Overview of Ecology and Aspects of Antibiotic Resistance in Campylobacter spp. Isolated from Free-Grazing Chicken Tissues in Rural Households
Abstract
1. Introduction
2. Materials and Methods
- (a)
- The following four criteria formed a total of 15 sampling study groups. These criteria were determined solely by deductive reasoning since other similar studies do not exist. The size of the flock, the co-existence of other poultry species, or of small ruminants and/or pigs in the same household and the feeding practices could be parameters able to shape the ecology of Campylobacter species by favoring (or not) various niches.
- (i)
- The size of the flock: up to 15 birds (Gallus domesticus), 16–40 birds, and 41 up to 60 birds.
- (ii)
- The presence or not in the same household of other poultry species like turkeys, ducks, etc.
- (ii)
- The presence or not in the same household of small ruminants (sheep and goats) and pigs.
- (iv)
- The administration of households’ leftovers of plant origin (potatoes, tomatoes, etc.) or the administration of industrial-grade concentrated feeds (corn, barley, etc.).
- (b)
- The following tissue sections were obtained from each bird:
- (i)
- Approximately 30 g of chicken skin.
- (ii)
- A total of 100 g of pectoralis muscle.
- (iii)
- Five swabs from the visceral cavity and the matching liver.
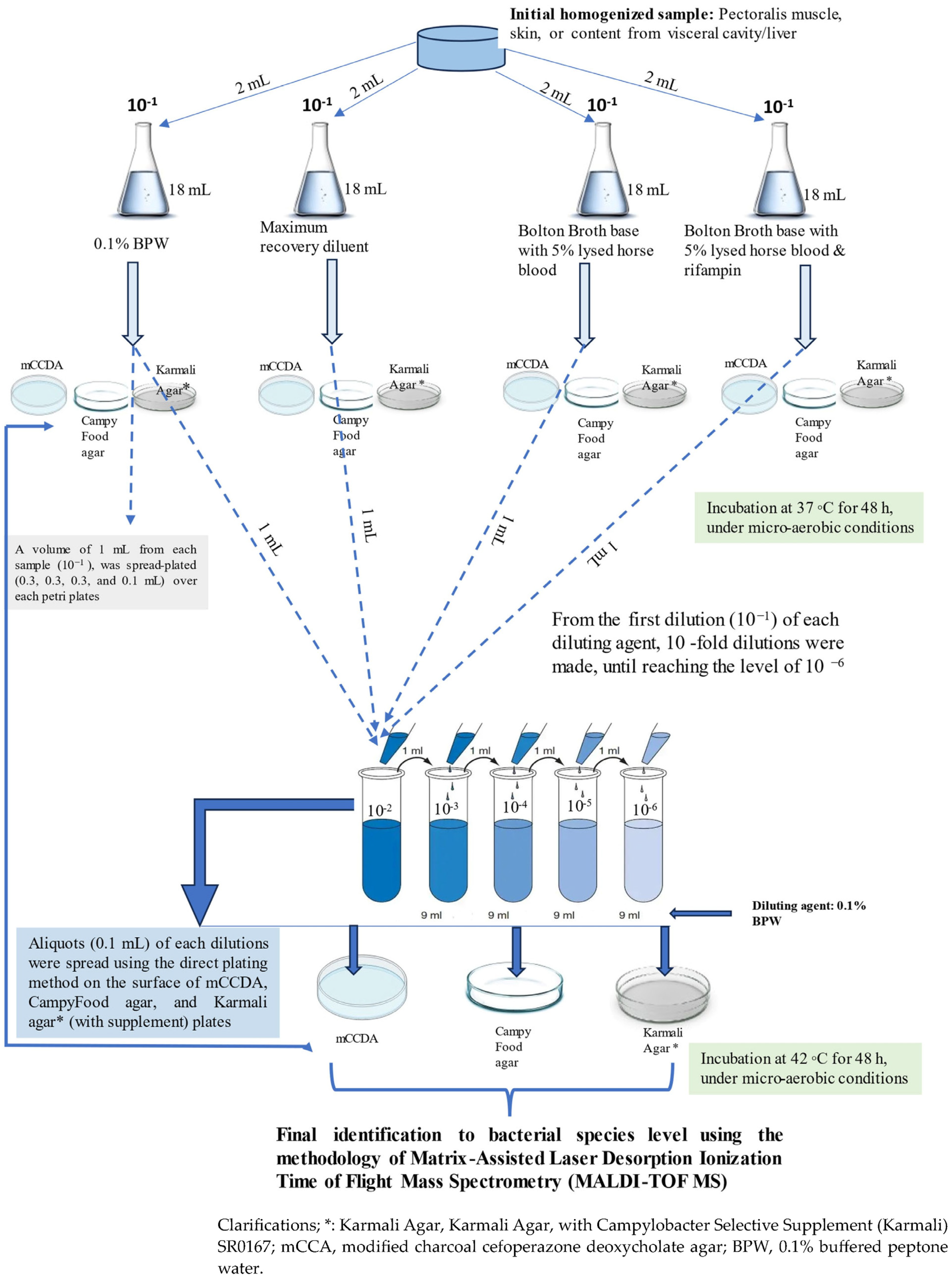
2.1. Microbiological Analyses for Isolation of Presumptive Campylobacter spp. Isolates
2.1.1. Quantitative Analysis
2.1.2. Species Identification
2.2. Screening the Antibiotic Susceptibility Pattern of Campylobacter Isolates
2.2.1. Phenotypic Characterization of Antibiotic-Resistant Profile
2.2.2. Inhibition of the Efflux Pump Factor and Its Effect on the Observed Resistance
2.2.3. Determination of Antibiotic Resistance-Encoded Genes
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
- -
- The Campylobacter species was found to have an impressive diversity in backyard chickens (17 species, 256 strains). As we extensively discussed in a previous study [26], these strains could be of environmental, anthropogenic or animal origin.
