Effects of Varied Sulfamethazine Dosage and Exposure Durations on Offspring Mice
Abstract
1. Introduction
2. Materials and Methods
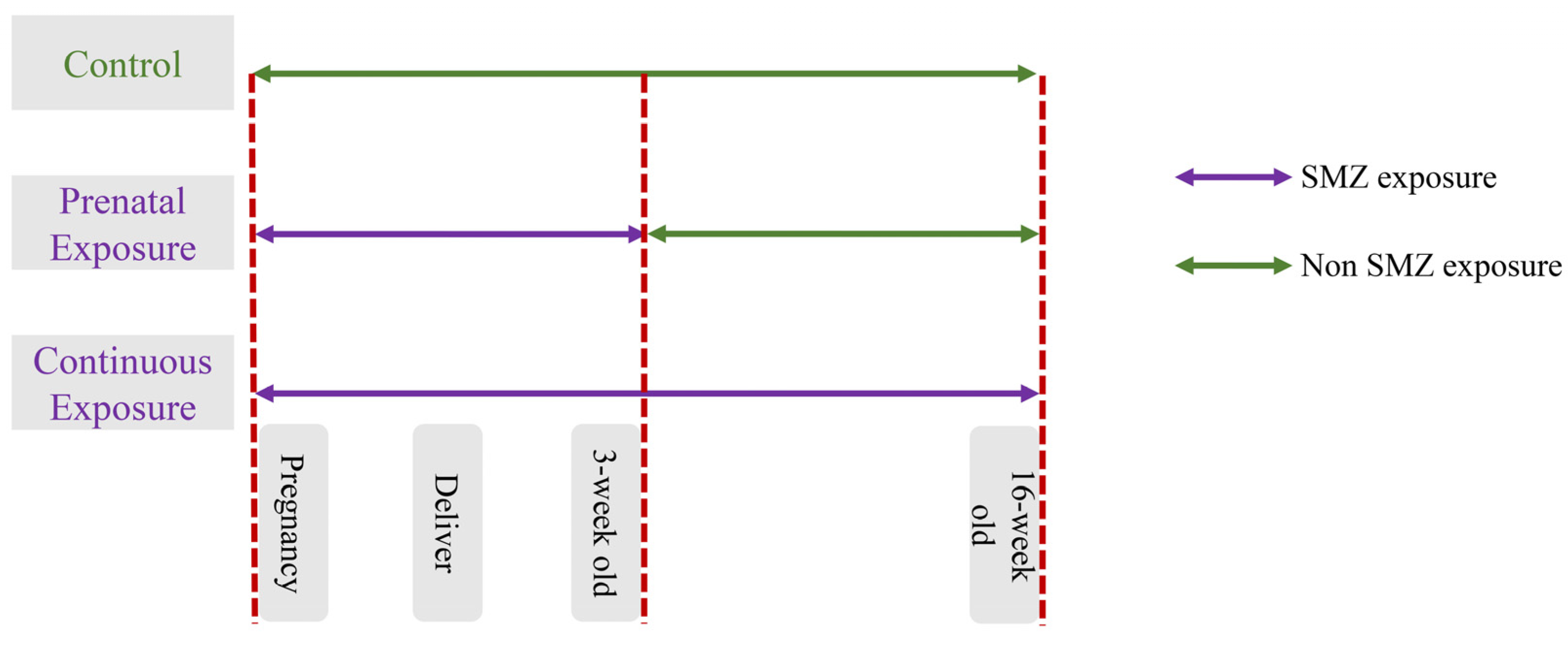
2.1. Study Design
2.2. Biochemical Analysis
2.3. Histological Analysis
2.4. DNA Extraction and Metagenomic Sequencing
2.5. Statistical Analysis
3. Results
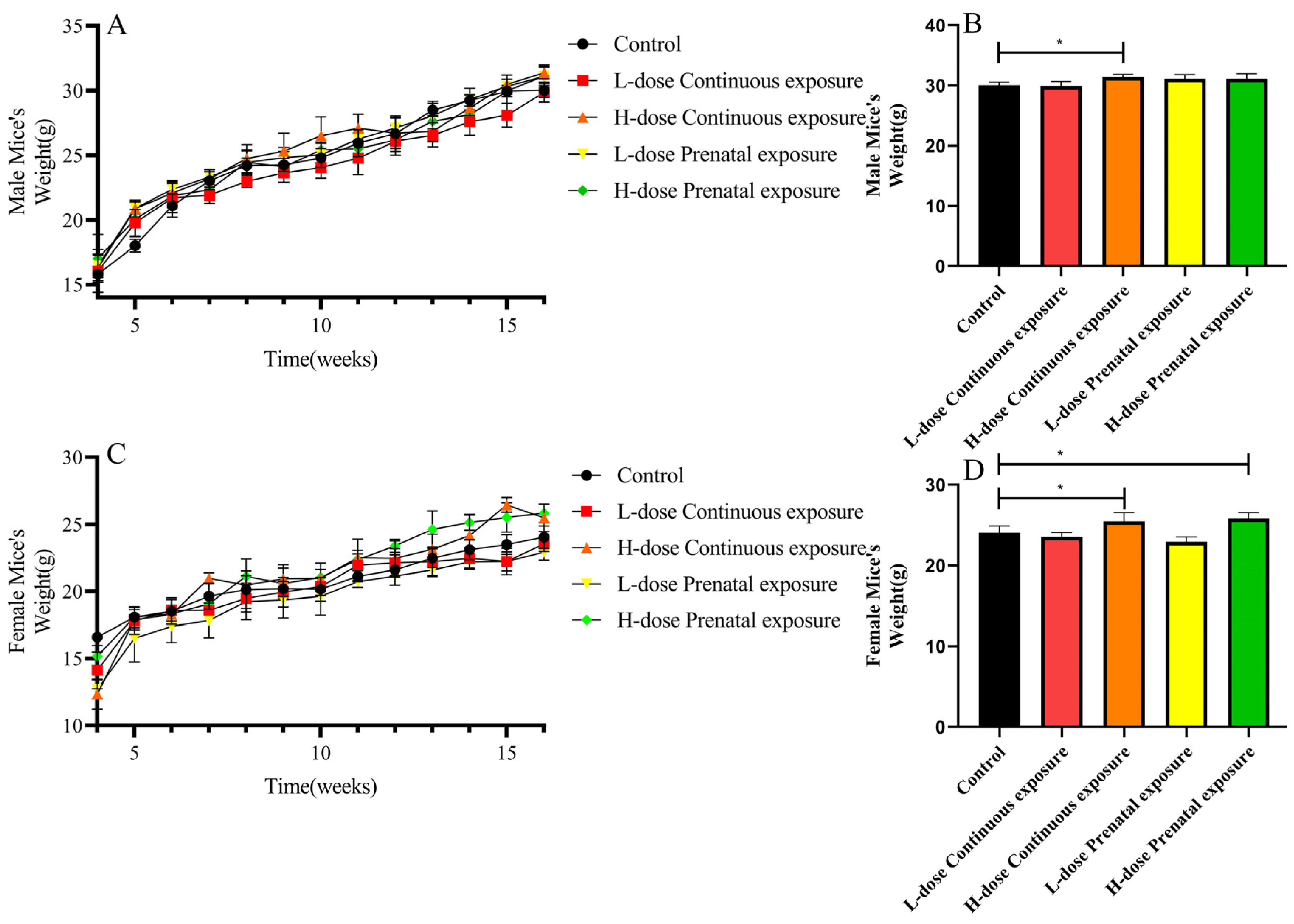
3.1. Effects of SMZ Exposure on Body Weight of Offspring Mice
3.2. Effects of SMZ Exposure on Blood Glucose of Offspring Mice
3.3. Effects of SMZ Exposure on Serum Lipids of Offspring Mice
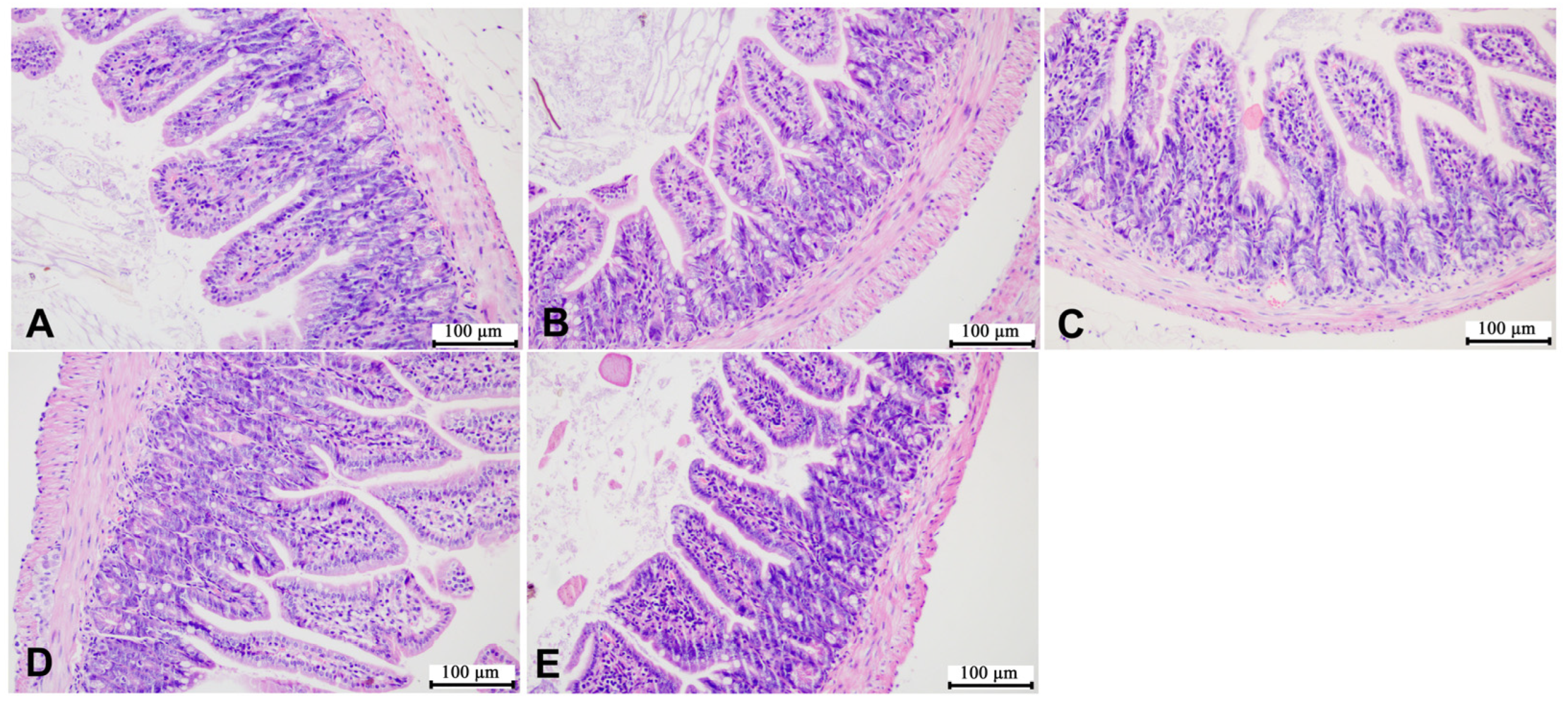
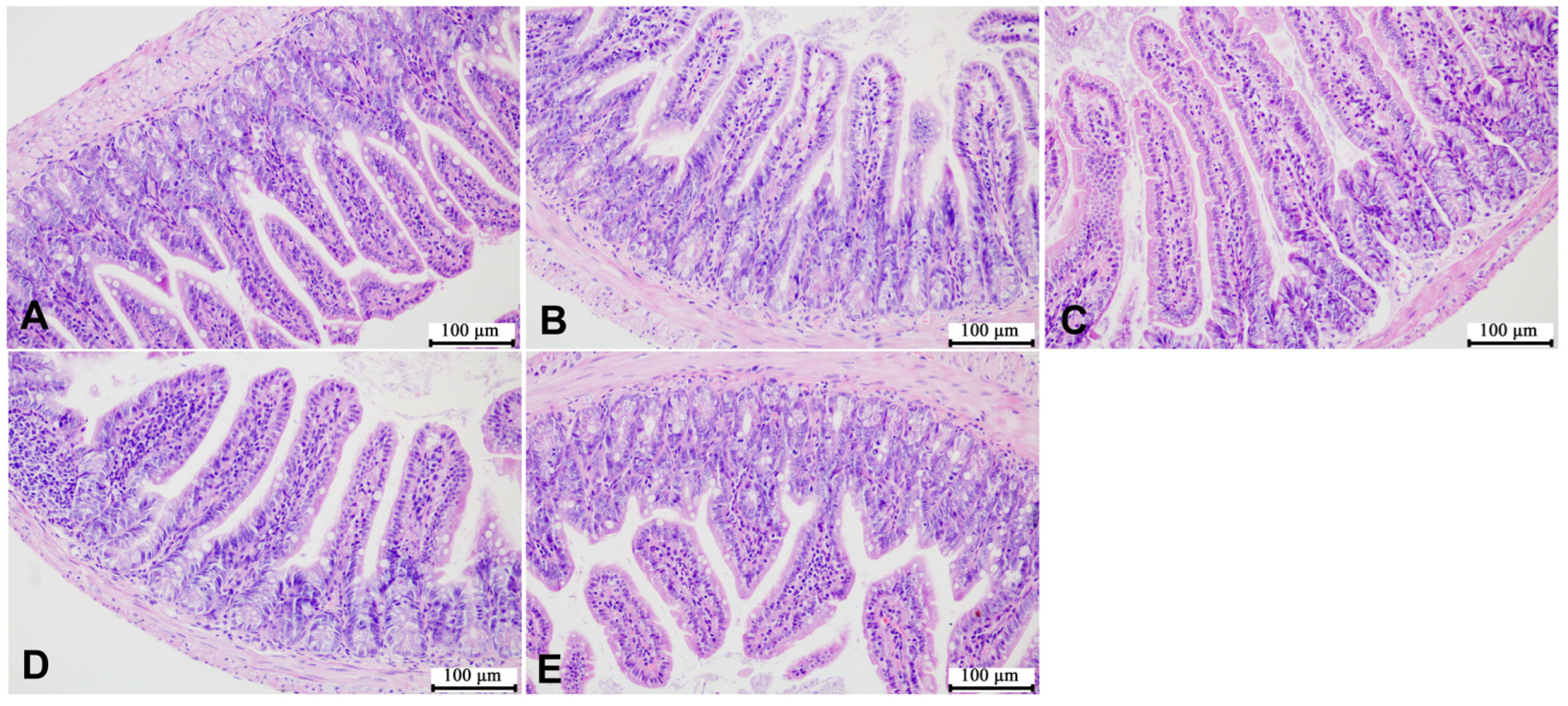
3.4. Effects of SMZ Exposure on Histopathology of Offspring Mice
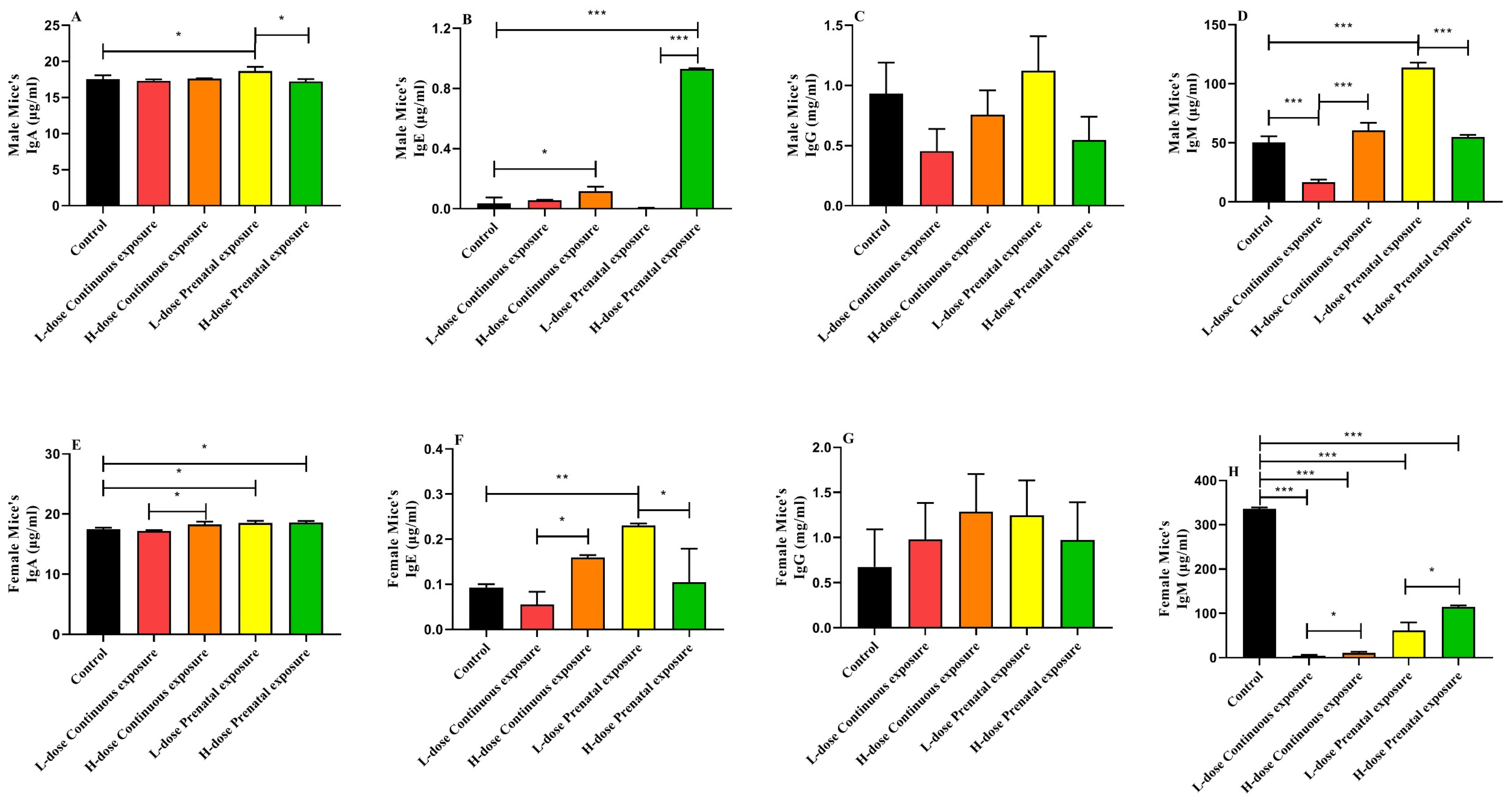
3.5. Effects of SMZ Exposure on Immunoglobulin of Offspring Mice
3.6. Effects of SMZ Exposure on Inflammatory Cytokines of Offspring Mice
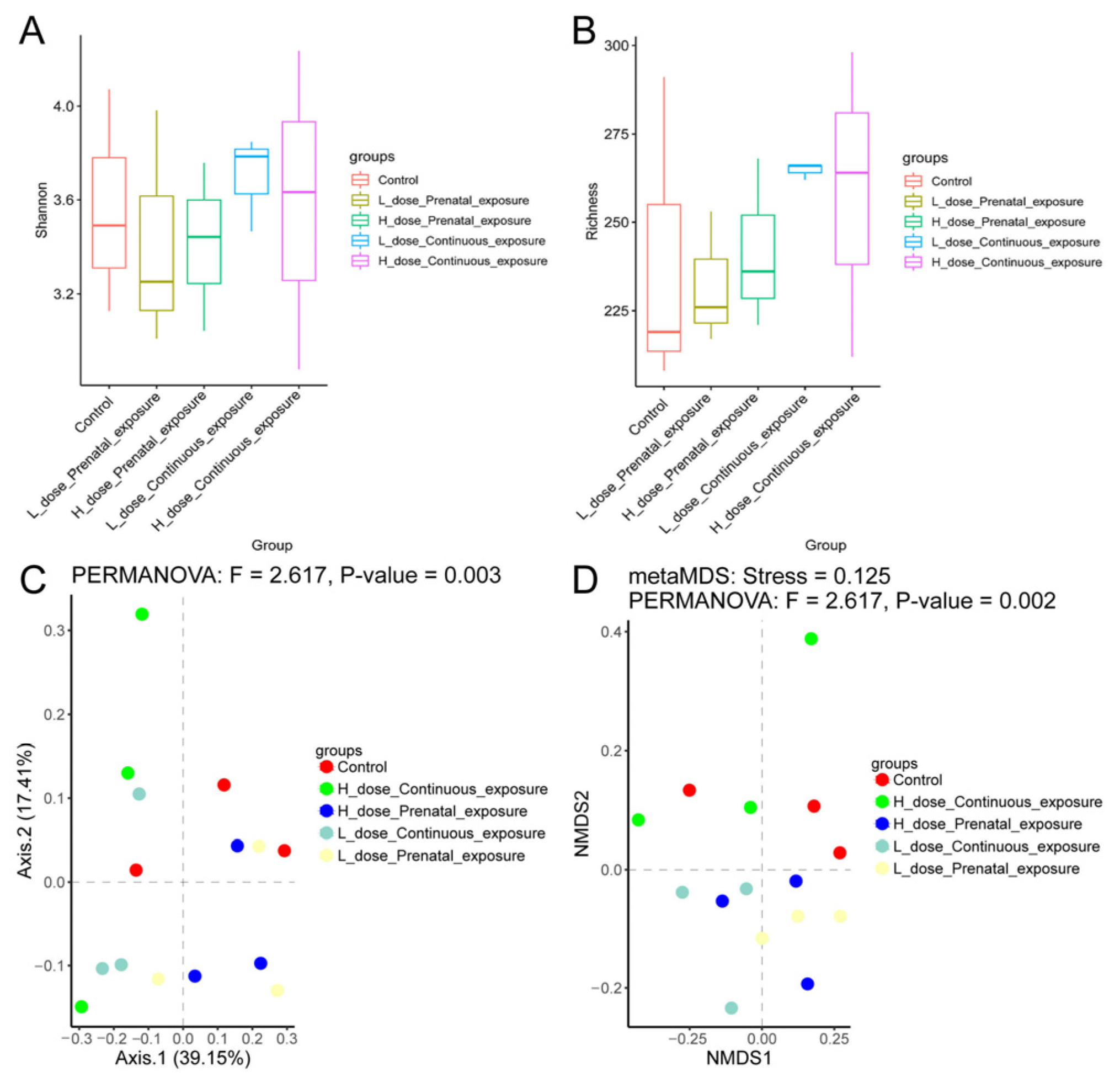
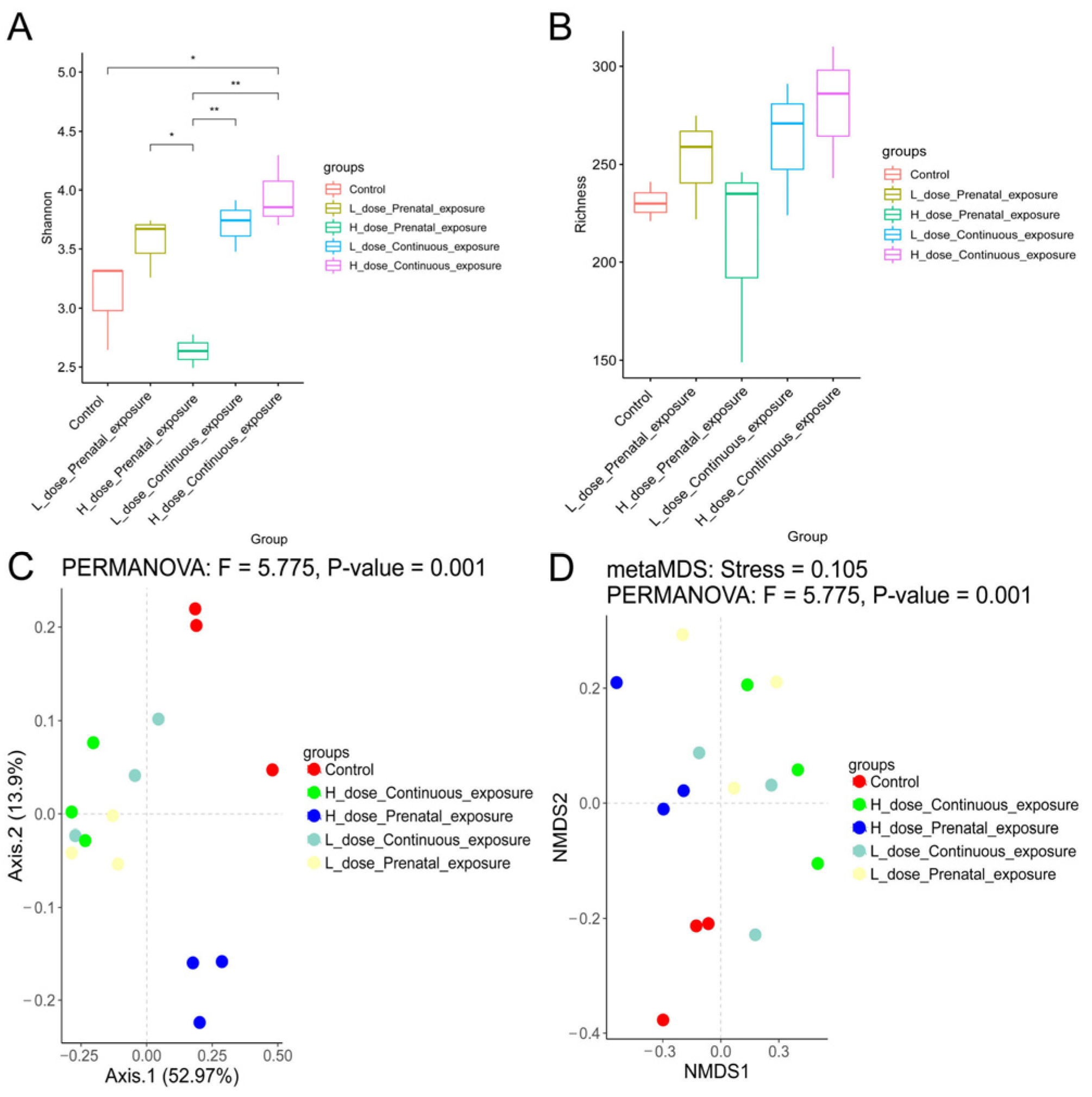
3.7. Effects of SMZ Exposure on Diversity of Gut Microbiota of Offspring Mice
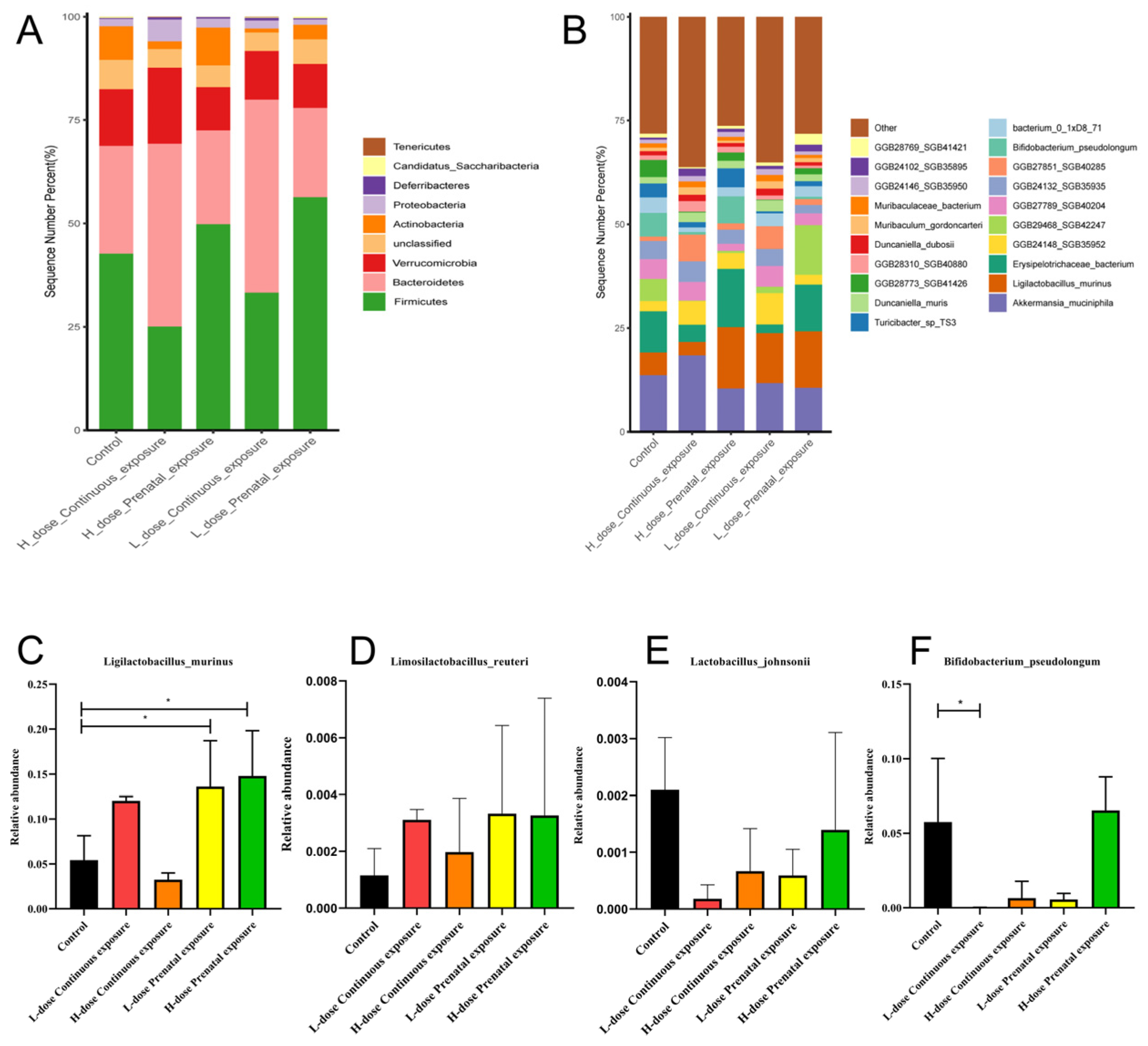
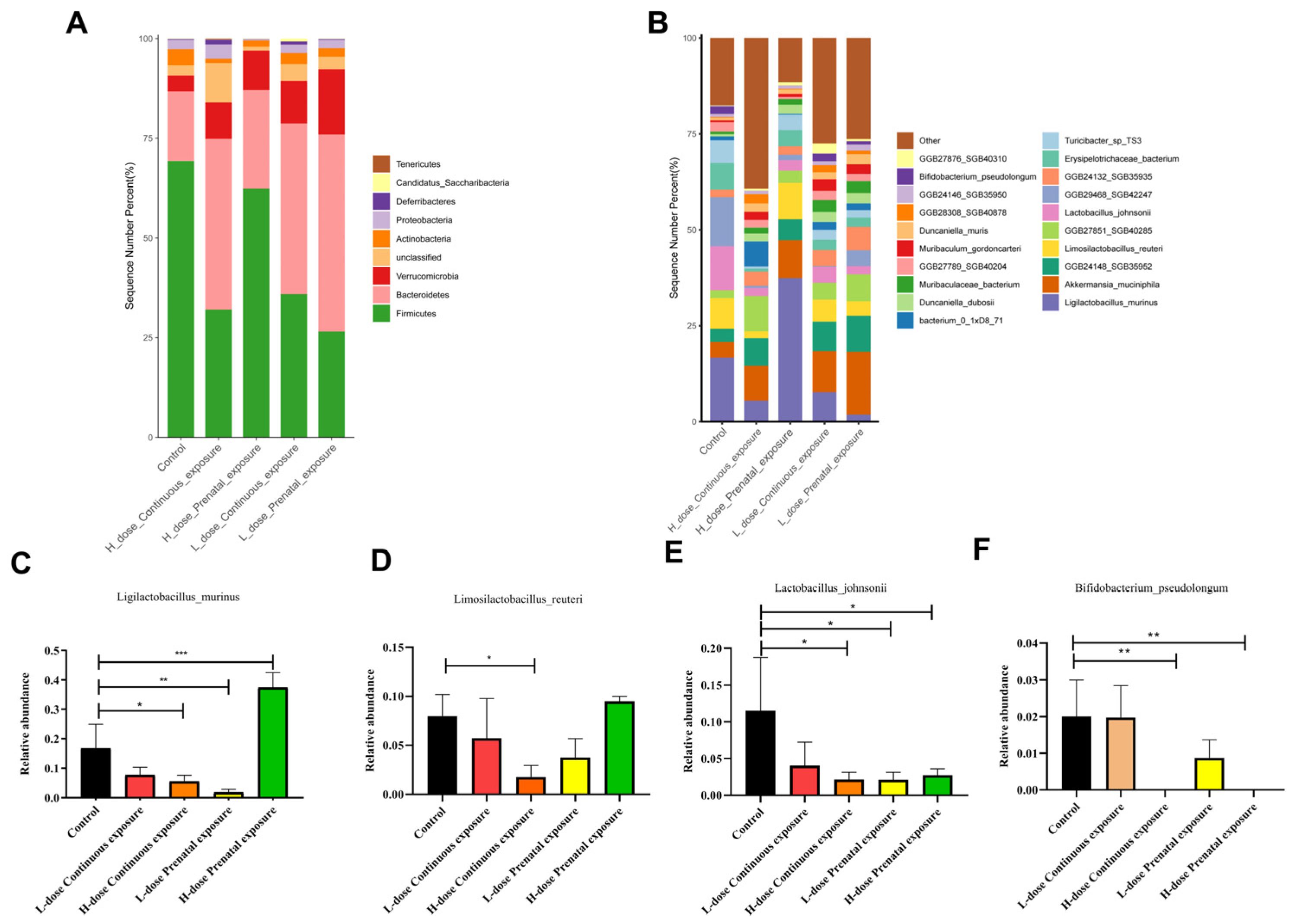
3.8. Effects of SMZ Exposure on the Composition of Gut Microbiota of Offspring Mice
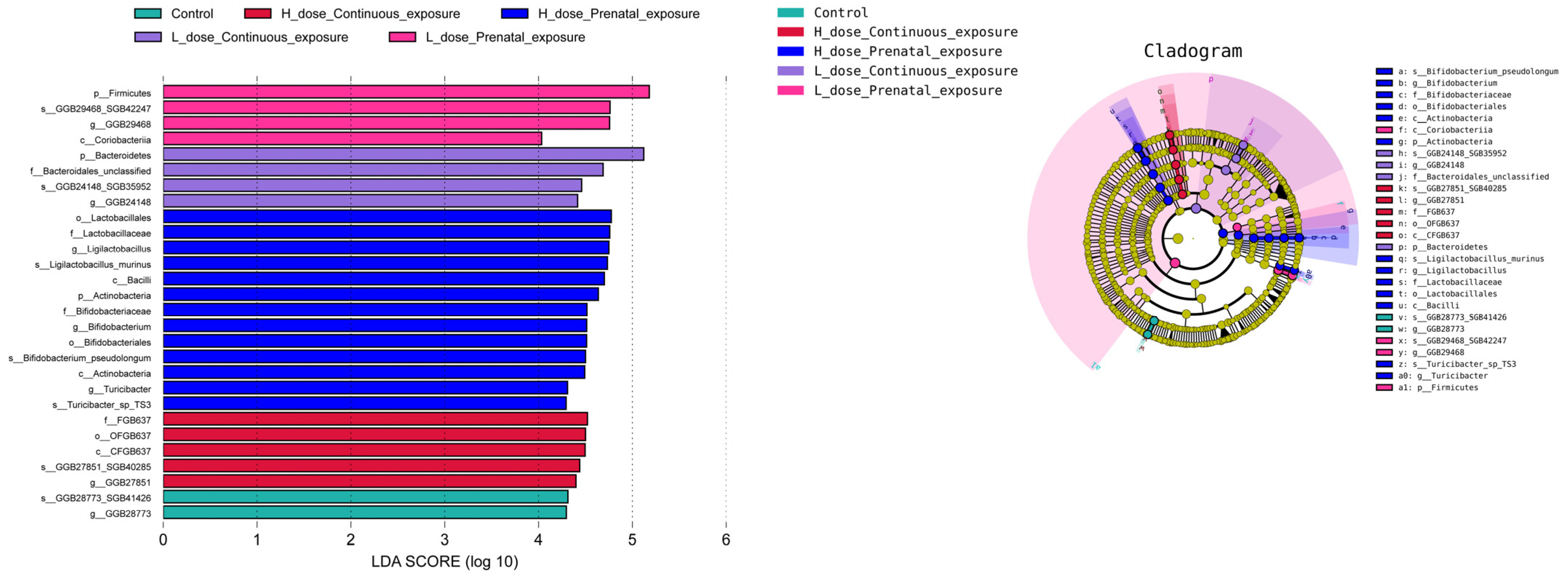
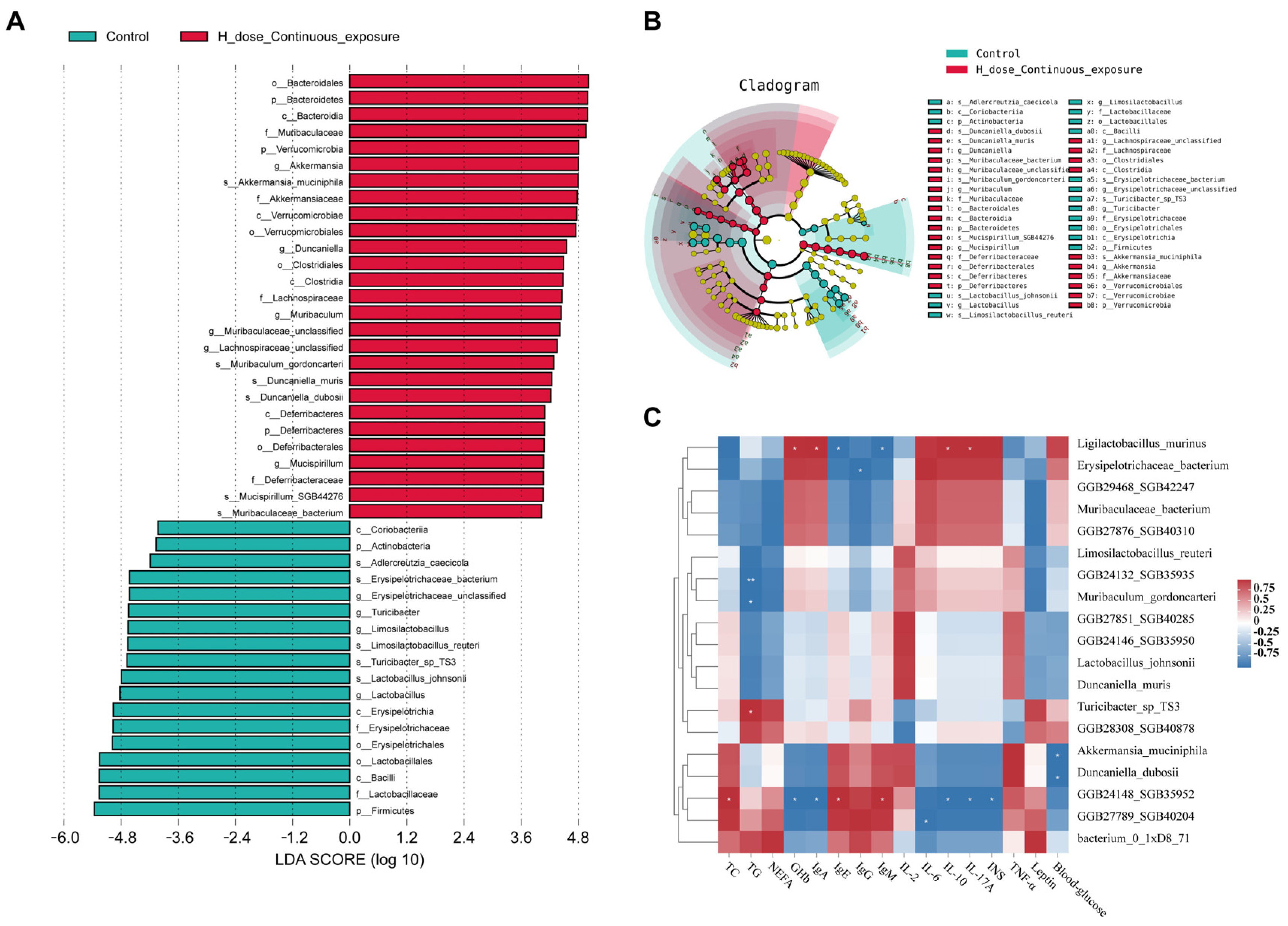
3.9. Effects of SMZ Exposure on Characteristic Microbiota of Offspring Mice
3.10. Effects of SMZ Exposure on Metabolic Pathways of Gut Microbiota of Offspring Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-K.; Yao, S.-K. Roles of Gut Microbiota and Metabolites in Pathogenesis of Functional Constipation. Evid.-Based Complement. Altern. Med. 2021, 2021, 5560310. [Google Scholar] [CrossRef]
