A Dual Pharmacological Strategy against COVID-19: The Therapeutic Potential of Metformin and Atorvastatin
Abstract
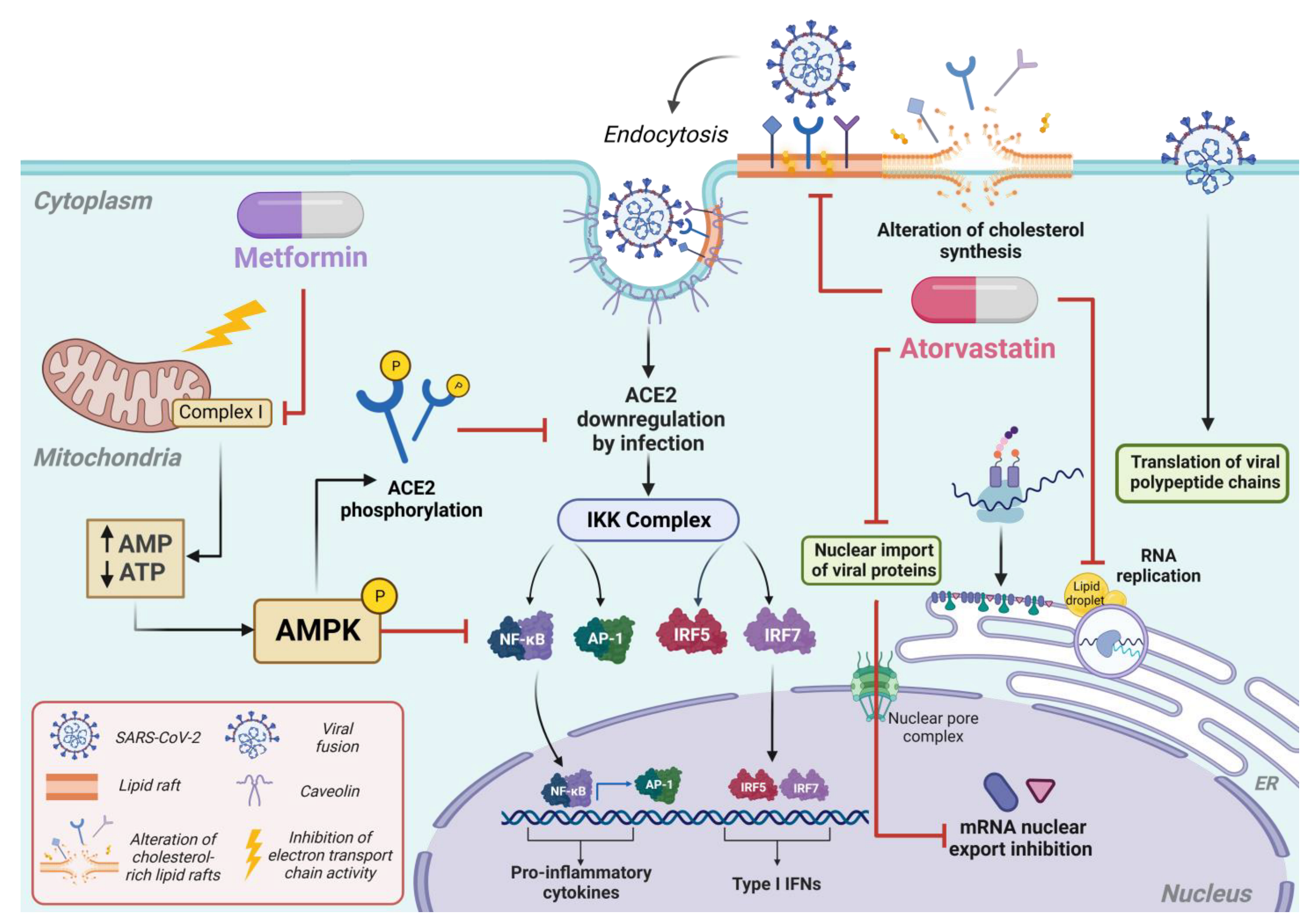
1. Introduction
2. Fundamentals and Mechanisms: An In-Depth Analysis of the Pharmacology of Metformin and Atorvastatin
3. Antiviral Properties of Metformin and Atorvastatin
| Group Baltimore (Class) | Virus (Family) | Model | Antiviral Effect |
|---|---|---|---|
| IV (ssRNA+) | SARS-CoV-2 | In silico, in vitro, and patients | Molecular docking analyses reveal that ATO could bind to the virus’s Mpro protease, Spike protein, and RNA-dependent RNA polymerase [59,60,61]. ATO showed antiviral activity against the D614G, Delta, and Mu variants of SARS-CoV-2 in Vero E6 cells through pre-post, pre-infection, and post-infection treatments [61,62,63]. |
| DENV | In vivo and in vitro | ATO reduced flaviviruses’ viral titer and cytopathic effect in Huh-7, Vero, MDCK, and neural stem/progenitor cells. ATO reduced clinical signs and increased survival of AG129 mice [53,64,65,66,67,68]. ATO inhibits nuclear-cytoplasmic transport of viral proteins, inhibiting DENV viral replication [65]. | |
| ZIKV | |||
| YFV | |||
| HCV | In vitro, and Patients | ATO suppresses HCV replication and has synergistic action with interferon. Additionally, it is associated with a 49% reduction in the incidence of hepatocellular carcinoma [69,70,71,72]. | |
| CV | In vivo | ATO reduced pathological features in the myocardium of mice infected with CVB3m and inhibited viral replication [73]. | |
| V (ssRNA-) | IAV | In vitro | ATO inhibits the formation of lipid droplets by IAV, suppressing virus replication [74,75]. |
| RABV | In vitro | ATO inhibits the formation of lipid droplets by RABV, suppressing virus replication [76]. | |
| VI (ssRNA-RT) | HIV | Patients | ATO is safe in patients with HIV, reduces virus-associated inflammation, reduces the activation of CD8+ and CD4+ T cells, and prevents viral rebound [75,76,77,78,79,80,81] [77,78,79,80,81,82,83]. |
4. The Impact of Metformin and Atorvastatin on the Immune Response
5. Retrospective Studies and Clinical Trials That Have Evaluated the Potential for Use of Metformin and Atorvastatin during COVID-19
5.1. Clinical Trials with Metformin
5.2. Clinical Trials with Atorvastatin
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Reyes-Ruiz, J.M.; García-Hernández, O.; Martínez-Mier, G.; Osuna-Ramos, J.F.; De Jesús-González, L.A.; Farfan-Morales, C.N.; Palacios-Rápalo, S.N.; Cordero-Rivera, C.D.; Ordoñez-Rodríguez, T.; Ángel, R.M.d. The Role of Aspartate Aminotransferase-to-Lymphocyte Ratio Index (ALRI) in Predicting Mortality in SARS-CoV-2 Infection. Microorganisms 2023, 11, 2894. [Google Scholar] [CrossRef]
- Li, G.; Hilgenfeld, R.; Whitley, R.; De Clercq, E. Therapeutic Strategies for COVID-19: Progress and Lessons Learned. Nat. Rev. Drug Discov. 2023, 22, 449–475. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Lu, R.-M.; Li, M.-C.; Huang, J.-L.; Hsu, F.-F.; Ko, S.-H.; Ke, F.-Y.; Su, S.-C.; Liang, K.-H.; Yuan, J.P.-Y.; et al. A Critical Overview of Current Progress for COVID-19: Development of Vaccines, Antiviral Drugs, and Therapeutic Antibodies. J. Biomed. Sci. 2022, 29, 68. [Google Scholar] [CrossRef] [PubMed]
- Akkiz, H. Implications of the Novel Mutations in the SARS-CoV-2 Genome for Transmission, Disease Severity, and the Vaccine Development. Front. Med. 2021, 8, 636532. [Google Scholar] [CrossRef] [PubMed]
- El-Mir, M.Y.; Nogueira, V.; Fontaine, E.; Avéret, N.; Rigoulet, M.; Leverve, X. Dimethylbiguanide Inhibits Cell Respiration via an Indirect Effect Targeted on the Respiratory Chain Complex I. J. Biol. Chem. 2000, 275, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Hawley, S.A.; Ross, F.A.; Chevtzoff, C.; Green, K.A.; Evans, A.; Fogarty, S.; Towler, M.C.; Brown, L.J.; Ogunbayo, O.A.; Evans, A.M.; et al. Use of Cells Expressing Gamma Subunit Variants to Identify Diverse Mechanisms of AMPK Activation. Cell Metab. 2010, 11, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Conde, K.; Pineda, G.; Newton, R.S.; Fernandez, M.L. Hypocholesterolemic Effects of 3-Hydroxy-3-Methylglutaryl Coenzyme A (HMG-CoA) Reductase Inhibitors in the Guinea Pig: Atorvastatin versus Simvastatin. Biochem. Pharmacol. 1999, 58, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.H.; Seeger, J.D. Atorvastatin Calcium: An Addition to HMG-CoA Reductase Inhibitors. Pharmacotherapy 1997, 17, 1157–1177. [Google Scholar] [CrossRef] [PubMed]
- Reis, G.; Silva, E.A.d.S.M.; Silva, D.C.M.; Thabane, L.; Milagres, A.C.; Ferreira, T.S.; dos Santos, C.V.Q.; Neto, A.D.d.F.; Callegari, E.D.; Savassi, L.C.M.; et al. Effect of Early Treatment with Metformin on Risk of Emergency Care and Hospitalization among Patients with COVID-19: The TOGETHER Randomized Platform Clinical Trial. Lancet Reg. Health Am. 2022, 6, 100142. [Google Scholar] [CrossRef]