- -
- In general, the size of the flock plays an important role in the prevalence of the various species, with the large size favoring the presence of the microorganisms. A similar effect occurs in the presence of small ruminants. The ecology of Campylobacter seems to be species-specific.
- -
- Phenotypical resistance against 14 antibacterial pharmaceutical substances was recorded for most of the strains. Only 13 strains were found to be sensitive to all antibiotics, while 48 strains were resistant to one or two antibacterials. MAR resistance was very high (154 strains out of 215, which is 71.62%). In general, the phenotypes of resistance were also affected by the size of the flock as well as by the presence of small ruminants.
- -
- Genes coding antimicrobial resistance were detected (blaOXA-61, tet(A), tet(B), mutative gyrA, cmeA, cmeB, and cmeC) in most of the isolated strains. Apart from the blaOxA-61 which moved independently, the other genes’ prevalence was positively related to each other in their distribution in the epidemiological groups, a finding suggesting the possibility of circulating together in mobile genetic elements. This conclusion is also supported by the finding that the epidemiological criteria affected the prevalence of all other genes except for blaOxA-61.
- -
- The MIC values were seriously affected when the function of efflux pumps was inhibited by CCCP (including in the case of bile acids), implying that these pumps are an essential mechanism of defense and survival for the Campylobacter genus in the presence of antibacterial substances.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, e07666. [Google Scholar]
- Hansson, I.; Sandberg, M.; Habib, I.; Lowman, R.; Engvall, E.O. Knowledge gaps in control of Campylobacter for prevention of campylobacteriosis. Transbound. Emerg. Dis. 2018, 65 (Suppl. S1), 30–48. [Google Scholar] [CrossRef]
- Igwaran, A.; Okoh, A.I. Human campylobacteriosis: A public health concern of global importance. Heliyon 2019, 5, e02814. [Google Scholar] [CrossRef]
- Santos-Ferreira, N.; Ferreira, V.; Teixeira, P. Occurrence and Multidrug Resistance of Campylobacter in Chicken Meat from Different Production Systems. Foods 2022, 11, 1827. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Rothrock, M.J.; Dev Kumar, G.; Mishra, A. Assessing the Risk of Seasonal Effects of Campylobacter Contaminated Broiler Meat Prepared In-Home in the United States. Foods 2023, 12, 2559. [Google Scholar] [CrossRef] [PubMed]
- Thames, H.T.; Fancher, C.A.; Colvin, M.G.; Mcanally, M.; Tucker, E.; Zhang, L.; Kiess, A.S.; Dinh, T.T.N.; Sukumaran, A.T. The Prevalence of Salmonella and Campylobacter on Broiler Meat at Different Stages of Commercial Poultry Processing. Animals 2022, 12, 2460. [Google Scholar] [CrossRef] [PubMed]
- Alders, R.G.; Pym, R.A.E. Village poultry: Still important to millions, eight thousand years after domestication. World’s Poult. Sci. J. 2009, 65, 181–190. Available online: https://api.semanticscholar.org/CorpusID:85885987 (accessed on 26 December 2023). [CrossRef]
- Otte, J.; Rushton, J.; Rukambile, E.; Alders, R.G. Biosecurity in Village and Other Free-Range Poultry—Trying to Square the Circle? Front. Vet. Sci. 2021, 8, 678419. [Google Scholar] [CrossRef] [PubMed]
- FAO. The Future of Food and Agriculture—Alternative Pathways to 2050; Licence: CC BY-NC-SA 3.0 IGO; FAO: Rome, Italy, 2018; 224p. [Google Scholar]
- Fischer, G.H.; Paterek, E. Campylobacter. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Facciolà, A.; Riso, R.; Avventuroso, E.; Visalli, G.; Delia, S.A.; Laganà, P. Campylobacter: From microbiology to prevention. J. Prev. Med. Hyg. 2017, 58, E79–E92. [Google Scholar] [PubMed]
- El-Saadony, M.T.; Saad, A.M.; Yang, T.; Salem, H.M.; Korma, S.A.; Ahmed, A.E.; Mosa, W.F.A.; Abd El-Mageed, T.A.; Selim, S.; Al Jaouni, S.K.; et al. Avian campylobacteriosis, prevalence, sources, hazards, antibiotic resistance, poultry meat contamination, and control measures: A comprehensive review. Poult. Sci. 2023, 102, 102786. [Google Scholar] [CrossRef]
- Olvera-Ramírez, A.M.; Mcewan, N.R.; Stanley, K.; Nava-Diaz, R.; Aguilar-Tipacamú, G. A Systematic Review on the Role of Wildlife as Carriers and Spreaders of Campylobacter spp. Animals 2023, 13, 1334. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Feye, K.M.; Shi, Z.; Pavlidis, H.O.; Kogut, M.; Ashworth, A.J.; Ricke, S.C. A Historical Review on Antibiotic Resistance of Foodborne Campylobacter. Front. Microbiol. 2019, 10, 1509. [Google Scholar] [CrossRef] [PubMed]
- Shakir, Z.M.; Alhatami, A.O.; Ismail Khudhair, Y.; Muhsen Abdulwahab, H. Antibiotic Resistance Profile and Multiple Antibiotic Resistance Index of Campylobacter Species Isolated from Poultry. Arch. Razi Inst. 2021, 76, 1677–1686. [Google Scholar]
- Paintsil, E.K.; Ofori, L.A.; Akenten, C.W.; Zautner, A.E.; Mbwana, J.; Jaeger, A.; Lamshöft, M.; May, J.; Obiri-Danso, K.; Philipps, R.O.; et al. Antibiotic-resistant Campylobacter coli and Campylobacter jejuni in commercial and smallholder farm animals in the Asante Akim North Municipality of Ghana. Front. Microbiol. 2022, 13, 983047. [Google Scholar] [CrossRef]
- Portes, A.B.; Panzenhagen, P.; Pereira dos Santos, A.M.; Junior, C.A.C. Antibiotic Resistance in Campylobacter: A Systematic Review of South American Isolates. Antibiotics 2023, 12, 548. [Google Scholar] [CrossRef] [PubMed]
- Zachariah, O.H.; Lizzy, M.A.; Rose, K.; Angela, M.M. Multiple drug resistance of Campylobacter jejuni and Shigella isolated from diarrhoeic children at Kapsabet County referral hospital, Kenya. BMC Infect. Dis. 2021, 21, 1–8. [Google Scholar] [CrossRef]
- Luangtongkum, T.; Jeon, B.; Han, J.; Plummer, P.; Logue, C.M.; Zhang, Q. Antibiotic resistance in Campylobacter: Emergence, transmission and persistence. Future Microbiol. 2009, 4, 189–200. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/news-room/fact-sheets/detail/campylobacter (accessed on 22 December 2023).
