Abstract
Gram-negative bacterial endotoxins can cause pathophysiological effects such as high fever when introduced into the bloodstream. Therefore, endotoxin testing is necessary when producing injectable pharmaceuticals. The pharmaceutical industry has widely used Limulus amebocyte lysate (LAL) to certify product quality. However, ethical concerns have been raised and the increasing scarcity of Limulus polyphemus necessitates the development of novel testing techniques. Recombinant factor C (rFC) was developed using genetic engineering techniques. The aim of this study was to investigate the validity of rFC testing and compare it with the LAL method. The specificity, linearity, accuracy, precision, and robustness of the rFC assay were evaluated. After validation, the rFC assay was found to be suitable for endotoxin detection. We compared the accuracy of the rFC and LAL assays using reference standard endotoxin. The rFC assay was as accurate as the LAL assay. We also compared the two assays using biopharmaceuticals. Greater interference occurred in some samples when the rFC assay was used than when the LAL assay was used. However, the rFC assay overcame the interference when the samples were diluted. Overall, we suggest that rFC can be applied to test biopharmaceuticals.
1. Introduction
Endotoxins (e.g., lipopolysaccharide, LPS) are present in the outer membrane of all Gram-negative bacteria and can cause toxic effects such as fever, septic shock, and multiorgan failure by producing proinflammatory cytokines and activating the coagulation cascade [1]. Therefore, elimination of endotoxins in final pharmaceutical products is an important challenge for manufacturers [2]. The Limulus amebocyte lysate (LAL) test is commonly used to detect endotoxins. The underlying mechanism of this method is based on the defense system of horseshoe crab hemocytes. When exposed to endotoxins, factor C is activated, and the coagulation cascade is initiated. Eventually, a coagulin gel is formed that traps the invading bacteria [3,4]. However, LAL contains many other proteins; thus, the coagulation cascade can be triggered by glucan, which is not pyrogenic, thereby producing false-positive endotoxin results [5,6].
Major issues with the LAL method related to ethical concerns and limited supplies have been raised. For example, the process of bleeding horseshoe crabs is associated with crab mortality rates of up to 30% [7]. To address these challenges, a recombinant factor C (rFC) assay was developed as an alternative to LAL. The cDNA sequence of factor C was cloned from the mangrove horseshoe crab (Carcinoscorpius rotundicauda) and expressed in several hosts by Ding et al. in 1997 [8,9,10]. In the rFC assay, the synthetic rFC molecule is activated by an endotoxin, and amino-methylcoumarin, a fluorogenic substrate, is then cleaved to yield a fluorogenic compound [11,12,13]. Because rFC is biotechnologically engineered, the rFC-based assay has high purity, endotoxin specificity, and low lot-to-lot variation [14,15]. In addition, the rFC assay does not contain the factor G protein; thus, it cannot lead to false positives or result in enhancement of β-glucan [16]. Recombinant proteins can be easily and sustainably produced in unlimited amounts without using animals [17]. In recent years, many studies on validation of the rFC assay and comparability of the LAL and rFC assays have been conducted [14,15,16,17].
rFC assays are now available from many manufacturers and suppliers. rFC assay was listed as a compendial method in European Pharmacopoeias [18]. Considering the need to examine whether rFC assay could be used as an alternative, in this study, we validated the rFC assay in compliance with the International Council on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use guidelines and Bioanalytical Method Validation Guidance or Industry issued by the Food and Drug Administration. We also compared the LAL and rFC assays using reference standard endotoxin (RSE) and biopharmaceuticals to determine the applicability of the rFC assay.
2. Materials and Methods
2.1. Reagents
LAL reagent water (W110), Control Standard Endotoxin (CSE) 10 ng/vial (E120), Kinetic Turbidimetric LAL 5.2 mL (R19000), Kinetic Chromogenic LAL 3.2 mL (R17100), and 96-well microplates (Falcon 353072, Becton Dickinson, Franklin Lakes, NJ, USA) were all purchased from Charles River Laboratories (Wilmington, MA, USA) and used for the LAL test. Endozyme II (890030), Endozyme II GO (890031), and Endolisa (609033), all purchased from bioMérieux (Marcy-l’Étoile, France), and PyroGene (50-658U) purchased from Lonza (Basel, Switzerland), were used for the rFC test. We used 96-well microplates provided with each kit from bioMérieux and a 96-well flat clear-bottomed black polystyrene microplate (3603, Corning, New York, NY, USA). All assays were performed using pyrogen-free tips and glass tubes.