- Li, Y.; Xia, S.; Jiang, X.; Feng, C.; Gong, S.; Ma, J.; Fang, Z.; Yin, J.; Yin, Y. Gut Microbiota and Diarrhea: An Updated Review. Front. Cell. Infect. Microbiol. 2021, 11, 625210. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Sears, C.L.; Maruthur, N. Gut microbiome and its role in obesity and insulin resistance. Ann. N. Y. Acad. Sci. 2020, 1461, 37–52. [Google Scholar] [CrossRef]
- Duan, H.; Yu, L.; Tian, F.; Zhai, Q.; Fan, L.; Chen, W. Antibiotic-induced gut dysbiosis and barrier disruption and the potential protective strategies. Crit. Rev. Food Sci. Nutr. 2022, 62, 1427–1452. [Google Scholar] [CrossRef]
- Kuhn, M.; Letunic, I.; Jensen, L.J.; Bork, P. The SIDER database of drugs and side effects. Nucleic Acids Res. 2016, 44, D1075–D1079. [Google Scholar] [CrossRef]
- Lange, K.; Buerger, M.; Stallmach, A.; Bruns, T. Effects of Antibiotics on Gut Microbiota. Dig. Dis. 2016, 34, 260–268. [Google Scholar] [CrossRef]
- Panda, S.; El Khader, I.; Casellas, F.; López Vivancos, J.; García Cors, M.; Santiago, A.; Cuenca, S.; Guarner, F.; Manichanh, C. Short-Term Effect of Antibiotics on Human Gut Microbiota. PLoS ONE 2014, 9, e95476. [Google Scholar] [CrossRef]
- Mor, G.; Aldo, P.; Alvero, A.B. The unique immunological and microbial aspects of pregnancy. Nat. Rev. Immunol. 2017, 17, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wong, F.S.; Wen, L. Antibiotics, gut microbiota, environment in early life and type 1 diabetes. Pharmacol. Res. 2017, 119, 219–226. [Google Scholar] [CrossRef]
- O’Connor, R.; Moloney, G.M.; Fulling, C.; O’Riordan, K.J.; Fitzgerald, P.; Bastiaanssen, T.F.S.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Maternal antibiotic administration during a critical developmental window has enduring neurobehavioural effects in offspring mice. Behav. Brain Res. 2021, 404, 113156. [Google Scholar] [CrossRef]