- Ahmed, N.A.; Gulhan, T. Campylobacter in Wild Birds: Is It an Animal and Public Health Concern? Front. Microbiol. 2022, 12, 812591. [Google Scholar] [CrossRef]
- Horrocks, S.M.; Anderson, R.C.; Nisbet, D.J.; Ricke, S.C. Incidence and ecology of Campylobacter jejuni and coli in animals. Anaerobe 2009, 15, 18–25. [Google Scholar] [CrossRef]
- Pillay, S.; Amoako, D.G.; Abia, A.L.K.; Somboro, A.M.; Shobo, C.O.; Perrett, K.; Bester, L.A.; Essack, S.Y. Characterisation of Campylobacter spp. Isolated from Poultry in KwaZulu-Natal, South Africa. Antibiotics 2020, 9, 42. [Google Scholar] [CrossRef]
- Hakeem, M.J.; Lu, X. Survival and Control of Campylobacter in Poultry Production Environment. Front. Cell. Infect. Microbiol. 2021, 10, 615049. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Jiang, Q.; Tang, H.; Wang, Z.; Yin, Y.; Ren, F.; Kong, L.; Jiao, X.; Huang, J. Characterization and Prevalence of Campylobacter spp. From Broiler Chicken Rearing Period to the Slaughtering Process in Eastern China. Front. Vet. Sci. 2020, 7, 227. [Google Scholar] [CrossRef]
- Dermatas, A.; Rozos, G.; Voidarou, C.; Akrida-Demertzi, K.; Demertzis, P. Biodiversity Dynamics of Campylobacter Species in Chicken Tissues in Rural Households in Region Epirus, Greece. Appl. Sci. 2023, 13, 6073. [Google Scholar] [CrossRef]
- Feucherolles, M.; Nennig, M.; Becker, S.L.; Martiny, D.; Losch, S.; Penny, C.; Cauchie, H.-M.; Ragimbeau, C. Investigation of MALDI-TOF Mass Spectrometry for Assessing the Molecular Diversity of Campylobacter jejuni and Comparison with MLST and cgMLST: A Luxembourg One-Health Study. Diagnostics 2021, 11, 1949. [Google Scholar] [CrossRef]
- Ziomek, M.; Gondek, M.; Torracca, B.; Marotta, F.; Garofolo, G.; Wieczorek, K.; Michalak, K.; Fratini, F.; Pedonese, F. Occurrence of Campylobacter in Faeces, Livers and Carcasses of Wild Boars Hunted in Tuscany (Italy) and Evaluation of MALDI-TOF MS for the Identification of Campylobacter Species. Foods 2023, 12, 778. [Google Scholar] [CrossRef]
- CLSI. Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria, 2nd ed.; Clinical and Laboratory Standards Institute (M45eA2): Wayne, PA, USA, 2010. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute (M100e): Wayne, PA, USA, 2021. [Google Scholar]
- Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_13.0_Breakpoint_Tables.pdf (accessed on 20 November 2023).