2.2. Bacterial Endotoxin Test (BET)
2.2.1. LAL Assays
The kinetic chromogenic assay (KCA) and kinetic turbidimetric assay (KTA) reagents were used for the LAL assay. The LAL assays were performed according to the manufacturer’s instructions and analyzed using an ELx808IU absorbance microplate reader (BioTek Instruments GmbH, Bad Friedrichshall, Germany) with Endoscan V software version 6.0.2 (Charles River Laboratories). The temperature of the instrument incubator was maintained at 37 °C during the measurement. Absorption was observed at 405 nm with an onset optical density of 0.1 for KCA and at 340 nm with an onset optical density of 0.05 for KTA.
2.2.2. rFC Assays
The rFC assays were performed according to the manufacturer’s instructions. Assays with rFC reagent from Biomerieux were analyzed using a BioTek Synergy HTX fluorescence reader (Biotek, Winooski, Vermont, USA) with ENDONEXT software version 1.1 (bioMérieux, Marcy-l’Étoile, France). Samples were preincubated in the plate reader at 37 °C for 5 min before adding the working reagent (a mixture of fluorogenic substrate, assay buffer, and the enzyme solution). Thereafter, the plates were measured with excitation/emission wavelengths at 380/445 nm and incubated at 37 °C during the measurement. Assays with rFC reagent from Lonza were analyzed using a BioTek FLx800 Fluorescence reader (Biotek, Winooski, Vermont, USA) with WinKQCL software version 6.0.1 (Lonza, Basel, Switzerland). The plate was preincubated in the plate reader at 37 °C for 10 min, and then the working reagent was dispensed to each well. The plate was incubated at 37 °C during the measurement, and the fluorescence was read with excitation/emission wavelengths at 380/440 nm.
2.3. Validation of rFC Assay
To detect endotoxins, an rFC-based assay was conducted using Endozyme II. Standard curves were generated by using diluted solutions prepared through repeated 1:10 dilutions of CSE stock solution (50 EU/mL) and via linear regression analysis. BET was carried out using four replicates of sample, half of which were spiked samples. CSE was added to the sample at a final concentration of 0.5 EU/mL The spiked samples were in a positive product control (PPC) group and prepared to ensure the presence of interference.
2.3.1. Linearity
Four concentrations of CSE (0.005, 0.05, 0.5, and 5 EU/mL) were prepared in duplicate for the standard curve. The acceptance criterion for linearity was a correlation coefficient (|R|) greater than 0.9800 for the standard curve.
2.3.2. Accuracy
Four endotoxin concentrations (0.05, 0.1, 0.5, and 1.0 EU/mL) were tested within the standard curve range. Accuracy was evaluated based on sample recovery (%), which should be between 50% and 200%.
2.3.3. Precision
Four endotoxin concentrations (0.05, 0.1, 0.5, and 1.0 EU/mL) were tested. Precision was assessed using the coefficient of variation (CV) obtained from repeated measurements of an assay (repeatability), analysts (intermediate precision), and laboratories (reproducibility). We assessed the CV (%) of both sample recovery and PPC recovery for precision. The acceptance criterion for precision and robustness was CV < 25%.
2.3.4. Robustness
Four endotoxin concentrations (0.05, 0.1, 0.5, and 1.0 EU/mL) were tested. Robustness was confirmed by the CV (%) obtained from the results of different reagent lots. We assessed the CV (%) of both sample recovery and PPC recovery. The acceptance criterion for precision and robustness was CV < 25%.