- Metsälä, J.; Lundqvist, A.; Virta, L.J.; Kaila, M.; Gissler, M.; Virtanen, S.M. Prenatal and post-natal exposure to antibiotics and risk of asthma in childhood. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2015, 45, 137–145. [Google Scholar] [CrossRef]
- Cho, I.; Yamanishi, S.; Cox, L.; Methé, B.A.; Zavadil, J.; Li, K.; Gao, Z.; Mahana, D.; Raju, K.; Teitler, I.; et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012, 488, 621–626. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Chung, J.; Battaglia, T.; Henderson, N.; Jay, M.; Li, H.; Lieber, A.D.; Wu, F.; Perez-Perez, G.I.; Chen, Y.; et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 2016, 8, 343ra382. [Google Scholar] [CrossRef] [PubMed]
- Korpela, K.; Blakstad, E.W.; Moltu, S.J.; Strømmen, K.; Nakstad, B.; Rønnestad, A.E.; Brække, K.; Iversen, P.O.; Drevon, C.A.; de Vos, W. Intestinal microbiota development and gestational age in preterm neonates. Sci. Rep. 2018, 8, 2453. [Google Scholar] [CrossRef] [PubMed]
- Turta, O.; Rautava, S. Antibiotics, obesity and the link to microbes—What are we doing to our children? BMC Med. 2016, 14, 57. [Google Scholar] [CrossRef]
- Lynn, M.A.; Eden, G.; Ryan, F.J.; Bensalem, J.; Wang, X.; Blake, S.J.; Choo, J.M.; Chern, Y.T.; Sribnaia, A.; James, J.; et al. The composition of the gut microbiota following early-life antibiotic exposure affects host health and longevity in later life. Cell Rep. 2021, 36, 109564. [Google Scholar] [CrossRef]