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, Twenty Second Informational Supplement; Clinical and Laboratory Standards Institute (M100e): Wayne, PA, USA, 2012. [Google Scholar]
- Sanchez-Carbonel, A.; Mondragón, B.; López-Chegne, N.; Peña-Tuesta, I.; Huayan-Dávila, G.; Blitchtein, D.; Carrillo-Ng, H.; Silva-Caso, W.; Aguilar-Luis, M.A.; Del Valle-Mendoza, J. The effect of the efflux pump inhibitor Carbonyl Cyanide m-Chlorophenylhydrazone (CCCP) on the susceptibility to imipenem and cefepime in clinical strains of Acinetobacter baumannii. PLoS ONE 2021, 16, e0259915. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zheng, X.C.; Huang, Y.Y.; Ge, Y.P.; Sun, M.; Chen, W.L.; Liu, W.B.; Li, X.F. Carbonyl cyanide 3-chlorophenylhydrazone induced the imbalance of mitochondrial homeostasis in the liver of Megalobrama amblycephala: A dynamic study. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 244, 109003. [Google Scholar] [CrossRef]
- Hungaro, H.M.; Mendonça, R.C.S.; Rosa, V.O.; Badaró, A.C.L.; Moreira, M.A.S.; Chaves, J.B.P. Low contamination of Campylobacter spp. on chicken carcasses in Minas Gerais state, Brazil: Molecular characterization and antimicrobial resistance. Food Control 2015, 51, 15–22. [Google Scholar] [CrossRef]
- Poudel, S.; Li, T.; Chen, S.; Zhang, X.; Cheng, W.H.; Sukumaran, A.T.; Kiess, A.S.; Zhang, L. Prevalence, Antimicrobial Resistance, and Molecular Characterization of Campylobacter Isolated from Broilers and Broiler Meat Raised without Antibiotics. Microbiol. Spectr. 2022, 10, e0025122. [Google Scholar] [CrossRef]
- Koolman, L.; Whyte, P.; Burgess, C.; Bolton, D. Distribution of Virulence-Associated Genes in a Selection of Campylobacter Isolates. Foodborne Pathog. Dis. 2015, 12, 424–432. [Google Scholar] [CrossRef]
- Gibreel, A.; Tracz, D.M.; Nonaka, L.; Ngo, T.M.; Connell, S.R.; Taylor, D.E. Incidence of antibiotic resistance in Campylobacter jejuni isolated in Alberta, Canada, from 1999 to 2002, with special reference to tet(O)-mediated tetracycline resistance. Antimicrob. Agents Chemother 2004, 48, 3442–3450. [Google Scholar] [CrossRef]
- Abdi Hachesoo, B.; Khoshbakht, R.; Sharifi Yazdi, H.; Tabatabaei, M.; Hosseinzadeh, S.; Asasi, K. Tetracycline Resistance Genes in Campylobacter jejuni and C. coli Isolated From Poultry Carcasses. Jundishapur J. Microbiol. 2014, 7, e12129. [Google Scholar] [CrossRef] [PubMed]
- Obeng, A.S.; Rickard, H.; Sexton, M.; Pang, Y.; Peng, H.; Barton, M. Antimicrobial susceptibilities and resistance genes in Campylobacter strains isolated from poultry and pigs in Australia. J. Appl. Microbiol. 2012, 113, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Zirnstein, G.; Li, Y.; Swaminathan, B.; Angulo, F. Ciprofloxacin Resistance in Campylobacter jejuni Isolates: Detection of gyrA Resistance Mutations by Mismatch Amplification Mutation Assay PCR and DNA Sequence Analysis. J. Clin. Microbiol. 1999, 37, 3276–3280. [Google Scholar] [CrossRef] [PubMed]
- Schreyer, M.E.; Olivero, C.R.; Rossler, E.; Soto, L.P.; Frizzo, L.S.; Zimmermann, J.A.; Signorini, M.L.; Virginia, Z.M. Prevalence and antimicrobial resistance of Campylobacter jejuni and C. coli identified in a slaughterhouse in Argentina. Curr. Res. Food Sci. 2022, 5, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Michel, L.O.; Zhang, Q. CmeABC Functions as a Multidrug Efflux System in Campylobacter jejuni. Antimicrob. Agents Chemother. 2002, 46, 2124–2131. [Google Scholar] [CrossRef] [PubMed]
- Fakhr, M.K.; Logue, C.M. Sequence Variation in the Outer Membrane Protein-Encoding Gene cmeC, Conferring Multidrug Resistance among Campylobacter jejuni and Campylobacter coli Strains Is. J. Clin. Microbiol. 2007, 45, 3381–3383. [Google Scholar] [CrossRef]
- Wagenaar, J.A.; Van Bergen, M.A.P.; Blaser, M.J.; Tauxe, R.V.; Newell, D.G.; Van Putten, J.P.M. Campylobacter fetus Infections in Humans: Exposure and Disease. Clin. Infect. Dis. 2014, 58, 1579–1586. [Google Scholar] [CrossRef]
- Zanoni, R.G.; Debruyne, L.; Rossi, M.; Revez, J.; Vandamme, P. Campylobacter cuniculorum sp. nov., from rabbits. Int. J. Syst. Evol. Microbiol. 2009, 59, 1666–1671. [Google Scholar] [CrossRef]
- Elmonir, W.; Vetchapitak, T.; Amano, T.; Taniguchi, T.; Misawa, N. Survival capability of Campylobacter upsaliensis under environmental stresses. BMC Res. Notes 2022, 15, 1–5. [Google Scholar] [CrossRef]
- Kakuta, R.; Hidaka, H.; Yano, H.; Okamoto, M.; Ozawa, D.; Endo, S.; Kaku, M.; Katori, Y. First report of severe acute otitis media caused by Campylobacter rectus and review of the literature. J. Infect. Chemother. 2016, 22, 800–803. [Google Scholar] [CrossRef]
- Pohjola, L.; Nykäsenoja, S.; Kivistö, R.; Soveri, T.; Huovilainen, A.; Hänninen, M.L.; Fredriksson-Ahomaa, M. Zoonotic Public Health Hazards in Backyard Chickens. Zoonoses Public Health 2016, 63, 420–430. [Google Scholar] [CrossRef]
- Nather, G.; Alter, T.; Martin, A.; Ellerbroek, L. Analysis of risk factors for Campylobacter species infection in broiler flocks. Poult. Sci. 2009, 88, 1299–1305. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Deng, F.; Shen, Z.; Wu, C.; Zhang, J.; Zhang, Q.; Shen, J. Emergence of Multidrug-Resistant Campylobacter Species Isolates with a Horizontally Acquired rRNA Methylase. Antimicrob. Agents Chemother. 2014, 58, 5405–5412. [Google Scholar] [CrossRef] [PubMed]
- Lekshmi, M.; Kumar, S.H.; Nayak, B.B.; Varela, M.F. Antimicrobial Resistance in Food-Borne Campylobacter spp. In Handbook on Antimicrobial Resistance: Current Status, Trends in Detection and Mitigation Measures; Springer Nature: Singapore, 2023; pp. 1–19. [Google Scholar]
- Available online: https://www.who.int/publications/i/item/9789241564601 (accessed on 20 December 2023).