- Ventura-López, C.; Cervantes-Luevano, K.; Aguirre-Sánchez, J.S.; Flores-Caballero, J.C.; Alvarez-Delgado, C.; Bernaldez-Sarabia, J.; Sánchez-Campos, N.; Lugo-Sánchez, L.A.; Rodríguez-Vázquez, I.C.; Sander-Padilla, J.G.; et al. Treatment with Metformin Glycinate Reduces SARS-CoV-2 Viral Load: An in Vitro Model and Randomized, Double-Blind, Phase IIb Clinical Trial. Biomed. Pharmacother. 2022, 152, 113223. [Google Scholar] [CrossRef]
- Bramante, C.T.; Ingraham, N.E.; Murray, T.A.; Marmor, S.; Hovertsen, S.; Gronski, J.; McNeil, C.; Feng, R.; Guzman, G.; Abdelwahab, N.; et al. Metformin and Risk of Mortality in Patients Hospitalised with COVID-19: A Retrospective Cohort Analysis. Lancet Healthy Longev. 2021, 2, e34–e41. [Google Scholar] [CrossRef] [PubMed]
- Bramante, C.T.; Huling, J.D.; Tignanelli, C.J.; Buse, J.B.; Liebovitz, D.M.; Nicklas, J.M.; Cohen, K.; Puskarich, M.A.; Belani, H.K.; Proper, J.L.; et al. Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for COVID-19. N. Engl. J. Med. 2022, 387, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Bramante, C.T.; Buse, J.B.; Liebovitz, D.M.; Nicklas, J.M.; Puskarich, M.A.; Cohen, K.; Belani, H.K.; Anderson, B.J.; Huling, J.D.; Tignanelli, C.J.; et al. Outpatient Treatment of COVID-19 and Incidence of Post-COVID-19 Condition over 10 Months (COVID-OUT): A Multicentre, Randomised, Quadruple-Blind, Parallel-Group, Phase 3 Trial. Lancet Infect. Dis. 2023, 23, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, L.; Jafarpour, H.; Oladi, Z.; Zakariaei, Z.; Tabarestani, M.; Ahmadi, B.M.; Razavi, A.; Hessami, A. Atorvastatin Therapy in COVID-19 Adult Inpatients: A Double-Blind, Randomized Controlled Trial. Int. J. Cardiol. Heart Vasc. 2021, 36, 100875. [Google Scholar] [CrossRef] [PubMed]
- Investigators, I.-S. Atorvastatin versus Placebo in Patients with COVID-19 in Intensive Care: Randomized Controlled Trial. BMJ 2022, 376, e068407. [Google Scholar] [CrossRef]
- Nasri, H.; Rafieian-Kopaei, M. Metformin: Current Knowledge. J. Res. Med. Sci. 2014, 19, 658–664. [Google Scholar] [PubMed]
- Bailey, C.J.; Turner, R.C. Metformin. N. Engl. J. Med. 1996, 334, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Sum, C.F.; Webster, J.M.; Johnson, A.B.; Catalano, C.; Cooper, B.G.; Taylor, R. The Effect of Intravenous Metformin on Glucose Metabolism during Hyperglycaemia in Type 2 Diabetes. Diabet. Med. 1992, 9, 61–65. [Google Scholar] [CrossRef]
- Scarpello, J.H.B.; Howlett, H.C.S. Metformin Therapy and Clinical Uses. Diab. Vasc. Dis. Res. 2008, 5, 157–167. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Goodman, A.M. Efficacy of Metformin in Patients with Non-Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1995, 333, 541–549. [Google Scholar] [CrossRef]
- Giannarelli, R.; Aragona, M.; Coppelli, A.; Del Prato, S. Reducing Insulin Resistance with Metformin: The Evidence Today. Diabetes Metab. 2003, 29, 6S28–6S35. [Google Scholar] [CrossRef]
- Madiraju, A.K.; Erion, D.M.; Rahimi, Y.; Zhang, X.-M.; Braddock, D.T.; Albright, R.A.; Prigaro, B.J.; Wood, J.L.; Bhanot, S.; MacDonald, M.J.; et al. Metformin Suppresses Gluconeogenesis by Inhibiting Mitochondrial Glycerophosphate Dehydrogenase. Nature 2014, 510, 542–546. [Google Scholar] [CrossRef]
- LaMoia, T.E.; Shulman, G.I. Cellular and Molecular Mechanisms of Metformin Action. Endocr. Rev. 2021, 42, 77–96. [Google Scholar] [CrossRef]
- Xiao, B.; Heath, R.; Saiu, P.; Leiper, F.C.; Leone, P.; Jing, C.; Walker, P.A.; Haire, L.; Eccleston, J.F.; Davis, C.T.; et al. Structural Basis for AMP Binding to Mammalian AMP-Activated Protein Kinase. Nature 2007, 449, 496–500. [Google Scholar] [CrossRef]
- Hawley, S.A.; Boudeau, J.; Reid, J.L.; Mustard, K.J.; Udd, L.; Mäkelä, T.P.; Alessi, D.R.; Hardie, D.G. Complexes between the LKB1 Tumor Suppressor, STRAD Alpha/Beta and MO25 Alpha/Beta Are Upstream Kinases in the AMP-Activated Protein Kinase Cascade. J. Biol. 2003, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- Hawley, S.A.; Pan, D.A.; Mustard, K.J.; Ross, L.; Bain, J.; Edelman, A.M.; Frenguelli, B.G.; Hardie, D.G. Calmodulin-Dependent Protein Kinase Kinase-Beta Is an Alternative Upstream Kinase for AMP-Activated Protein Kinase. Cell Metab. 2005, 2, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.J.; Lamia, K.A.; Vasquez, D.; Koo, S.-H.; Bardeesy, N.; Depinho, R.A.; Montminy, M.; Cantley, L.C. The Kinase LKB1 Mediates Glucose Homeostasis in Liver and Therapeutic Effects of Metformin. Science 2005, 310, 1642–1646. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, M.D.; Galic, S.; Marcinko, K.; Sikkema, S.; Pulinilkunnil, T.; Chen, Z.-P.; O’Neill, H.M.; Ford, R.J.; Palanivel, R.; O’Brien, M.; et al. Single Phosphorylation Sites in Acc1 and Acc2 Regulate Lipid Homeostasis and the Insulin-Sensitizing Effects of Metformin. Nat. Med. 2013, 19, 1649–1654. [Google Scholar] [CrossRef]
- Badr, D.; Kurban, M.; Abbas, O. Metformin in Dermatology: An Overview. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 1329–1335. [Google Scholar] [CrossRef]
- Frid, A.; Sterner, G.N.; Löndahl, M.; Wiklander, C.; Cato, A.; Vinge, E.; Andersson, A. Novel Assay of Metformin Levels in Patients with Type 2 Diabetes and Varying Levels of Renal Function: Clinical Recommendations. Diabetes Care 2010, 33, 1291–1293. [Google Scholar] [CrossRef]