- Dodiya, H.B.; Kuntz, T.; Shaik, S.M.; Baufeld, C.; Leibowitz, J.; Zhang, X.; Gottel, N.; Zhang, X.; Butovsky, O.; Gilbert, J.A.; et al. Sex-specific effects of microbiome perturbations on cerebral Aβ amyloidosis and microglia phenotypes. J. Exp. Med. 2019, 216, 1542–1560. [Google Scholar] [CrossRef]
- Yin, X.; Zheng, J.; Liu, Y.; Li, Y.; Yu, X.; Li, Y.; Wang, X. Metagenomic evidence for increasing antibiotic resistance in progeny upon parental antibiotic exposure as the cost of hormesis. Chemosphere 2022, 309, 136738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, F.; Liu, X.; Xie, L.; Zhang, Y.T.; Mu, J. Polylactic acid (PLA), polyethylene terephthalate (PET), and polystyrene (PS) microplastics differently affect the gut microbiota of marine medaka (Oryzias melastigma) after individual and combined exposure with sulfamethazine. Aquat. Toxicol. 2023, 259, 106522. [Google Scholar] [CrossRef] [PubMed]
- Vidovic, N.; Vidovic, S. Antimicrobial Resistance and Food Animals: Influence of Livestock Environment on the Emergence and Dissemination of Antimicrobial Resistance. Antibiotics 2020, 9, 52. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Flach, C.-F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
- Dubourg, G.; Lagier, J.C.; Robert, C.; Armougom, F.; Hugon, P.; Metidji, S.; Dione, N.; Dangui, N.P.; Pfleiderer, A.; Abrahao, J.; et al. Culturomics and pyrosequencing evidence of the reduction in gut microbiota diversity in patients with broad-spectrum antibiotics. Int. J. Antimicrob. Agents 2014, 44, 117–124. [Google Scholar] [CrossRef]
- Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact of antibiotics on the human microbiome and consequences for host health. MicrobiologyOpen 2022, 11, e1260. [Google Scholar] [CrossRef] [PubMed]