- Obaidat, M.; Alshdaifat, R.M. High prevalence of multidrug-resistant Campylobacter jejuni in sheep and goats milk in Jordan. Int. Dairy J. 2023, 143, 105676. [Google Scholar] [CrossRef]
- Bai, J.; Chen, Z.; Luo, K.; Zeng, F.; Qu, X.; Zhang, H.; Chen, K.; Lin, Q.; He, H.; Liao, M.; et al. Highly Prevalent Multidrug-Resistant Campylobacter spp. Isolated From a Yellow-Feathered Broiler Slaughterhouse in South China. Front. Microbiol. 2021, 12, 682741. [Google Scholar] [CrossRef]
- Montgomery, M.P.; Robertson, S.; Koski, L.; Salehi, E.; Stevenson, L.M.; Silver, R.; Sundararaman, P.; Singh, A.; Joseph, L.A.; Weisner, M.B.; et al. Multidrug-Resistant Campylobacter jejuni Outbreak Linked to Puppy Exposure—United States, 2016–2018. MMWR. Morb. Mortal. Wkly. Rep. 2018, 67, 1032–1035. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, J.N.; Eghnatios, E.; El Roz, A.; Fardoun, T.; Ghssein, G. Prevalence, antimicrobial resistance and risk factors for campylobacteriosis in Lebanon. J. Infect. Dev. Ctries. 2019, 13, 11–20. [Google Scholar] [CrossRef]
- Xiao, J.; Cheng, Y.; Zhang, W.; Lu, Q.; Guo, Y.; Hu, Q.; Wen, G.; Shao, H.; Luo, Q.; Zhang, T. Genetic characteristics, antimicrobial susceptibility, and virulence genes distribution of Campylobacter isolated from local dual-purpose chickens in central China. Front. Cell. Infect. Microbiol. 2023, 13, 1236777. [Google Scholar] [CrossRef]
- Beheshti, M.; Ardebili, A.; Beheshti, F.; Lari, A.R.; Siyadatpanah, A.; Pournajaf, A.; Gautam, D.; Dolma, K.G.; Nissapatorn, V. Tetracycline resistance mediated by tet efflux pumps in clinical isolates of Acinetobacter baumannii. Rev. Inst. Med. Trop. São Paulo 2020, 62, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Butucel, E.; Balta, I.; Bundurus, I.A.; Popescu, C.A.; Iancu, T.; Venig, A.; Pet, I.; Stef, D.; Mccleery, D.; Stef, L.; et al. Natural Antimicrobials Promote the Anti-Oxidative Inhibition of COX-2 Mediated Inflammatory Response in Primary Oral Cells Infected with Staphylococcus aureus, Streptococcus pyogenes and Enterococcus faecalis. Antioxidants 2023, 12, 1017. [Google Scholar] [CrossRef] [PubMed]
- Ge, B.; Mcdermott, P.F.; White, D.G.; Meng, J. Role of Efflux Pumps and Topoisomerase Mutations in Fluoroquinolone Resistance in Campylobacter jejuni and Campylobacter coli. Antimicrob. Agents Chemother. 2005, 49, 3347–3354. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yao, Y.; Huang, J.; Sun, Y.; Wu, Q.; Guo, D.; Wang, S. Effect of dietary bile acids supplementation on growth performance, feed utilization, intestinal digestive enzyme activity and fatty acid transporters gene expression in juvenile leopard coral grouper (Plectropomus leopardus). Front. Mar. Sci. 2023, 10, 1171344. [Google Scholar] [CrossRef]
- Urdaneta, V.; Casadesús, J. Interactions between Bacteria and Bile Salts in the Gastrointestinal and Hepatobiliary Tracts. Front. Med. 2017, 4, 1171344. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Cagliero, C.; Guo, B.; Barton, Y.-W.; Maurel, M.-C.; Payot, S.; Zhang, Q. Bile Salts Modulate Expression of the CmeABC Multidrug Efflux Pump in Campylobacter jejuni. J. Bacteriol. 2005, 187, 7417–7424. [Google Scholar] [CrossRef]
- Mavri, A.; Smole Možina, S. Effects of efflux-pump inducers and genetic variation of the multidrug transporter cmeB in biocide resistance of Campylobacter jejuni and Campylobacter coli. J. Med. Microbiol. 2013, 62 Pt 3, 400–411. [Google Scholar] [CrossRef]