- Endo, A. The Discovery and Development of HMG-CoA Reductase Inhibitors. J. Lipid Res. 1992, 33, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; Brown, M.S. Regulation of the Mevalonate Pathway. Nature 1990, 343, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Roglans, N.; Verd, J.C.; Peris, C.; Alegret, M.; Vázquez, M.; Adzet, T.; Díaz, C.; Hernández, G.; Laguna, J.C.; Sánchez, R.M. High Doses of Atorvastatin and Simvastatin Induce Key Enzymes Involved in VLDL Production. Lipids 2002, 37, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicki, A.S. Atorvastatin. Expert Opin. Pharmacother. 2001, 2, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Carro, A.C.; Damonte, E.B. Requirement of Cholesterol in the Viral Envelope for Dengue Virus Infection. Virus Res. 2013, 174, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Xun, Y.-H.; Zhang, Y.-J.; Pan, Q.-C.; Mao, R.-C.; Qin, Y.-L.; Liu, H.-Y.; Zhang, Y.-M.; Yu, Y.-S.; Tang, Z.-H.; Lu, M.-J.; et al. Metformin Inhibits Hepatitis B Virus Protein Production and Replication in Human Hepatoma Cells. J. Viral. Hepat. 2014, 21, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Hoppe-Seyler, K.; Herrmann, A.L.; Däschle, A.; Kuhn, B.J.; Strobel, T.D.; Lohrey, C.; Bulkescher, J.; Krijgsveld, J.; Hoppe-Seyler, F. Effects of Metformin on the Virus/Host Cell Crosstalk in Human Papillomavirus-Positive Cancer Cells. Int. J. Cancer 2021, 149, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Feng, C.; Luo, D.; Zhao, R.; Kannan, P.R.; Yin, Y.; Iqbal, M.Z.; Hu, Y.; Kong, X. Metformin Hydrochloride Significantly Inhibits Rotavirus Infection in Caco2 Cell Line, Intestinal Organoids, and Mice. Pharmaceuticals 2023, 16, 1279. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, H. Metformin Inhibits Tumorigenesis in HBV-Induced Hepatocellular Carcinoma by Suppressing HULC Overexpression Caused by HBX. J. Cell Biochem. 2018, 119, 4482–4495. [Google Scholar] [CrossRef]
- Farfan-Morales, C.N.; Cordero-Rivera, C.D.; Osuna-Ramos, J.F.; Monroy-Muñoz, I.E.; De Jesús-González, L.A.; Muñoz-Medina, J.E.; Hurtado-Monzón, A.M.; Reyes-Ruiz, J.M.; del Ángel, R.M. The Antiviral Effect of Metformin on Zika and Dengue Virus Infection. Sci. Rep. 2021, 11, 8743. [Google Scholar] [CrossRef]
- Parthasarathy, H.; Tandel, D.; Siddiqui, A.H.; Harshan, K.H. Metformin Suppresses SARS-CoV-2 in Cell Culture. Virus Res. 2022, 323, 199010. [Google Scholar] [CrossRef]
- Curry, J.M.; Johnson, J.; Mollaee, M.; Tassone, P.; Amin, D.; Knops, A.; Whitaker-Menezes, D.; Mahoney, M.G.; South, A.; Rodeck, U.; et al. Metformin Clinical Trial in HPV+ and HPV- Head and Neck Squamous Cell Carcinoma: Impact on Cancer Cell Apoptosis and Immune Infiltrate. Front. Oncol. 2018, 8, 436. [Google Scholar] [CrossRef] [PubMed]
- Abdelmoneim, M.; Eissa, I.R.; Aboalela, M.A.; Naoe, Y.; Matsumura, S.; Sibal, P.A.; Bustos-Villalobos, I.; Tanaka, M.; Kodera, Y.; Kasuya, H. Metformin Enhances the Antitumor Activity of Oncolytic Herpes Simplex Virus HF10 (Canerpaturev) in a Pancreatic Cell Cancer Subcutaneous Model. Sci. Rep. 2022, 12, 21570. [Google Scholar] [CrossRef] [PubMed]
- Granato, M.; Gilardini Montani, M.S.; Romeo, M.A.; Santarelli, R.; Gonnella, R.; D’Orazi, G.; Faggioni, A.; Cirone, M. Metformin Triggers Apoptosis in PEL Cells and Alters Bortezomib-Induced Unfolded Protein Response Increasing Its Cytotoxicity and Inhibiting KSHV Lytic Cycle Activation. Cell. Signal. 2017, 40, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.; Yi, P.; Baker, C.; Casey, G.; Xie, X.; Luo, H.; Cai, J.; Fan, X.; Soong, L.; et al. Metformin Restrains ZIKV Replication and Alleviates Virus-Induced Inflammatory Responses in Microglia. Int. Immunopharmacol. 2023, 121, 110512. [Google Scholar] [CrossRef]
- del Campo, J.A.; García Valdecasas, M.; Gil Gómez, A.; Rojas Alvarez-Ossorio, M.A.; Gallego, P.; Ampuero Herrojo, J.; Gallego Durán, R.; Pastor, H.; Grande, L.; Padillo Ruiz, F.J.; et al. Simvastatin and Metformin Inhibit Cell Growth in Hepatitis C Virus Infected Cells via mTOR Increasing PTEN and Autophagy. PLoS ONE 2018, 13, e0191805. [Google Scholar] [CrossRef]
- Tsai, W.-L.; Chang, T.-H.; Sun, W.-C.; Chan, H.-H.; Wu, C.-C.; Hsu, P.-I.; Cheng, J.-S.; Yu, M.-L. Metformin Activates Type I Interferon Signaling against HCV via Activation of Adenosine Monophosphate-Activated Protein Kinase. Oncotarget 2017, 8, 91928–91937. [Google Scholar] [CrossRef]
- Lee, H.S.; Noh, J.Y.; Song, J.Y.; Cheong, H.J.; Kim, W.J. Metformin Reduces the Risk of Developing Influenza A Virus Related Cardiovascular Disease. Heliyon 2023, 9, e20284. [Google Scholar] [CrossRef]
- Planas, D.; Pagliuzza, A.; Ponte, R.; Fert, A.; Marchand, L.R.; Massanella, M.; Gosselin, A.; Mehraj, V.; Dupuy, F.P.; Isnard, S.; et al. LILAC Pilot Study: Effects of Metformin on mTOR Activation and HIV Reservoir Persistence during Antiretroviral Therapy. EBioMedicine 2021, 65, 103270. [Google Scholar] [CrossRef]
