | 0 | 0 | 0 | 2 | 6 | 2 | 1 | |
| Severely disturbed EM (n = 36) | 1 | 8 | 2 | 1 | 6 | 9 | 9 | 9 | 4 | |
| Total RPL | 3 | 8 | 2 | 5 | 6 | 11 | 15 | 29 | 11 |
| RIF | RPL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal EM n = 22 | Low Biomass n = 29 | Disturbed EM n = 15 | Severely Disturbed EM n = 41 | Total RIF n = 107 | Normal EM n = 19 | Low Biomass n = 28 | Disturbed EM n = 10 | Severely Disturbed EM n = 36 | Total RPL n = 93 | |
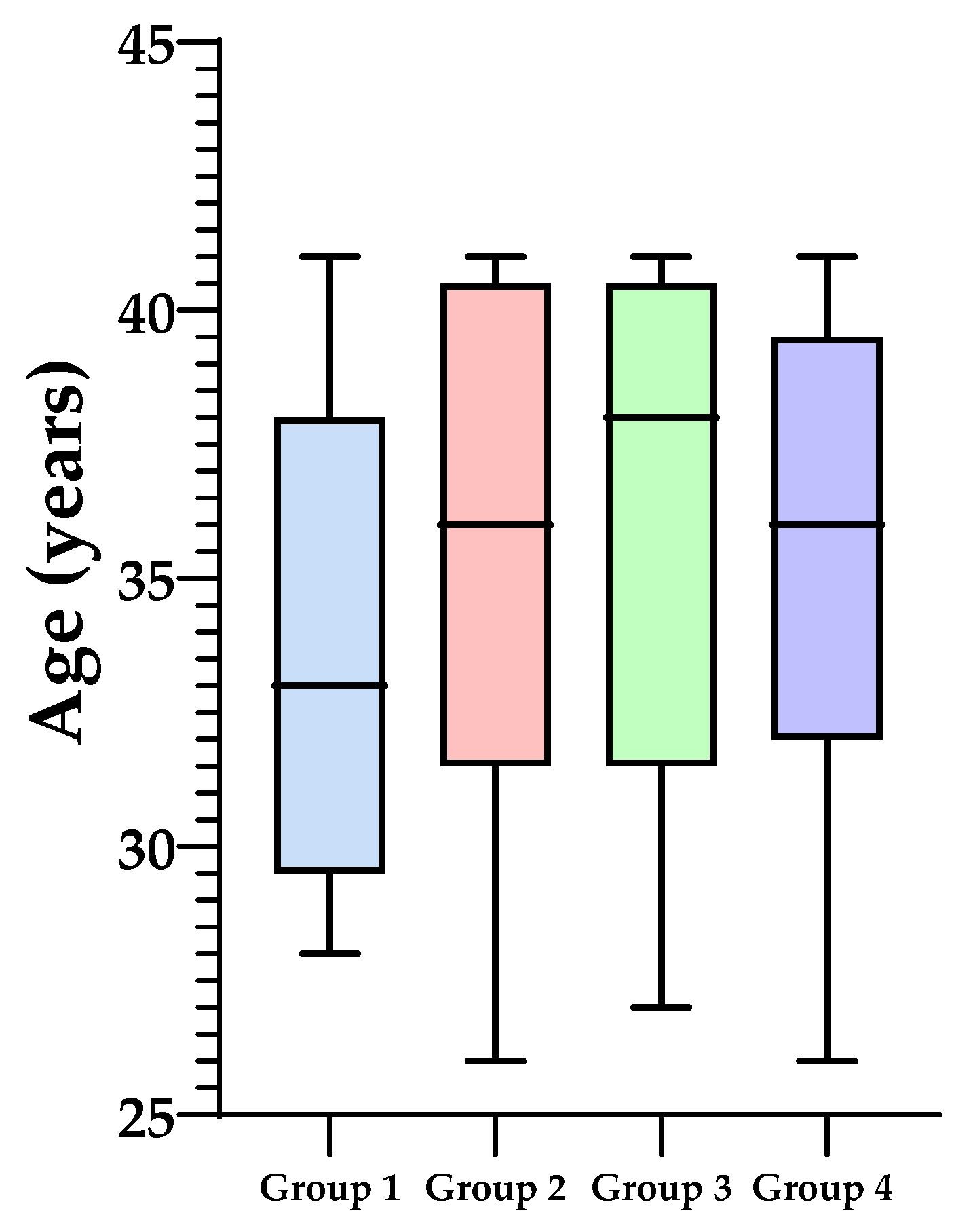
| Age (years) | 35.86 ± 4.27 | 35.17 ± 4.88 | 37.07 ± 4.717 | 36.49 ± 3.99 | 36.08 ± 4.39 | 32.21 ± 3.9 | 35.46 ± 4.78 | 33.5 ± 4.58 | 34.78 ± 4.41 | 34.32 ± 4.54 |
| Leucocytes (%) | 17.71 ± 10.98 | 25.09 ± 15.73 | 23.12 ± 16.33 | 25.56 ± 15.29 | 18.26 ± 12.88 | 19.34 ± 14.99 | 22.92 ± 13.81 | 12.13 ± 9.72 | 16.62 ± 11.04 | 23.55 ± 14.85 |
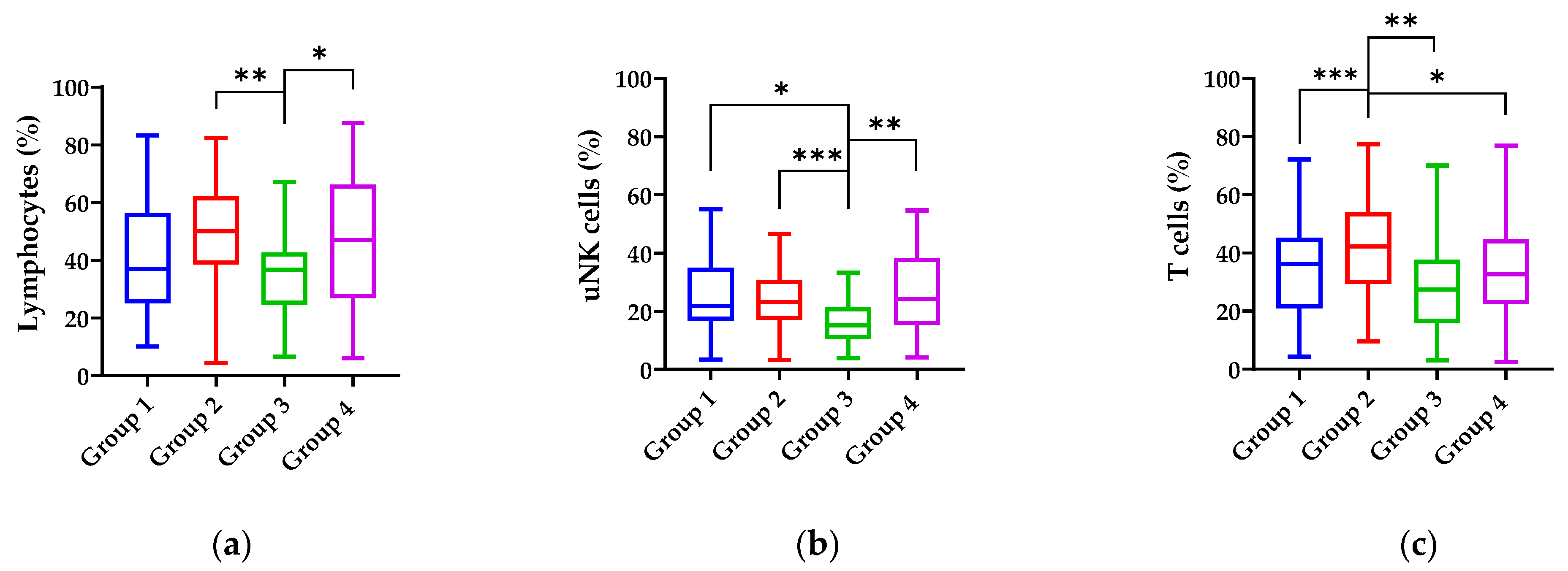
| Lymphocytes (%) | 43.35 ± 19.64 | 50.77± 18.47 | 33.68 ± 11.42 | 44.61 ± 24.75 | 44.49 ± 21.04 | 38.94 ± 20.14 | 45.96 ± 18.77 | 38.46 ± 24.79 | 46.96 ± 21.76 | 44.11 ± 20.89 |
| Macrophages (%) | 31.60± 19.67 | 31.22 ± 18.86 | 36.32 ± 22.01 | 32.74 ± 21.96 | 32.6 ± 20.48 | 42.61 ± 27.17 | 26.72 ± 14.5 | 29.59 ± 24.63 | 27.89 ± 17.72 | 30.72 ± 20.55 |