2.4. Comparison of LAL and rFC Test Methods
To compare the LAL and rFC assays, we used KCA and KTA reagents and Endozyme II, Endozyme II GO, Endolisa, and PyroGene kits. For each assay, a standard curve was prepared from 0.005–5 EU/mL CSE (fresh CSE for each assay) and generated using a linear regression model. RSE (10/178, NIBSC) was used as the test sample. Five RSE concentrations (1, 0.5, 0.1, 0.05, and 0.01 EU/mL) were diluted with endotoxin-free water. For each of the six types of assay, CSE was added to the PPC group so that the final endotoxin concentration of CSE was 0.5 EU/mL. Unlike the other reagents, the standard curve of Endolisa was 0.05–50 EU/mL and fitted using a 4-parameter logistic regression model. Therefore, four different RSE concentrations (5, 1, 0.5, and 0.1 EU/mL) were tested. All samples were measured in duplicate, and the means of sample recovery (%) and percentage difference were calculated for each reagent. The values for all reagents were compared using the Kruskal–Wallis H test, and then Dunn’s test was performed for the post hoc analysis. The values of the LAL and rFC assays were compared using the Wilcoxon rank-sum test.
2.5. Application of rFC Assay to Biopharmaceuticals
BET was conducted using Endozyme II, PyroGene, KTA, and KCA kits. Fourteen recombinant products, including antibody therapeutics (Ab) and recombinant protein products (P), two botulinum toxin products, and 14 vaccines, including a bacterial (BV) and viral (VV) vaccine, were used as test samples. The samples were diluted with endotoxin-free water, and the dilution factors were determined using an inhibition/enhancement test. CSE was added to the PPC group so that the final endotoxin concentration was 0.5 EU/mL. As the PPC recovery must be within 50–200%, serial dilution was performed to not exceed the maximum valid dilution (MVD). Four different testing methods were used to evaluate each biopharmaceutical type. Each test was performed once, and three sets of duplicate samples were prepared for each assay.
3. Results
3.1. Validation of rFC Assay
3.1.1. Linearity
Linearity was analyzed based on the results obtained using the four CSE concentrations. A standard curve was calculated using a regression model by fitting a linear model log(Y) = Alog(X) + B. The |R| values of assays 1, 2, and 3 were 1.000, 0.9991, and 0.9999, respectively, which met the target criterion (|R| ≥ 0.980).
3.1.2. Accuracy
Four different concentrations were prepared with three sets of duplicate samples, and three assays were conducted by a single analyst. The actual endotoxin levels in the samples were compared with the expected CSE concentrations. The sample recoveries (%) were 97.4–121.0%, which was within the acceptable range of 50–200% (Table 1).

Table 1.
Sample recovery (%) of three assays.
3.1.3. Precision (Repeatability)
One analyst performed three assays, and three sets of duplicate samples were measured in each assay. The CV (%) of the actual endotoxin concentration and PPC recovery (%) among the three sets was calculated. All CV results (%) were within 0.6–11.2%, and within the acceptance criterion of <25% (Table 2).

Table 2.
CV (%) of the actual concentration and PPC recovery from different assays.
3.1.4. Precision (Intermediate)
Two analysts each performed all three assays. Three duplicate samples were prepared for each assay. The average endotoxin concentration and PPC recovery (%) were calculated for each assay. The CV (%) values obtained by the two different analysts were within 3.6–6.1% and 2.2–14.8% for the actual endotoxin concentration and PPC recovery (%), respectively. All CV results (%) met the acceptance criterion of <25% (Table 3).

Table 3.
Results of actual concentration and PPC recovery (%) from two different analysts.
3.1.5. Precision (Reproducibility)
Three different laboratories performed all three assays. Four different concentrations of CSE were prepared in duplicate. The CV (%) of the actual concentration and PPC recovery (%) between laboratories was within 4.7–12.2% and 5.5–14.3%, respectively. All CV results (%) met the acceptance criterion of <25% (Table 4).

Table 4.
Results of actual concentration and PPC recovery (%) from three different laboratories.
3.1.6. Robustness
Robustness was assessed using the CV (%) obtained from three different reagent lots. The CV (%) of the actual endotoxin concentration and PPC recovery (%) were 5.6–10.7% and 4.7–7.9%, respectively. All CV (%) results met the acceptance criterion of <25% (Table 5).

Table 5.
Actual concentration and PPC recovery (%) from different lot numbers.
3.2. Comparison of LAL and rFC Test Methods
The rFC (Endozyme II, Endozyme II GO, Endolisa, and PyroGene) and LAL (KCA and KTA) reagents were used for equivalence testing. The rFC reagents were labeled A–D. Five different concentrations (1, 0.5, 0.1, 0.05, and 0.01 EU/mL) of RSE were used. Each sample was prepared in duplicate, and the average sample recovery (%) and percentage difference for each reagent were calculated (Table 6).