- Masich, A.M.; Thompson, L.; Fulco, P.P. Bictegravir and Metformin Drug-Drug Interaction in People with Human Immunodeficiency Virus (HIV). Infect. Dis. Rep. 2023, 15, 231–237. [Google Scholar] [CrossRef]
- Farfan-Morales, C.N.; Cordero-Rivera, C.D.; Reyes-Ruiz, J.M.; Hurtado-Monzón, A.M.; Osuna-Ramos, J.F.; González-González, A.M.; De Jesús-González, L.A.; Palacios-Rápalo, S.N.; del Ángel, R.M. Anti-Flavivirus Properties of Lipid-Lowering Drugs. Front. Physiol. 2021, 12, 749770. [Google Scholar] [CrossRef]
- Osuna-Ramos, J.F.; Farfan-Morales, C.N.; Cordero-Rivera, C.D.; De Jesús-González, L.A.; Reyes-Ruiz, J.M.; Hurtado-Monzón, A.M.; Palacios-Rápalo, S.N.; Jiménez-Camacho, R.; Meraz-Ríos, M.A.; Del Ángel, R.M. Cholesterol-Lowering Drugs as Potential Antivirals: A Repurposing Approach against Flavivirus Infections. Viruses 2023, 15, 1465. [Google Scholar] [CrossRef]
- Herrera-Moro Huitron, L.; De Jesús-González, L.A.; Martínez-Castillo, M.; Ulloa-Aguilar, J.M.; Cabello-Gutierrez, C.; Helguera-Repetto, C.; Garcia-Cordero, J.; León Juárez, M. Multifaceted Nature of Lipid Droplets in Viral Interactions and Pathogenesis. Microorganisms 2023, 11, 1851. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.R.; Doran, E.; Halestrap, A.P. Evidence That Metformin Exerts Its Anti-Diabetic Effects through Inhibition of Complex 1 of the Mitochondrial Respiratory Chain. Biochem. J. 2000, 348 Pt 3, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, E. Metformin-Induced Mitochondrial Complex I Inhibition: Facts, Uncertainties, and Consequences. Front. Endocrinol. 2018, 9, 753. [Google Scholar] [CrossRef] [PubMed]
- Soto-Acosta, R.; Bautista-Carbajal, P.; Cervantes-Salazar, M.; Angel-Ambrocio, A.H.; Del Angel, R.M. DENV Up-Regulates the HMG-CoA Reductase Activity through the Impairment of AMPK Phosphorylation: A Potential Antiviral Target. PLoS Pathog. 2017, 13, e1006257. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Shirasaki, T.; Terashima, T.; Kawaguchi, K.; Nakamura, M.; Oishi, N.; Wang, X.; Shimakami, T.; Okada, H.; Arai, K.; et al. Hepatitis B Virus (HBV) Core-Related Antigen During Nucleos(t)Ide Analog Therapy Is Related to Intra-Hepatic HBV Replication and Development of Hepatocellular Carcinoma. J. Infect. Dis. 2016, 213, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Abohashrh, M.; Baig, M.H.; Dong, J.-J.; Alam, M.M.; Ahmad, I.; Irfan, S. Screening of Drug Databank against WT and Mutant Main Protease of SARS-CoV-2: Towards Finding Potential Compound for Repurposing against COVID-19. Saudi J. Biol. Sci. 2021, 28, 3152–3159. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Liu, H.; Wu, C. Drug Repurposing against SARS-CoV-2 Receptor Binding Domain Using Ensemble-Based Virtual Screening and Molecular Dynamics Simulations. Comput. Biol. Med. 2021, 135, 104634. [Google Scholar] [CrossRef]
- Duarte, R.R.R.; Copertino, D.C.; Iñiguez, L.P.; Marston, J.L.; Bram, Y.; Han, Y.; Schwartz, R.E.; Chen, S.; Nixon, D.F.; Powell, T.R. Identifying FDA-Approved Drugs with Multimodal Properties against COVID-19 Using a Data-Driven Approach and a Lung Organoid Model of SARS-CoV-2 Entry. Mol. Med. 2021, 27, 105. [Google Scholar] [CrossRef]
- Zapata-Cardona, M.I.; Flórez-Álvarez, L.; Zapata-Builes, W.; Guerra-Sandoval, A.L.; Guerra-Almonacid, C.M.; Hincapié-García, J.; Rugeles, M.T.; Hernandez, J.C. Atorvastatin Effectively Inhibits Ancestral and Two Emerging Variants of SARS-CoV-2 in Vitro. Front. Microbiol. 2022, 13, 721103. [Google Scholar] [CrossRef]
- Segatori, V.I.; Garona, J.; Caligiuri, L.G.; Bizzotto, J.; Lavignolle, R.; Toro, A.; Sanchis, P.; Spitzer, E.; Krolewiecki, A.; Gueron, G.; et al. Effect of Ivermectin and Atorvastatin on Nuclear Localization of Importin Alpha and Drug Target Expression Profiling in Host Cells from Nasopharyngeal Swabs of SARS-CoV-2-Positive Patients. Viruses 2021, 13, 2084. [Google Scholar] [CrossRef]
- Bryan-Marrugo, O.L.; Arellanos-Soto, D.; Rojas-Martinez, A.; Barrera-Saldaña, H.; Ramos-Jimenez, J.; Vidaltamayo, R.; Rivas-Estilla, A.M. The Anti-dengue Virus Properties of Statins May Be Associated with Alterations in the Cellular Antiviral Profile Expression. Mol. Med. Rep. 2016, 14, 2155–2163. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Rápalo, S.N.; Farfan-Morales, C.N.; Cordero-Rivera, C.D.; De Jesús-González, L.A.; Reyes-Ruiz, J.M.; Meraz-Ríos, M.A.; Del Ángel, R.M. An Ivermectin—Atorvastatin Combination Impairs Nuclear Transport Inhibiting Dengue Infection in Vitro and in Vivo. iScience 2023, 26, 108294. [Google Scholar] [CrossRef] [PubMed]
- Españo, E.; Kim, J.-K. Effects of Statin Combinations on Zika Virus Infection in Vero Cells. Pharmaceutics 2022, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Stoyanova, G.; Jabeen, S.; Landazuri Vinueza, J.; Ghosh Roy, S.; Lockshin, R.A.; Zakeri, Z. Zika Virus Triggers Autophagy to Exploit Host Lipid Metabolism and Drive Viral Replication. Cell Commun. Signal. 2023, 21, 114. [Google Scholar] [CrossRef]
- Wani, M.A.; Mukherjee, S.; Mallick, S.; Akbar, I.; Basu, A. Atorvastatin Ameliorates Viral Burden and Neural Stem/Progenitor Cell (NSPC) Death in an Experimental Model of Japanese Encephalitis. J. Biosci. 2020, 45, 77. [Google Scholar] [CrossRef]