| Neutrophils (%) | 21.14 ± 9.74 | 17.78 ± 2.45 | 29.75 ±15.98 | 22.02 ± 19.84 | 21.77 ± 17.72 | 17.30 ± 16.73 | 25.14 ± 18.69 | 31.12 ± 27.22 | 24.58 ± 19.72 | 23.97 ± 19.82 |
| T cells (%) | 34.28 ± 14.85 | 44.63 ± 15.2 | 26.86 ± 15.95 | 35.03 ± 18.09 | 36.33 ± 17.17 | 34.69 ± 18.54 | 40.27 ± 16.98 | 33.41 ± 20.39 | 34.18 ± 17.46 | 36.03 ± 17.79 |
| uNK cells (%) | 27.93 ± 12.44 | 23.93 ± 10.69 | 16.87 ± 9.81 | 26.78 ± 13.99 | 24.85 ± 12.65 | 23.08 ± 12.04 | 25.44 ± 13.74 | 17 ± 6.19 | 28.29 ± 15.98 | 25.15 ± 14.02 |
| CD34+ uNK cells (%) | 3.63 ± 4.14 | 2.41 ± 3.23 | 1.22 ± 2.18 | 2.1 ± 2.49 | 2.37 ± 3.11 | 2.44 ± 3.52 | 2.46 ± 3.51 | 2.07 ± 1.59 | 1.98 ± 2.35 | 2.23 ± 2.91 |
| Plasma cells * (%) | 2.70 ± 4.03 | 3.46 ± 6.48 | 6.31 ± 10.57 | 2.26 ± 5.38 | 3.24 ± 6.46 | 2.2 ± 2.44 | 1.18 ± 1.52 | 2.18 ± 5.39 | 2.12 ± 2.9 | 1.86 ± 2.84 |
| Plasma cells ** (%) | 0.64 ± 1.2 | 0.59 ± 1.19 | 0.92 ± 2.07 | 0.56 ± 1.93 | 0.64 ± 1.62 | 0.39 ± 0.51 | 0.29 ± 0.54 | 0.8 ± 2.04 | 0.53± 0.77 | 0.46 ± 0.89 |
| Patients | Negative hCG Test | Pregnancy Loss | Ongoing Pregnancy | Delivery | Cumulative Birth Rate |
|---|---|---|---|---|---|
| Normal EM n = 41 | 18 | 1 | 3 | 19 | 46.34% |
| Low biomass n = 57 | 29 | 8 | 8 | 12 | 21.05% |
| Disturbed EM n = 25 | 13 | 3 | 2 | 7 | 28.0% |
| Severely disturbed EM n = 77 | 41 | 7 | 11 | 18 | 23.37% |