Table 6.
Sample recovery (%) and percentage difference of LAL and rFC reagents.
Only data that met the inclusion criteria were used in the statistical analysis. Figure 1 shows the data distribution and outliers. The sample recovery (%) increased in the order KCA < KTA < D < B < A < C. However, there was no significant difference between reagents B and D or A and C.
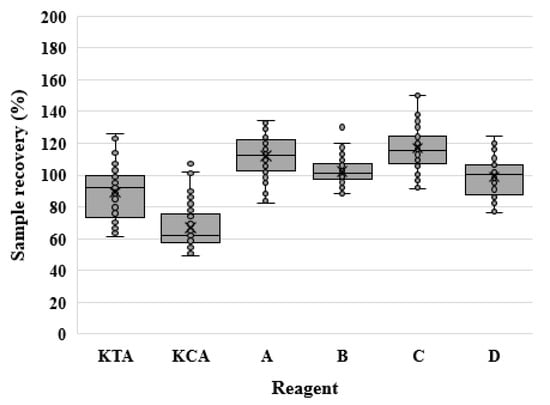
Figure 1.
Sample recovery (%) for LAL and rFC assays. The sample recovery (%) for each reagent is graphically represented using a box plot. LAL reagents contain KTA and KCA, and rFC reagents are labeled A–D.
We also analyzed the absolute value of the difference between sample recovery (%) and 100%. The percentage difference was higher in the order of B < D < A < KTA < C < KCA. When the results of the percentage difference were statistically analyzed using the Kruskal–Wallis H test, the reagents showed a significant difference (p < 0.001). Then, Dunn’s tests were performed for the post hoc analysis. No significant difference was found among KTA, A, and C; however, KCA showed significant differences compared with all rFC reagents. The mean percentage difference of the rFC assay was significantly lower (p < 0.001) than that of the LAL assay in Wilcoxon rank-sum test analysis when the reagents were grouped according to the test method (LAL and rFC) (Table 7).

Table 7.
Sample recovery (%) and percentage difference of LAL and rFC assays.
The data distribution and outliers are shown in Figure 2.
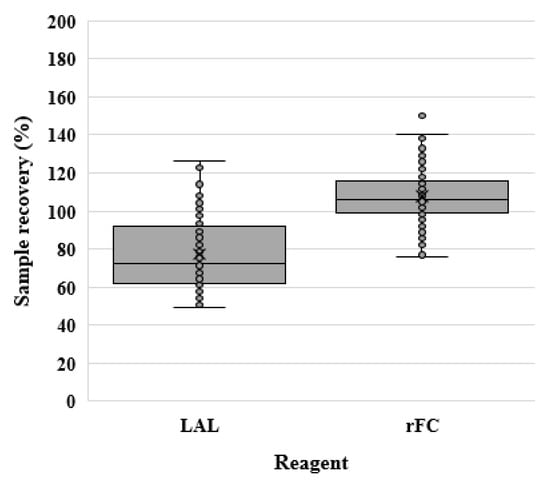
Figure 2.
Sample recovery (%) for the LAL and rFC assays. The sample recovery (%) for each reagent is graphically represented using a box plot.
3.3. Application of rFC Assay to Biopharmaceuticals
We compared the LAL and rFC test methods using biopharmaceuticals including vaccines, recombinant products, and toxin products. An inhibition/enhancement test was performed on each product to determine the appropriate dilution factor. Each diluted product was analyzed in three sets of duplicate samples. All recombinant products and toxin products had the same dilution factors regardless of the assay type, whereas some bacterial (BV4 and BV6) and viral (VV2, VV3, VV4, VV5, and VV6) vaccines needed additional dilution when using the rFC assay compared to when using the LAL assay to overcome interference. Furthermore, rFC reagents from two different manufacturers had different dilution factors for BV4, VV4, and VV5, although they were used in the same rFC-based assay. None of the biopharmaceuticals exceeded the endotoxin limits, and the average PPC recovery (%) values of the samples were within 50–200% (Table 8, Table 9 and Table 10).