- Yurkovetskiy, L.; Burrows, M.; Khan, A.A.; Graham, L.; Volchkov, P.; Becker, L.; Antonopoulos, D.; Umesaki, Y.; Chervonsky, A.V. Gender bias in autoimmunity is influenced by microbiota. Immunity 2013, 39, 400–412. [Google Scholar] [CrossRef]
- Li, T.; Chiang, J.Y. Bile acids as metabolic regulators. Curr. Opin. Gastroenterol. 2015, 31, 159–165. [Google Scholar] [CrossRef]
- Perelmuter, K.; Fraga, M.; Zunino, P. In vitro activity of potential probiotic Lactobacillus murinus isolated from the dog. J. Appl. Microbiol. 2008, 104, 1718–1725. [Google Scholar] [CrossRef]
- Rossi, M.; Martínez-Martínez, D.; Amaretti, A.; Ulrici, A.; Raimondi, S.; Moya, A. Mining metagenomic whole genome sequences revealed subdominant but constant Lactobacillus population in the human gut microbiota. Environ. Microbiol. Rep. 2016, 8, 399–406. [Google Scholar] [CrossRef]
- Singer, J.R.; Blosser, E.G.; Zindl, C.L.; Silberger, D.J.; Conlan, S.; Laufer, V.A.; DiToro, D.; Deming, C.; Kumar, R.; Morrow, C.D.; et al. Preventing dysbiosis of the neonatal mouse intestinal microbiome protects against late-onset sepsis. Nat. Med. 2019, 25, 1772–1782. [Google Scholar] [CrossRef] [PubMed]
- Lebovitz, Y.; Kowalski, E.A.; Wang, X.; Kelly, C.; Lee, M.; McDonald, V.; Ward, R.; Creasey, M.; Mills, W.; Gudenschwager Basso, E.K.; et al. Lactobacillus rescues postnatal neurobehavioral and microglial dysfunction in a model of maternal microbiome dysbiosis. Brain Behav. Immun. 2019, 81, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Deng, F.; Zhao, B.; Lin, Z.; Sun, Q.; Yang, X.; Wu, M.; Qiu, S.; Chen, Y.; Yan, Z.; et al. Lactobacillus murinus alleviate intestinal ischemia/reperfusion injury through promoting the release of interleukin-10 from M2 macrophages via Toll-like receptor 2 signaling. Microbiome 2022, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhou, X.; Zhao, J.; Ross, R.P.; Stanton, C.; Chen, W.; Yang, B. Comparative genomics of Lactobacillus johnsonii reveals extensive intraspecific genetic variation. Food Biosci. 