- Možina, S.S.; Kurinčič, M.; Klančnik, A.; Mavri, A. Campylobacter and its multi-resistance in the food chain. Trends Food Sci. Technol. 2011, 22, 91–98. [Google Scholar] [CrossRef]
| Study Group | Epidemiological Criteria Parameters | |||
|---|---|---|---|---|
| Size of the Flock (Birds) | Presence of Other Poultry Species | Presence of Small Ruminants and/or Pigs | Feeding Approach: (a) Fed Leftovers or (b) Mainly Concentrates | |
| 1 (n * = 20) | 3–15 | no | No | (a) |
| 2 (n = 20) | 3–15 | yes | No | (a) |
| 3 (n = 20) | 3–15 | no | Yes | (a) |
| 4 (n = 20) | 3–15 | yes | Yes | (a) |
| 5 (n = 20) | 3–15 | yes | Yes | (b) |
| 6 (n = 40) | 16–40 | no | No | (a) |
| 7 (n = 40) | 16–40 | yes | No | (a) |
| 8 (n = 40) | 16–40 | no | Yes | (a) |
| 9 (n = 40) | 16–40 | yes | Yes | (a) |
| 10 (n = 40) | 16–40 | yes | Yes | (b) |
| 11 (n = 60) | 41–60 | no | No | (a) |
| 12 (n = 60) | 41–60 | yes | No | (a) |
| 13 (n = 60) | 41–60 | no | Yes | (a) |
| 14 (n = 60) | 41–60 | yes | Yes | (a) |
| 15 (n = 60) | 41–60 | yes | Yes | (b) |
| Target Gene | Primer Sequence (5′ to 3′) | Amplicon Length (bp) | References |
|---|---|---|---|
| tet(O) | F: GCGTTTTGTTTATGTGCG R: ATGGACAACCCGACAGAAG | 559 | [37,38] |
| tet(A) | F: GTGAAACCCAACATACCCC R: GAAGGCAAGCAGGATGTAG | 888 | [39] |
| blaOxA-61 | F: AGAGTATAATACAAGCG R: TAGTGAGTTGTCAAGCC | 372 | [35,40] |
| gyrA (Thr-86-Ile mutation) | F: TTTTTAGCAAAGATTCTGAT R: CAAAGCATCATAAACTGCAA | 265 | [36,41] |
| cmeA | F: TGTGCATCAGCTCCTGTGTAA R: ACGGACAAGCTTTGATGGCT | 957 | [37,42] |
| cmeB | F: GGTACAGATCCTGATCAAGCC R: AGGAATAAGTGTTGCACGGAAATT | 820 | [42,43] |
| cmeC | F: AGATGAAGCTTTTGTAAATT R: TATAAGCAATTTTATCATTT | 500 | [42,44] |
| Study Group a | Isolated Campylobacter Species | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Je b | co | la | fe | av | hp | hl | ur | sp | hy | gr | cc | sh | cu | Mu | re | up | Total | |
| 1 | - | 3 | - | - | - | 1 | - | - | - | - | - | - | - | - | 1 | 1 | 6 | |
| 2 | 2 | 3 | - | - | 2 | 2 | - | - | - | - | - | - | - | - | - | - | - | 9 |
| 3 | 5 | 2 | - | - | - | - | - | - | 1 | - | - | - | - | - | 1 | - | - | 9 |
| 4 | 3 | 1 | 2 | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | 7 |
| 5 | 3 | 1 | 2 | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | 7 |
| 6 | 3 | 2 | - | - | 3 | 1 | - | 1 | - | - | 1 | 1 | - | - | - | 1 | - | 13 |
| 7 | 2 | 4 | - | - | 3 | - | - | - | - | - | 1 | - | - | 1 | - | - | - | 11 |
| 8 | 4 | 3 | - | 5 | - | - | - | - | 1 | 1 | - | - | - | - | - | - | - | 14 |
| 9 | 9 | 5 | 3 | - | 5 | - | - | - | - | - | - | - | - | - | - | - | - | 22 |
| 10 | 7 | 7 | 1 | 3 | - | - | - | - | - | - | - | - | - | - | - | - | - | 18 |
| 11 | - | 9 | 4 | - | 5 | 3 | - | 4 | - | - | - | 1 | 1 | - | - | - | - | 27 |
| 12 | 11 | 8 | 4 | - | 2 | 2 | - | - | - | - | - | - | - | - | - | - | - | 27 |
| 13 | 13 | 5 | 2 | 2 | - | - | - | - | 2 | 2 | - | - | - | - | - | - | - | 26 |
| 14 | 18 | 7 | 2 | 2 | - | - | - | - | - | 1 | - | - | - | - | - | - | - | 30 |
| 15 | 18 | 4 | 3 | - | 2 | 2 | - | - | - | - | - | - | - | - | - | - | 1 | 30 |
| Total | 98 | 64 | 23 | 14 | 22 | 10 | 1 | 5 | 4 | 4 | 2 | 2 | 1 | 1 | 1 | 2 | 2 | 256 |
| Study Group a | Isolated Campylobacter Species | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Je b | co | la | fe | av | hp | hl | ur | sp | hy | gr | cc | sh | cu | Mu | re | up | Total | |
| 1 | - | 2 | - | - | - | 1 | - | - | - | - | - | - | - | - | - | 1 | 4 | |