- Ikeda, M.; Kato, N. Life Style-Related Diseases of the Digestive System: Cell Culture System for the Screening of Anti-Hepatitis C Virus (HCV) Reagents: Suppression of HCV Replication by Statins and Synergistic Action with Interferon. J. Pharmacol. Sci. 2007, 105, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Abe, K.; Yamada, M.; Dansako, H.; Naka, K.; Kato, N. Different Anti-HCV Profiles of Statins and Their Potential for Combination Therapy with Interferon. Hepatology 2006, 44, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Todorovska, B.; Caloska-Ivanova, V.; Dimitrova-Genadieva, M.; Trajkovska, M.; Popova-Jovanovska, R.; Grivceva-Stardelova, K.; Licoska-Josifovic, F.; Andreevski, V.; Curakova-Ristovska, E.; Joksimovic, N. Atorvastatin in Combination with Pegylated Interferon and Ribavirin Provided High Rate of Sustained Virological Response in Patients with Genotype 3 Hepatitis C Virus. Open Access Maced. J. Med. Sci. 2019, 7, 1641–1648. [Google Scholar] [CrossRef]
- Simon, T.G.; Bonilla, H.; Yan, P.; Chung, R.T.; Butt, A.A. Atorvastatin and Fluvastatin Are Associated with Dose-Dependent Reductions in Cirrhosis and Hepatocellular Carcinoma, among Patients with Hepatitis C Virus: Results from ERCHIVES. Hepatology 2016, 64, 47–57. [Google Scholar] [CrossRef]
- Guan, J.; Sun, X.; Liang, Y.; Dong, W.; Zhang, L.; Zhu, J.; Wang, G. Atorvastatin Attenuates Coxsackie Virus B3m-Induced Viral Myocarditis in Mice. J. Cardiovasc. Pharmacol. 2010, 56, 540–547. [Google Scholar] [CrossRef]
- Episcopio, D.; Aminov, S.; Benjamin, S.; Germain, G.; Datan, E.; Landazuri, J.; Lockshin, R.A.; Zakeri, Z. Atorvastatin Restricts the Ability of Influenza Virus to Generate Lipid Droplets and Severely Suppresses the Replication of the Virus. FASEB J. 2019, 33, 9516–9525. [Google Scholar] [CrossRef]
- Ianevski, A.; Yao, R.; Zusinaite, E.; Lysvand, H.; Oksenych, V.; Tenson, T.; Bjørås, M.; Kainov, D. Active Components of Commonly Prescribed Medicines Affect Influenza A Virus-Host Cell Interaction: A Pilot Study. Viruses 2021, 13, 1537. [Google Scholar] [CrossRef]
- Zhao, J.; Zeng, Z.; Chen, Y.; Liu, W.; Chen, H.; Fu, Z.F.; Zhao, L.; Zhou, M. Lipid Droplets Are Beneficial for Rabies Virus Replication by Facilitating Viral Budding. J. Virol. 2022, 96, e0147321. [Google Scholar] [CrossRef] [PubMed]
- Mystakelis, H.A.; Wilson, E.; Laidlaw, E.; Poole, A.; Krishnan, S.; Rupert, A.; Welker, J.L.; Gorelick, R.J.; Lisco, A.; Manion, M.; et al. An Open Label Randomized Controlled Trial of Atorvastatin versus Aspirin in Elite Controllers and Antiretroviral-Treated People with HIV. AIDS 2023, 37, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Overton, E.T.; Sterrett, S.; Westfall, A.O.; Kahan, S.M.; Burkholder, G.; Zajac, A.J.; Goepfert, P.A.; Bansal, A. Effects of Atorvastatin and Pravastatin on Immune Activation and T-Cell Function in Antiretroviral Therapy-Suppressed HIV-1-Infected Patients. AIDS 2014, 28, 2627–2631. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.; Crum-Cianflone, N.; Higgins, J.; Qin, J.; Rehm, C.; Metcalf, J.; Brandt, C.; Vita, J.; Decker, C.F.; Sklar, P.; et al. High Dose Atorvastatin Decreases Cellular Markers of Immune Activation without Affecting HIV-1 RNA Levels: Results of a Double-Blind Randomized Placebo Controlled Clinical Trial. J. Infect. Dis. 2011, 203, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Negredo, E.; Clotet, B.; Puig, J.; Pérez-Alvarez, N.; Ruiz, L.; Romeu, J.; Moltó, J.; Rey-Joly, C.; Blanco, J. The Effect of Atorvastatin Treatment on HIV-1-Infected Patients Interrupting Antiretroviral Therapy. AIDS 2006, 20, 619–621. [Google Scholar] [CrossRef] [PubMed]
- Riestenberg, R.A.; Furman, A.; Cowen, A.; Pawlowksi, A.; Schneider, D.; Lewis, A.A.; Kelly, S.; Taiwo, B.; Achenbach, C.; Palella, F.; et al. Differences in Statin Utilization and Lipid Lowering by Race, Ethnicity, and HIV Status in a Real-World Cohort of Persons with Human Immunodeficiency Virus and Uninfected Persons. Am. Heart J. 2019, 209, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Negredo, E.; Puigdomènech, I.; Marfil, S.; Puig, J.; Pérez-Alvarez, N.; Ruiz, L.; Rey-Joly, C.; Clotet, B.; Blanco, J. Association between HIV Replication and Cholesterol in Peripheral Blood Mononuclear Cells in HIV-Infected Patients Interrupting HAART. J. Antimicrob. Chemother. 2008, 61, 400–404. [Google Scholar] [CrossRef]
- Calza, L.; Trapani, F.; Bartoletti, M.; Manfredi, R.; Colangeli, V.; Borderi, M.; Grossi, G.; Motta, R.; Viale, P. Statin Therapy Decreases Serum Levels of High-Sensitivity C-Reactive Protein and Tumor Necrosis Factor-α in HIV-Infected Patients Treated with Ritonavir-Boosted Protease Inhibitors. HIV Clin. Trials 2012, 13, 153–161. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, J.; Martin, M.; He, M.; Gongol, B.; Marin, T.L.; Chen, L.; Shi, X.; Yin, Y.; Shang, F.; et al. AMP-Activated Protein Kinase Phosphorylation of Angiotensin-Converting Enzyme 2 in Endothelium Mitigates Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2018, 198, 509–520. [Google Scholar] [CrossRef]