2023, 56, 103190. [Google Scholar] [CrossRef]
- Jia, D.J.; Wang, Q.W.; Hu, Y.Y.; He, J.M.; Ge, Q.W.; Qi, Y.D.; Chen, L.Y.; Zhang, Y.; Fan, L.N.; Lin, Y.F.; et al. Lactobacillus johnsonii alleviates colitis by TLR1/2-STAT3 mediated CD206(+) macrophages(IL-10) activation. Gut Microbes 2022, 14, 2145843. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.L.; Martoni, C.J.; Prakash, S. Cholesterol lowering and inhibition of sterol absorption by Lactobacillus reuteri NCIMB 30242: A randomized controlled trial. Eur. J. Clin. Nutr. 2012, 66, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Mangifesta, M.; Mancabelli, L.; Lugli, G.A.; James, K.; Duranti, S.; Turroni, F.; Ferrario, C.; Ossiprandi, M.C.; van Sinderen, D.; et al. Unveiling bifidobacterial biogeography across the mammalian branch of the tree of life. ISME J. 2017, 11, 2834–2847. [Google Scholar] [CrossRef] [PubMed]
- Bo, T.B.; Wen, J.; Zhao, Y.C.; Tian, S.J.; Zhang, X.Y.; Wang, D.H. Bifidobacterium pseudolongum reduces triglycerides by modulating gut microbiota in mice fed high-fat food. J. Steroid Biochem. Mol. Biol. 2020, 198, 105602. [Google Scholar] [CrossRef]
- Jess, T.; Morgen, C.S.; Harpsøe, M.C.; Sørensen, T.I.A.; Ajslev, T.A.; Antvorskov, J.C.; Allin, K.H. Antibiotic use during pregnancy and childhood overweight: A population-based nationwide cohort study. Sci. Rep. 2019, 9, 11528. [Google Scholar] [CrossRef]