| 2 | 1 | 3 | - | - | 2 | 2 | - | - | - | - | - | - | - | - | - | - | - | 8 |
| 3 | 5 | 2 | - | - | - | - | - | - | 1 | - | - | - | - | - | 1 | - | - | 9 |
| 4 | 3 | 1 | 2 | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | 7 |
| 5 | 3 | 1 | 2 | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | 7 |
| 6 | 3 | 2 | - | - | 4 | 1 | - | 2 | - | - | 1 | 1 | - | - | - | 1 | - | 15 |
| 7 | 2 | 4 | 1 | - | 3 | - | - | - | - | - | 1 | - | - | 1 | - | - | - | 12 |
| 8 | 4 | 3 | - | 5 | - | - | - | - | 1 | 1 | - | - | - | - | - | - | - | 14 |
| 9 | 9 | 5 | 3 | - | 5 | - | - | - | - | - | - | - | - | - | - | - | - | 22 |
| 10 | 7 | 7 | 1 | 3 | - | - | - | - | - | - | - | - | - | - | - | - | - | 18 |
| 11 | - | 9 | 4 | - | 5 | 3 | - | 4 | - | - | - | 1 | 1 | - | - | - | - | 27 |
| 12 | 11 | 8 | 4 | - | 2 | 2 | - | - | - | - | - | - | - | - | - | - | - | 27 |
| 13 | 13 | 5 | 2 | 2 | - | - | - | - | 2 | 2 | - | - | - | - | - | - | - | 26 |
| 14 | 18 | 7 | 2 | 2 | - | - | - | - | - | 1 | - | - | - | - | - | - | - | 30 |
| 15 | 17 | 4 | 3 | - | 2 | 2 | - | - | - | - | - | - | - | - | - | - | 1 | 29 |
| Total | 97 | 63 | 24 | 14 | 23 | 10 | 1 | 6 | 3 | 4 | 2 | 2 | 1 | 1 | 1 | 1 | 2 | 255 |
| Study Group a | AMC b | AMP | CIP | NAL | CHL | ERY | TER | GEN | STM | SUT | CFL | CFU | CFT | CFE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 4 | 0 | 0 |
| 2 | 1 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 7 | 0 | 0 |
| 3 | 0 | 6 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 6 | 0 | 0 | 0 |
| 4 | 2 | 9 | 3 | 4 | 0 | 1 | 1 | 0 | 0 | 3 | 9 | 5 | 3 | 0 |
| 5 | 1 | 1 | 0 | 3 | 0 | 0 | 2 | 0 | 0 | 0 | 6 | 5 | 3 | 0 |
| 6 | 3 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 8 | 1 | 0 |
| 7 | 1 | 6 | 1 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 1 | 0 | 0 |
| 8 | 1 | 6 | 4 | 1 | 0 | 0 | 5 | 0 | 1 | 2 | 8 | 5 | 1 | 0 |
| 9 | 2 | 13 | 11 | 6 | 1 | 5 | 13 | 3 | 4 | 11 | 16 | 12 | 2 | 0 |
| 10 | 5 | 11 | 7 | 6 | 0 | 4 | 9 | 0 | 1 | 10 | 14 | 8 | 2 | 0 |
| 11 | 1 | 9 | 9 | 0 | 0 | 2 | 9 | 3 | 2 | 15 | 15 | 3 | 1 | 1 |
| 12 | 0 | 6 | 16 | 1 | 0 | 0 | 14 | 2 | 1 | 19 | 21 | 11 | 2 | 0 |
| 13 | 2 | 10 | 15 | 5 | 1 | 2 | 13 | 1 | 13 | 16 | 17 | 16 | 9 | 0 |
| 14 | 2 | 15 | 21 | 1 | 1 | 1 | 17 | 0 | 9 | 18 | 23 | 23 | 5 | 0 |
| 15 | 5 | 17 | 18 | 3 | 0 | 5 | 15 | 0 | 12 | 15 | 25 | 20 | 13 | 0 |
| Total | 26 | 122 | 109 | 32 | 3 | 20 | 101 | 10 | 43 | 110 | 182 | 128 | 42 | 1 |
| Study Group b | Number of Antibiotics a | MAR Index c | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total | ||
| 1 | - | 2 | 5 | - | - | - | - | - | - | - | - | - | 7 | 0 |
| 2 | - | 2 | 7 | - | - | - | - | - | - | - | - | - | 9 | 0 |
| 3 | 1 | 4 | 1 | 1 | 2 | - | - | - | - | - | - | - | 9 | 3 |
| 4 | - | 1 | 2 | 1 | - | 4 | 2 | - | - | - | - | - | 10 | 7 |
| 5 | - | 1 | 1 | 1 | 1 | 1 | 1 | - | - | - | - | - | 6 | 4 |
| 6 | 2 | 2 | 2 | 5 | 2 | - | - | - | - | - | - | - | 13 | 7 |
| 7 | 5 | 1 | 4 | 2 | - | - | - | - | - | - | - | - | 12 | 2 |
| 8 | 4 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | - | - | - | - | 12 | 6 |
| 9 | - | - | - | - | 3 | 3 | 4 | 1 | 4 | 1 | - | - | 16 | 16 |
| 10 | - | - | 1 | 3 | 1 | 1 | 2 | 4 | 1 | 1 | - | - | 14 | 13 |
| 11 | 1 | 2 | 4 | 5 | 6 | 1 | 1 | - | - | - | 1 | - | 21 | 13 |
| 12 | - | 1 | 3 | 3 | 7 | 4 | 2 | - | 2 | - | - | - | 22 | 18 |
| 13 | - | - | - | - | - | 7 | 1 | 2 | 3 | - | 3 | 1 | 17 | 18 |