Table 8.
PPC recovery (%) and endotoxin concentrations of recombinant products.

Table 9.
PPC recovery (%) and endotoxin concentrations of toxin products.

Table 10.
PPC recovery (%) and endotoxin concentrations of vaccines.
4. Discussion
Over the past five decades, horseshoe crab hemolymph has been used as a source of lysate reagents for endotoxin detection and quantification. However, this dependence on horseshoe crabs has led to limited reagent supplies owing to decreases in the horseshoe crab population [4]. Ethical issues surrounding the continued use of animals have also brought global attention to the need for alternatives to animal sources. Therefore, the rFC assay has been proposed as an alternative to LAL.
We determined the linearity, accuracy, precision, and robustness of the rFC assay through method validation. The rFC assay satisfied the acceptance criteria for all parameters. The results indicated that the rFC assay is reliable for endotoxin detection. We then compared the LAL and rFC assays using RSE and biologics. We tested five concentrations (1, 0.5, 0.1, 0.05, and 0.01 EU/mL) of RSE to investigate the accuracy of the assays. All rFC reagents were substantially different from the LAL reagents when sample recovery (%) was statistically analyzed. The sample recovery of the rFC reagents was 98.9–116.9%. The sample recoveries of all rFC reagents were closer to 100% than the sample recovery (%) of KCA. Moreover, Piehler et al. demonstrated that rFC assays are accurate, with sample recoveries of nearly 100% [17]. The percentage difference of all rFC reagents, except for reagent C, was lower than that of KTA. In addition, no significant difference was found in percentage difference between KTA and rFC reagents A and C, although the average sample recovery (%) using KTA was less than 100%, whereas those using A and C were more than 100%. Among all reagents, rFC reagent B showed the lowest percentage difference, whereas the percentage difference of KCA was the highest. Overall, our results demonstrated that the rFC test method is as reliable as the LAL method.
We also investigated the performance of the rFC assay for the endotoxin test of biopharmaceuticals and compared it with that of the LAL assay. Generally, in bacterial endotoxin test, pH, salt, detergents, chelating agents, and proteins are potential influencing agents [19]. Therefore, adjusting pH, diluting samples, or neutralizing the influencing agent is required to overcome product interference. In this study, interference was overcome by diluting the samples with endotoxin-free water within the MVD. Then, we compared the sample dilution factors and PPC recovery (%) results obtained from each reagent. Many recombinant products contain polysorbates, which may cause false-negative results [20,21]. However, our results showed that there was no severe interference with the PPC recovery (%), because all the samples, except for P2 and P4, were diluted less than 50-fold. This is probably owing to the small amount of polysorbate in the products. Moreover, the concurrent existence of a chelator and nonionic surfactant is enough to reduce the ability of endotoxin detection. However, the existence of only one of the formulation constituents does not cause a remarkable disruption in endotoxin recovery [22]. Any interferences in P2 and P4 were probably caused by components other than polysorbate, which were also overcome through further dilution. Toxin products also showed no severe interference because the samples were only diluted 10-fold. However, some vaccines contained different dilution factors for each reagent. When using rFC rather than LAL, more interference occurred in some of the samples containing aluminum hydroxide or amorphous aluminum hydroxyphosphate sulfate. Aluminum hydroxide interfered greatly with both KCA and KTA assays [23]. Polymers in metal are used as carriers in complicated pharmaceutical formulations; however, the structure and size of endotoxin can also be interfered with by aluminum salt [24]. We identified that the rFC assay is also susceptible to interference by aluminum, as the LAL assay is; however, the problem of interference may be reduced as the rFC assay can withstand more dilution than the LAL assay.
All biopharmaceuticals, including recombinant products, toxin products, and vaccines, satisfied the criteria. However, we found some samples with different dilution factors between reagents or differences in PPC recovery (%) between reagents, although an identical dilution factor was used for the samples. In the case of LAL assays, KTA is linked to the rate of turbidity, and KCA uses a synthetic chromogenic substrate. The rFC assay detects endotoxins using fluorescence. Thus, differences may occur between assays. However, there were also considerable differences in the PPC recovery (%) of some products between rFC reagents A and B, even though they used the same rFC-based assay. Specifically, P4 showed the largest difference in PPC recovery (57%) between reagents A and B. Furthermore, BV4, VV4, and VV5 showed a 100-fold difference in dilution factor when using the rFC reagents A and B. Minimum dilution factors of injectable drugs differ by at least two- to fourfold between PyroGene and EndoZyme II [16]. We assumed that the difference in the manufacturing process or combination ratio of working reagents of rFC reagents A and B induced the disparity in the results of PPC recovery (%).