- Jin, Y.; Wu, Y.; Zeng, Z.; Jin, C.; Wu, S.; Wang, Y.; Fu, Z. From the Cover: Exposure to Oral Antibiotics Induces Gut Microbiota Dysbiosis Associated with Lipid Metabolism Dysfunction and Low-Grade Inflammation in Mice. Toxicol. Sci. 2016, 154, 140–152. [Google Scholar] [CrossRef]
- Su, Y.; Gan, X.P.; Li, F.F.; Zhang, D.Y.; Chen, L.; Cao, Y.N.; Qiu, H.H.; Cheng, D.C.; Zu, J.F.; Liu, W.Y.; et al. Effect of exposure to antibiotics on the gut microbiome and biochemical indexes of pregnant women. BMJ Open Diabetes Res. Care 2021, 9, e002321. [Google Scholar] [CrossRef]
- Bito, M.; Tomita, T.; Komori, M.; Taogoshi, T.; Kimura, Y.; Kihira, K. The mechanisms of insulin secretion and calcium signaling in pancreatic β-cells exposed to fluoroquinolones. Biol. Pharm. Bull. 2013, 36, 31–35. [Google Scholar] [CrossRef]
- Kendall, C.; Wooltorton, E. People with diabetes should avoid antibiotic gatifloxacin. CMAJ Can. Med. Assoc. J. 2006, 174, 1089–1090. [Google Scholar] [CrossRef] [PubMed]
- Wlodarska, M.; Willing, B.; Keeney, K.M.; Menendez, A.; Bergstrom, K.S.; Gill, N.; Russell, S.L.; Vallance, B.A.; Finlay, B.B. Antibiotic treatment alters the colonic mucus layer and predisposes the host to exacerbated Citrobacter rodentium-induced colitis. Infect. Immun. 2011, 79, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Tulic, M.K.; Vivinus-Nébot, M.; Rekima, A.; Rabelo Medeiros, S.; Bonnart, C.; Shi, H.; Walker, A.; Dainese, R.; Boyer, J.; Vergnolle, N.; et al. Presence of commensal house dust mite allergen in human gastrointestinal tract: A potential contributor to intestinal barrier dysfunction. Gut 2016, 65, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Ran, Y.; Fukui, H.; Xu, X.; Wang, X.; Ebisutani, N.; Tanaka, Y.; Maeda, A.; Makizaki, Y.; Ohno, H.; Kondo, T.; et al. Alteration of Colonic Mucosal Permeability during Antibiotic-Induced Dysbiosis. Int. J. Mol. Sci. 2020, 21, 6108. [Google Scholar] [CrossRef] [PubMed]