| Total n = 200 | 101 | 19 | 24 | 56 | 28.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blazheva, S.; Pachkova, S.; Bodurska, T.; Ivanov, P.; Blazhev, A.; Lukanov, T.; Konova, E. Unlocking the Uterine Code: Microbiota, Immune Cells, and Therapy for Recurrent Reproductive Failure. Microorganisms 2024, 12, 547. https://doi.org/10.3390/microorganisms12030547
Blazheva S, Pachkova S, Bodurska T, Ivanov P, Blazhev A, Lukanov T, Konova E. Unlocking the Uterine Code: Microbiota, Immune Cells, and Therapy for Recurrent Reproductive Failure. Microorganisms. 2024; 12(3):547. https://doi.org/10.3390/microorganisms12030547
Chicago/Turabian StyleBlazheva, Svetla, Svetlana Pachkova, Tatyana Bodurska, Petar Ivanov, Alexander Blazhev, Tzvetan Lukanov, and Emiliana Konova. 2024. "Unlocking the Uterine Code: Microbiota, Immune Cells, and Therapy for Recurrent Reproductive Failure" Microorganisms 12, no. 3: 547. https://doi.org/10.3390/microorganisms12030547
APA StyleBlazheva, S., Pachkova, S., Bodurska, T., Ivanov, P., Blazhev, A., Lukanov, T., & Konova, E. (2024). Unlocking the Uterine Code: Microbiota, Immune Cells, and Therapy for Recurrent Reproductive Failure. Microorganisms, 12(3), 547. https://doi.org/10.3390/microorganisms12030547