- Simões e Silva, A.; Silveira, K.; Ferreira, A.; Teixeira, M. ACE2, Angiotensin-(1-7) and Mas Receptor Axis in Inflammation and Fibrosis. Br. J. Pharmacol. 2013, 169, 477–492. [Google Scholar] [CrossRef]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.-C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef]
- Bian, J.; Li, Z. Angiotensin-Converting Enzyme 2 (ACE2): SARS-CoV-2 Receptor and RAS Modulator. Acta Pharm. Sin. B 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Chi, X.; Wang, S.; Huang, Y.; Stamnes, M.; Chen, J.-L. Roles of Rho GTPases in Intracellular Transport and Cellular Transformation. Int. J. Mol. Sci. 2013, 14, 7089–7108. [Google Scholar] [CrossRef]
- Marcianò, G.; Palleria, C.; Casarella, A.; Rania, V.; Basile, E.; Catarisano, L.; Vocca, C.; Bianco, L.; Pelaia, C.; Cione, E.; et al. Effect of Statins on Lung Cancer Molecular Pathways: A Possible Therapeutic Role. Pharmaceuticals 2022, 15, 589. [Google Scholar] [CrossRef]
- Wagstaff, K.M.; Sivakumaran, H.; Heaton, S.M.; Harrich, D.; Jans, D.A. Ivermectin Is a Specific Inhibitor of Importin α/β-Mediated Nuclear Import Able to Inhibit Replication of HIV-1 and Dengue Virus. Biochem. J. 2012, 443, 851–856. [Google Scholar] [CrossRef]
- Chen, L.Y.C.; Quach, T.T.T. Combining Immunomodulators and Antivirals for COVID-19. Lancet Microbe 2021, 2, e233. [Google Scholar] [CrossRef]
- Chen, X.; Guo, H.; Qiu, L.; Zhang, C.; Deng, Q.; Leng, Q. Immunomodulatory and Antiviral Activity of Metformin and Its Potential Implications in Treating Coronavirus Disease 2019 and Lung Injury. Front. Immunol. 2020, 11, 2056. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From Mechanisms of Action to Therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef]
- Chaudhary, S.C.; Kurundkar, D.; Elmets, C.A.; Kopelovich, L.; Athar, M. Metformin, an Antidiabetic Agent Reduces Growth of Cutaneous Squamous Cell Carcinoma by Targeting mTOR Signaling Pathway†. Photochem. Photobiol. 2012, 88, 1149–1156. [Google Scholar] [CrossRef]
- Marcucci, F.; Romeo, E.; Caserta, C.A.; Rumio, C.; Lefoulon, F. Context-Dependent Pharmacological Effects of Metformin on the Immune System. Trends Pharmacol. Sci. 2020, 41, 162–171. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Mills, C. M1 and M2 Macrophages: Oracles of Health and Disease. CRI 2012, 32, 463–488. [Google Scholar] [CrossRef]
- Kelly, B.; Tannahill, G.M.; Murphy, M.P.; O’Neill, L.A.J. Metformin Inhibits the Production of Reactive Oxygen Species from NADH:Ubiquinone Oxidoreductase to Limit Induction of Interleukin-1β (IL-1β) and Boosts Interleukin-10 (IL-10) in Lipopolysaccharide (LPS)-Activated Macrophages *. J. Biol. Chem. 2015, 290, 20348–20359. [Google Scholar] [CrossRef]
- Ding, L.; Liang, G.; Yao, Z.; Zhang, J.; Liu, R.; Chen, H.; Zhou, Y.; Wu, H.; Yang, B.; He, Q. Metformin Prevents Cancer Metastasis by Inhibiting M2-like Polarization of Tumor Associated Macrophages. Oncotarget 2015, 6, 36441–36455. [Google Scholar] [CrossRef]
- Saito, A.; Koinuma, K.; Kawashima, R.; Miyato, H.; Ohzawa, H.; Horie, H.; Yamaguchi, H.; Kawahira, H.; Mimura, T.; Kitayama, J.; et al. Metformin May Improve the Outcome of Patients with Colorectal Cancer and Type 2 Diabetes Mellitus Partly through Effects on Neutrophil Extracellular Traps. BJC Rep. 2023, 1, 20. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Zhang, K.; Zhang, Y.; Gu, Z.; Huang, C. Neutrophils in COVID-19: Recent Insights and Advances. Virol. J. 2023, 20, 169. [Google Scholar] [CrossRef]
- Sun, Y.; Tian, T.; Gao, J.; Liu, X.; Hou, H.; Cao, R.; Li, B.; Quan, M.; Guo, L. Metformin Ameliorates the Development of Experimental Autoimmune Encephalomyelitis by Regulating T Helper 17 and Regulatory T Cells in Mice. J. Neuroimmunol. 2016, 292, 58–67. [Google Scholar] [CrossRef]
- Liu, X.; Yu, P.; Xu, Y.; Wang, Y.; Chen, J.; Tang, F.; Hu, Z.; Zhou, J.; Liu, L.; Qiu, W.; et al. Metformin Induces Tolerogenicity of Dendritic Cells by Promoting Metabolic Reprogramming. Cell Mol. Life Sci. 2023, 80, 283. [Google Scholar] [CrossRef]
- Cortés, M.; Brischetto, A.; Martinez-Campanario, M.C.; Ninfali, C.; Domínguez, V.; Fernández, S.; Celis, R.; Esteve-Codina, A.; Lozano, J.J.; Sidorova, J.; et al. Inflammatory Macrophages Reprogram to Immunosuppression by Reducing Mitochondrial Translation. Nat. Commun. 2023, 14, 7471. [Google Scholar] [CrossRef]
- Wiklund, O.; Mattsson-Hultén, L.; Hurt-Camejo, E.; Oscarsson, J. Effects of Simvastatin and Atorvastatin on Inflammation Markers in Plasma. J. Intern. Med. 2002, 251, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Saad, H.M.; Batiha, G.E.-S. The Potential Therapeutic Effect of Statins in Multiple Sclerosis: Beneficial or Detrimental Effects. Inflammopharmacology 2023, 31, 1671–1682. [Google Scholar] [CrossRef] [PubMed]