In summary, we identified that the rFC is reliable for detecting endotoxins through the validation of the rFC assay and comparison with the LAL assay using RSE. Our results provide new evidence for using rFC assay in replacement of laboratory animals. Moreover, our data demonstrated that the rFC assay would be applicable to biopharmaceuticals because it met the acceptance criteria for PPC recovery (%) by diluting samples within the MVD. However, we also encountered some interference. In this study, we overcame the interference by diluting samples with water alone. However, buffers such as saline can also be used to prevent interference with the LAL assay [25]. Further studies are required to investigate other methods for reducing the dilution factor as an alternative to diluting samples with water in cases in which severe interference with the rFC assay is encountered. In conclusion, our data suggest that the rFC assay is accurate and reliable for detection of endotoxins and can be used in testing biopharmaceuticals. Also, when the rFC assay is applied to products in the biopharmaceutical industry, the validation should be adequately performed, taking the components of the product into consideration.
Author Contributions
Conceptualization, K.M.L., K.E.Y. and S.E.; methodology, D.H.K. and S.Y.Y.; investigation, D.H.K. and S.Y.Y.; data curation, D.H.K. and S.Y.Y.; writing—original draft preparation, D.H.K. and S.Y.Y.; writing—review and editing, D.H.K., S.Y.Y., S.-R.R., C.L. and H.-R.H.; visualization, K.M.L., K.E.Y., and S.E.; supervision, K.M.L., K.E.Y., S.E., D.H.K. and S.Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Food and Drug Safety, grant numbers 21171MFDS183 and 23201MFDS138.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank all members from Biologics Research Division in National institute of Food and Drug Safety Evaluation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Schneier, M.; Razdan, S.; Miller, A.M.; Briceno, M.E.; Barua, S. Current technologies to endotoxin detection and removal for biopharmaceutical purification. Biotechnol. Bioeng. 2020, 117, 2588–2609. [Google Scholar] [CrossRef] [PubMed]
- Franco, E.; Gracia-Recio, V. Endotoxins from a Pharmacopoeial Point of View. Toxins 2018, 10, 331. [Google Scholar] [CrossRef]
- Muta, T.; Oda, T.; Iwanaga, S. Horseshoe crab coagulation factor B. A unique serine protease zymogen activated by cleavage of an Ile-Ile bond. J. Biol. Chem. 1993, 268, 21384–21388. [Google Scholar] [CrossRef]
- Ding, J.L.; Ho, B. Endotoxin detection—From Limulus amebocyte lysate to recombinant factor C. In Endotoxins: Structure, Function and Recognition; Springer: Dordrecht, The Netherlands, 2010; pp. 187–208. [Google Scholar] [CrossRef]
- Kawabata, S.; Koshiba, T.; Shibata, T. The lipopolysaccharide-activated innate immune response network of the horseshoe crab. Invertebr. Surviv. J. 2009, 6, 59–77. [Google Scholar]
- Perdomo-Morales, R.; Pardo-Ruiz, Z.; Spreitzer, I.; Lagarto, A.; Montag, T. Monocyte activation test (MAT) reliably detects pyrogens in parenteral formulations of human serum albumin. ALTEX-Altern. Anim. Exp. 2011, 28, 227–235. [Google Scholar] [CrossRef]
- Krisfalusi-Gannon, J.; Ali, W.; Dellinger, K.; Robertson, L.; Brady, T.E.; Goddard, M.K.M.; Tinker-Kulberg, R.; Kepley, C.L.; Dellinger, A.L. The role of horseshoe crabs in the biomedical industry and recent trends impacting species sustainability. Front. Mar. Sci. 2018, 5, 185. [Google Scholar] [CrossRef]