- Cua, D.J.; Tato, C.M. Innate IL-17-producing cells: The sentinels of the immune system. Nat. Rev. Immunol. 2010, 10, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.E.; Louie, S.; Shi, H.N.; Nagler-Anderson, C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J. Immunol. 2004, 172, 6978–6987. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.L.; Gold, M.J.; Hartmann, M.; Willing, B.P.; Thorson, L.; Wlodarska, M.; Gill, N.; Blanchet, M.R.; Mohn, W.W.; McNagny, K.M.; et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 2012, 13, 440–447. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Dang, D.; Zhu, L.; Pan, M.; Zhu, J.; Lu, W.; Lu, S.; Zhao, J. Effects of Varied Sulfamethazine Dosage and Exposure Durations on Offspring Mice. Microorganisms 2024, 12, 381. https://doi.org/10.3390/microorganisms12020381
Wang H, Dang D, Zhu L, Pan M, Zhu J, Lu W, Lu S, Zhao J. Effects of Varied Sulfamethazine Dosage and Exposure Durations on Offspring Mice. Microorganisms. 2024; 12(2):381. https://doi.org/10.3390/microorganisms12020381
Chicago/Turabian StyleWang, Hongchao, Danting Dang, Leilei Zhu, Mingluo Pan, Jinlin Zhu, Wenwei Lu, Shourong Lu, and Jianxin Zhao. 2024. "Effects of Varied Sulfamethazine Dosage and Exposure Durations on Offspring Mice" Microorganisms 12, no. 2: 381. https://doi.org/10.3390/microorganisms12020381
APA StyleWang, H., Dang, D., Zhu, L., Pan, M., Zhu, J., Lu, W., Lu, S., & Zhao, J. (2024). Effects of Varied Sulfamethazine Dosage and Exposure Durations on Offspring Mice. Microorganisms, 12(2), 381. https://doi.org/10.3390/microorganisms12020381