- Blankier, S.; McCrindle, B.W.; Ito, S.; Yeung, R.S.M. The Role of Atorvastatin in Regulating the Immune Response Leading to Vascular Damage in a Model of Kawasaki Disease. Clin. Exp. Immunol. 2011, 164, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Sorathia, N.; Al-Rubaye, H.; Zal, B. The Effect of Statins on the Functionality of CD4+CD25+FOXP3+ Regulatory T-Cells in Acute Coronary Syndrome: A Systematic Review and Meta-Analysis of Randomised Controlled Trials in Asian Populations. Eur. Cardiol. 2019, 14, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Fessler, M.B. Regulation of Adaptive Immunity in Health and Disease by Cholesterol Metabolism. Curr. Allergy Asthma Rep. 2015, 15, 48. [Google Scholar] [CrossRef]
- Sheridan, A.; Wheeler-Jones, C.P.D.; Gage, M.C. The Immunomodulatory Effects of Statins on Macrophages. Immuno 2022, 2, 317–343. [Google Scholar] [CrossRef]
- Pedrosa, A.R.; Martins, D.C.; Rizzo, M.; Silva-Nunes, J. Metformin in SARS-CoV-2 Infection: A Hidden Path—From Altered Inflammation to Reduced Mortality. A Review from the Literature. J. Diabetes Complicat. 2023, 37, 108391. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. Metformin and COVID-19: From Cellular Mechanisms to Reduced Mortality. Diabetes Metab. 2020, 46, 423–426. [Google Scholar] [CrossRef]
- Petakh, P.; Griga, V.; Mohammed, I.B.; Loshak, K.; Poliak, I.; Kamyshnyiy, A. Effects of Metformin, Insulin on Hematological Parameters of COVID-19 Patients with Type 2 Diabetes. Med. Arch. 2022, 76, 329–332. [Google Scholar] [CrossRef]
- Moreno-Corona, N.C.; López-Ortega, O.; Pérez-Martínez, C.A.; Martínez-Castillo, M.; De Jesús-González, L.A.; León-Reyes, G.; León-Juárez, M. Dynamics of the Microbiota and Its Relationship with Post-COVID-19 Syndrome. Int. J. Mol. Sci. 2023, 24, 14822. [Google Scholar] [CrossRef]
- Petakh, P.; Kamyshna, I.; Nykyforuk, A.; Yao, R.; Imbery, J.F.; Oksenych, V.; Korda, M.; Kamyshnyi, A. Immunoregulatory Intestinal Microbiota and COVID-19 in Patients with Type Two Diabetes: A Double-Edged Sword. Viruses 2022, 14, 477. [Google Scholar] [CrossRef]
- Petakh, P.; Kamyshna, I.; Oksenych, V.; Kainov, D.; Kamyshnyi, A. Metformin Therapy Changes Gut Microbiota Alpha-Diversity in COVID-19 Patients with Type 2 Diabetes: The Role of SARS-CoV-2 Variants and Antibiotic Treatment. Pharmaceuticals 2023, 16, 904. [Google Scholar] [CrossRef]
- Rodriguez-Nava, G.; Trelles-Garcia, D.P.; Yanez-Bello, M.A.; Chung, C.W.; Trelles-Garcia, V.P.; Friedman, H.J. Atorvastatin Associated with Decreased Hazard for Death in COVID-19 Patients Admitted to an ICU: A Retrospective Cohort Study. Crit. Care 2020, 24, 429. [Google Scholar] [CrossRef]
- Kouhpeikar, H.; Khosaravizade Tabasi, H.; Khazir, Z.; Naghipour, A.; Mohammadi Moghadam, H.; Forouzanfar, H.; Abbasifard, M.; Kirichenko, T.V.; Reiner, Ž.; Banach, M.; et al. Statin Use in COVID-19 Hospitalized Patients and Outcomes: A Retrospective Study. Front. Cardiovasc. Med. 2022, 9, 820260. [Google Scholar] [CrossRef] [PubMed]
- Haji Aghajani, M.; Moradi, O.; Azhdari Tehrani, H.; Amini, H.; Pourheidar, E.; Hatami, F.; Rabiei, M.M.; Sistanizad, M. Promising Effects of Atorvastatin on Mortality and Need for Mechanical Ventilation in Patients with Severe COVID-19; a Retrospective Cohort Study. Int. J. Clin. Pract. 2021, 75, e14434. [Google Scholar] [CrossRef] [PubMed]
- Schieffer, E.; Schieffer, B. The Rationale for the Treatment of Long-Covid Symptoms—A Cardiologist’s View. Front. Cardiovasc. Med. 2022, 9, 992686. [Google Scholar] [CrossRef]
- Visos-Varela, I.; Zapata-Cachafeiro, M.; Pintos-Rodríguez, S.; Bugarín-González, R.; González-Barcala, F.J.; Herdeiro, M.T.; Piñeiro-Lamas, M.; Figueiras, A.; Salgado-Barreira, Á. Outpatient Atorvastatin Use and Severe COVID-19 Outcomes: A Population-Based Study. J. Med. Virol. 2023, 95, e28971. [Google Scholar] [CrossRef] [PubMed]
- Boulware, D.R.; Bramante, C.; Pullen, M.; Buse, J.; Odde, D.; Mehta, T.; Murray, T.A. Metformin Reduced SARS-CoV-2 Viral Load in a Phase 3 Randomized Clinical Trial. Top. Antivir. Med. 2023, 31, 70. [Google Scholar]
- Yu, J.-W.; Sun, L.-J.; Zhao, Y.-H.; Kang, P.; Yan, B.-Z. The Effect of Metformin on the Efficacy of Antiviral Therapy in Patients with Genotype 1 Chronic Hepatitis C and Insulin Resistance. Int. J. Infect. Dis. 2012, 16, e436–e441. [Google Scholar] [CrossRef]
- Lv, Z.; Guo, Y. Metformin and Its Benefits for Various Diseases. Front. Endocrinol. 2020, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Tárraga López, P.J.; Celada Rodríguez, A.; Cerdán Oliver, M.; Solera Albero, J.; Ocaña López, J.M.; de Miguel Clavé, J. Análisis coste-efectividad de atorvastatina frente a simvastatina como tratamiento hipolipemiante en pacientes hipercolesterolémicos en atención primaria. Aten Primaria 2001, 27, 18–24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pepperrell, T.; Ellis, L.; Wang, J.; Hill, A. Barriers to Worldwide Access for Paxlovid, a New Treatment for COVID-19. Open Forum Infect. Dis. 2022, 9, ofac174. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, C.; Jacobs, T.F. Metformin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- DOF—Diario Oficial de La Federación. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5588819&fecha=10/03/2020#gsc.tab=0 (accessed on 13 June 2023).