- Ding, J.L.; Chai, C.; Pui, A.W.M.; Ho, B. Expression of full length and deletion homologues of Carcinoscorpius rotundicauda factor C in Saccharomyces cerevisiae: Immunoreactivity and endotoxin binding. J. Endotoxin Res. 1997, 4, 33–43. [Google Scholar] [CrossRef]
- Pui, A.W.M.; Ho, B.; Ding, J.L. Yeast recombinant factor C from horseshoe crab binds endotoxin and causes bacteriostasis. J. Endotoxin Res. 1997, 4, 391–400. [Google Scholar] [CrossRef]
- Roopashree, S.D.; Ho, B.; Ding, J.L. Expression of Carcinoscorpius rotundicauda factor C in Pichia pastoris. Mol. Mar. Biol. Biotechnol. 1996, 5, 334–343. [Google Scholar]
- Maloney, T.; Phelan, R.; Simmons, N. Saving the horseshoe crab: A synthetic alternative to horseshoe crab blood for endotoxin detection. PLoS Biol. 2018, 16, e2006607. [Google Scholar] [CrossRef]
- Ding, J.L.; Ho, B. A New era in pyrogen testing. Trends Biotechnol. 2001, 19, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Loverock, B.; Simon, B.; Burgenson, A.; Baines, A. A recombinant factor C procedure for the detection of gram-negative bacterial endotoxin. Pharmacopeial Forum 2010, 36, 321–329. [Google Scholar]
- Abate, W.; Sattar, A.A.; Liu, J.; Conway, M.E.; Jackson, S.K. Evaluation of recombinant factor C assay for the detection of divergent lipopolysaccharide structural species and comparison with Limulus amebocyte lysate-based assays and a human monocyte activity assay. J. Med. Microbiol. 2017, 66, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Bolden, J.; Smith, K. Application of recombinant factor C reagent for the detection of bacterial endotoxins in pharmaceutical products. PDA J. Pharm. Sci. Technol. 2017, 71, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Muroi, M.; Ogura, N.; Mizumura, H.; Aketagawa, J.; Oda, T.; Tanamoto, K.I. Application of a recombinant three-factor chromogenic reagent, PyroSmart, for bacterial endotoxins test filed in the pharmacopeias. Biol. Pharm. Bull. 2019, 42, 2024–2037. [Google Scholar] [CrossRef] [PubMed]
- Piehler, M.; Roeder, R.; Blessing, S.; Reich, J. Comparison of LAL and rFC assays-Participation in a proficiency test program between 2014 and 2019. Microorganisms 2020, 8, 418. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia, 10th ed; European Directorate for the Quality of Medicines & HeathCare (EDQM): Strasbourg, France, 2021.
- Tsuchiya, M. Mechanism of low endotoxin recovery caused by a solution containing a chelating agent and a detergent. Immunome Res. 2019, 15, 166. [Google Scholar] [CrossRef]
- Schneider, C. Overcoming low endotoxin recovery. Pharm. Technol. Eur. 2016, 28, 40. [Google Scholar]
- Schwarz, H.; Gornicec, J.; Neuper, T.; Parigiani, M.A.; Wallner, M.; Duschl, A.; Horejs-Hoeck, J. Biological activity of masked endotoxin. Sci. Rep. 2017, 7, 44750. [Google Scholar] [CrossRef]
- Reich, J.; Lang, P.; Grallert, H.; Motschmann, H. Masking of endotoxin in surfactant samples: Effects on Limulus-based detection systems. Biologicals 2016, 44, 417–422. [Google Scholar] [CrossRef]
- Park, C.Y.; Jung, S.H.; Bak, J.P.; Lee, S.S.; Rhee, D.K. Comparison of the rabbit pyrogen test and Limulus amoebocyte lysate (LAL) assay for endotoxin in hepatitis B vaccines and the effect of aluminum hydroxide. Biologicals 2005, 33, 145–151. [Google Scholar] [CrossRef]
- McCullough, K.Z.; Parenteral Drug Association. The Bacterial Endotoxins Test: A Practical Approach; River Grove: Bethesda, MD, USA, 2011; pp. 195–214. [Google Scholar]
- Fujita, Y.; Tokunaga, T.; Kataoka, H. Saline and buffers minimize the action of interfering factors in the bacterial endotoxins test. Anal. Biochem. 2011, 409, 46–53. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).