| Group Baltimore (Class) | Virus (Family) | Model | Antiviral Effect |
|---|---|---|---|
| I (dsDNA) | HPV | In vitro, in vivo, and patients | MET promotes HPV-HNSCC and cervical cancer cell apoptosis. It can also increase CD8+ Teff and FoxP3+ Tregs in the TME, suggesting an immunomodulatory effect [38,43]. |
| HSV | In vitro | Co-administration of C-REV (attenuated oncolytic HSV-1) and MET produces an antitumor effect and prolonged survival in mice. Additionally, it improves systemic antitumor immunity [44]. | |
| KSHV | In vitro | MET induces apoptosis in primary effusion lymphoma (KSHV-associated aggressive B-cell lymphoma) cells [45]. | |
| III (dsRNA) | Rotavirus (Reoviridae) | In vitro and in vivo | MET inhibits the Rotavirus gene and protein expression in Caco-2 cells. Furthermore, MET mitigates intestinal lesions caused by Rotavirus [39]. |
| IV (ssRNA+) | SARS-CoV-2 | In vitro and patients | MET effectively inhibits viral replication of SARS-CoV-2 after 48 h of drug exposure without cytotoxic effect in Vero [11], Calu3, and Caco2 cells by up to 99% [42]. |
| DENV | In vitro and in vivo | MET inhibited ZIKV infection in Huh-7, C20, and U-87 cells but inhibited DENV and YFV infection in Huh-7 cells more effectively. During DENV infection alone, MET increased the survival of male AG129 mice, reducing severe signs of the disease [41,46]. | |
| ZIKV | |||
| YFV | |||
| HCV | In vitro | Simvastatin and MET inhibited cell growth and HCV infection in Huh7.5 cells. Furthermore, MET increased cell death markers, activated type I interferon signaling, and inhibited HCV replication through AMPK activation [47,48]. | |
| V (ssRNA-) | IAV | In vitro, in vivo, and patients | Studies showed that MET inhibits viral replication and cytokine expression induced by IAV. MET treatment was associated with decreased influenza-related mortality in diabetic patients [49]. |
| VI (ssRNA-RT) | HIV | Patients | Co-administration of antiretrovirals and MET in patients with HIV decreased the infiltration of CD4 + T cells in the colon and the activation/phosphorylation of mTOR. Additionally, decreased HIV-RNA/HIV-DNA ratios, a surrogate marker of viral transcription [50]. Co-administration of dolutegravir and MET in patients with HIV and diabetes improves the control of both conditions, with a reduction in viral load and control of HgbA1C [51]. |
| VII (dsDNA-RT) | HBV | In vitro | MET shows an HBV-associated inhibitory effect by negatively regulating the HULC/p18/miR-200a/ZEB1 signaling pathway [40]. |
| Authors | Sample size (Patients) | Dosage | Primary Outcomes |
|---|---|---|---|
| Reis et al., 2022 [10]. | 421 | 750 mg (extended-release) twice daily for ten days | Presence of side effects and interruptions. Hospitalization in 8 of 168 patients (4.8%) (MET group) vs. 14 of 179 patients (7.8%) (control group). |
| Ventura-López et al., 2022 [11]. | 20 | 620 mg twice daily for 14 days vs. placebo * | MET significantly reduced viral load (93.2%) in 3.3 days and decreased hospitalization time to 8.8 days and supplemental oxygen requirement. Changes were observed in AST, lymphocytes, neutrophils, D-dimer, CRP, DHL, and IgG biomarkers. The average hospitalization time was 8.8 days for the MET group and 9.8 days for the placebo group. Safety was comparable between groups, with no serious adverse events or hypoglycemia. |
| Bramante et al., 2022 [13]. | 1431 | They tested three drugs. Immediate-release MET was administered for 1–6 days (750 mg) and 7–14 days (1500 mg per day), ivermectin 390 to 470 μg per kilogram per day for three days, and fluvoxamine at 50 mg twice a day for 14 days. | No adverse events occurred. None of the medications were effective in preventing hypoxia, ICU visits, hospitalization, or death. However, the authors mention that in the case of MET, there was a trend of benefits for preventing the severe form. |
| Bramante et al., 2023 [14]. | 1126 | MET reduced the incidence of post-COVID-19 (4.1% absolute reduction) | |
| Boulware et al., 2023 [122]. | 1323 | MET 1000 mg/day on days 2 to 5; 1500 mg/day on days 6 to 14 * | 42% reduction in ER visits, hospitalizations and deaths by day 14, and 58% reduction in hospitalizations and deaths by day 28 42% reduction in long COVID in 10 months Decrease in viral load compared to placebo |
| Authors | Sample Size | Dosage | Primary Outcomes |
|---|---|---|---|
| Davoodi et al., 2021 [15]. | 40 | 40 mg ATO + 400/100 mg lopinavir/ritonavir (group 1) or 400/100 mg lopinavir/ritonavir for 5 days * | The duration of hospitalization was significantly reduced in the lopinavir/ritonavir + ATO group. |
| BMJ 2022 [16]. | 605 | 20 mg oral ATO once daily and placebo each for 30 days | 90 (31%) patients died in the ATO group and 103 (35%) in the placebo group. Venous thromboembolism rates were 2% (n = 6) in the ATO group and 3% (n = 9) in the placebo group. ATO is safe with few adverse effects. Reduction in levels of white blood cells, platelets, and D-dimer in patients treated with ATO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Jesús-González, L.A.; del Ángel, R.M.; Palacios-Rápalo, S.N.; Cordero-Rivera, C.D.; Rodríguez-Carlos, A.; Trujillo-Paez, J.V.; Farfan-Morales, C.N.; Osuna-Ramos, J.F.; Reyes-Ruiz, J.M.; Rivas-Santiago, B.; et al. A Dual Pharmacological Strategy against COVID-19: The Therapeutic Potential of Metformin and Atorvastatin. Microorganisms 2024, 12, 383. https://doi.org/10.3390/microorganisms12020383
De Jesús-González LA, del Ángel RM, Palacios-Rápalo SN, Cordero-Rivera CD, Rodríguez-Carlos A, Trujillo-Paez JV, Farfan-Morales CN, Osuna-Ramos JF, Reyes-Ruiz JM, Rivas-Santiago B, et al. A Dual Pharmacological Strategy against COVID-19: The Therapeutic Potential of Metformin and Atorvastatin. Microorganisms. 2024; 12(2):383. https://doi.org/10.3390/microorganisms12020383
Chicago/Turabian StyleDe Jesús-González, Luis Adrián, Rosa María del Ángel, Selvin Noé Palacios-Rápalo, Carlos Daniel Cordero-Rivera, Adrián Rodríguez-Carlos, Juan Valentin Trujillo-Paez, Carlos Noe Farfan-Morales, Juan Fidel Osuna-Ramos, José Manuel Reyes-Ruiz, Bruno Rivas-Santiago, and et al. 2024. "A Dual Pharmacological Strategy against COVID-19: The Therapeutic Potential of Metformin and Atorvastatin" Microorganisms 12, no. 2: 383. https://doi.org/10.3390/microorganisms12020383
APA StyleDe Jesús-González, L. A., del Ángel, R. M., Palacios-Rápalo, S. N., Cordero-Rivera, C. D., Rodríguez-Carlos, A., Trujillo-Paez, J. V., Farfan-Morales, C. N., Osuna-Ramos, J. F., Reyes-Ruiz, J. M., Rivas-Santiago, B., León-Juárez, M., García-Herrera, A. C., Ramos-Cortes, A. C., López-Gándara, E. A., & Martínez-Rodríguez, E. (2024). A Dual Pharmacological Strategy against COVID-19: The Therapeutic Potential of Metformin and Atorvastatin. Microorganisms, 12(2), 383. https://doi.org/10.3390/microorganisms12020383