| 14 | - | - | 1 | - | 4 | 3 | 8 | 5 | 1 | 1 | - | - | 23 | 22 |
| 15 | - | - | - | 1 | 6 | 5 | 2 | 5 | 5 | 1 | - | - | 25 | 25 |
| Total | 13 | 17 | 32 | 25 | 33 | 30 | 27 | 18 | 18 | 4 | 4 | 1 | 215 | 154 |
| Antibiotics Agents | Criteria with Their Individual Recombination’s Shaped the Sampling Study Groups | |||||
|---|---|---|---|---|---|---|
| Small Size (the Size of the Flock: Up to 15 Birds) | Medium Size (the Size of the Flock: 15–40 Birds) | Large Size (the Size of the Flock: More than 40 Birds up to 60) | Presence of Other Poultry Species | Presence of Small Ruminants/or and Pigs | Feeding Approach: Mainly Administration of Concentrated Foods | |
| AMC a | (−) b | NA | (+) | NA | NA | (+) |
| AMP | (−) | NA | NA | NA | (+) | NA |
| CIP | NA | NA | (+) | NA | (+) | NA |
| NAL | NA | NA | NA | (+) | NA | NA |
| ERY | NA | NA | NA | NA | NA | (−) |
| TER | (−) | NA | (+) | NA | (+) | NA |
| GEN | NA | NA | (+) | NA | NA | NA |
| STM | NA | NA | (+) | NA | (+) | NA |
| SUT | NA | NA | (+) | NA | NA | NA |
| CFT | NA | NA | (+) | NA | (+) | NA |
| CFL | NA | NA | (+) | NA | NA | NA |
| CFU | NA | NA | (+) | (+) | NA | NA |
| Study Group | Isolated Campylobacter Species | |||||
|---|---|---|---|---|---|---|
| C. jejuni | C. coli | C. lari | C. avium | C. fetus | C. hepaticus | |
| 1 | - * | 2 **,2,2 | - | 2 | - | - |
| 2 | 2,2 | 2,2,2 | - | 2,1 | - | 2,1 |
| 3 | 2,3,1,1,4 | 4,0 | - | - | - | - |
| 4 | 6,5,6 | 5,5 | - | - | 3,5 | - |
| 5 | 2,3 | 6 | 5,4 | - | 1 | - |
| 6 | 2,3,2 | 3 | - | 3,3,3 | - | 1 |
| 7 | 0,3 | 2,2,1,2 | 0 | 3,0,2 | - | - |
| 8 | 4,2,6,7 | 3,6,1 | - | - | 0,5,0 | - |
| 9 | 7,8,6,8,8 | 6,5,6 | 9,5,8 | 5 | 6,4,4,4 | - |
| 10 | 9,7,6,7,4 | 7,5,3,3,8 | 7 | - | 2,3,7 | - |
| 11 | - | 6,2,4,2,4, 4,4,4 | 2,4 | 4,3,2,3 | - | 1,3,0 |
| 12 | 3,2,5,4,5, 4,6,8,4 | 1,3,2,4,8 | 4,4,4,6 | 2,3 | - | 5,5 |
| 13 | 11,10,6,7, 5,5,5,6 | 8,6,10,8,10 | 5,5 | - | 5 | - |
| 14 | 7,7,8,7,6,6, 6,6,5,7,2,7 | 9,6,4,6,5,4 | 6,5 | - | 4,4 | - |
| 15 | 9,7,5,4,4,4, 8,7,6,8,5,8,8 | 7,7,5,8 | 4,5 | 5,4 | - | 6,7 |
| Study Group a | Antibiotic Resistance-Encoded Genes/Mutations | ||||||
|---|---|---|---|---|---|---|---|
| blaOxA-61 | tet(O) | tet(A) | gyrA (Thr-86-Ile Mutation) | cmeA | cmeB | cmeC | |
| 1 | 1 b | 2 | 3 | 0 | 5 | 3 | 1 |
| 2 | 6 | 0 | 3 | 0 | 7 | 6 | 3 |
| 3 | 5 | 0 | 4 | 5 | 8 | 4 | 4 |
| 4 | 8 | 3 | 5 | 6 | 7 | 5 | 6 |
| 5 | 1 | 1 | 2 | 4 | 4 | 5 | 3 |
| 6 | 8 | 1 | 2 | 10 | 8 | 11 | 8 |
| 7 | 6 | 1 | 4 | 7 | 11 | 9 | 6 |
| 8 | 5 | 4 | 4 | 7 | 12 | 11 | 6 |
| 9 | 13 | 7 | 9 | 11 | 15 | 15 | 14 |
| 10 | 11 | 6 | 7 | 11 | 11 | 14 | 14 |
| 11 | 11 | 6 | 4 | 8 | 15 | 14 | 12 |
| 12 | 6 | 10 | 6 | 17 | 21 | 13 | 7 |
| 13 | 3 | 10 | 6 | 11 | 15 | 19 | 17 |
| 14 | 6 | 7 | 7 | 18 | 12 | 23 | 24 |
| 15 | 7 | 16 | 9 | 14 | 18 | 24 | 24 |
| Total | 97 | 74 | 75 | 129 | 169 | 176 | 149 |
| Study Group | Number of Genes/Mutations Coding Resistance to Antibiotics | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Total | |
| 1 | 1 * | 1 | 2 | 1 | 2 | 7 | |||
| 2 | 1 | 1 | 3 | 1 | 3 | 9 | |||
| 3 | 2 | 2 | 2 | 2 | 1 | 9 | |||
| 4 | 1 | 3 | 3 | 2 | 1 | 10 | |||
| 5 | 1 | 2 | 3 | 6 | |||||
| 6 | 2 | 3 | 5 | 3 | 13 | ||||
| 7 | 3 | 1 | 1 | 2 | 2 | 3 | 12 | ||
| 8 | 3 | 2 | 1 | 3 | 3 | 12 | |||
| 9 | 2 | 2 | 3 | 8 | 1 | 16 | |||
| 10 | 1 | 1 | 6 | 5 | 1 | 14 | |||
| 11 | 5 | 2 | 2 | 4 | 4 | 17 | |||
| 12 | 1 | 5 | 5 | 3 | 5 | 3 | 22 | ||
| 13 | 3 | 3 | 7 | 5 | 2 | 20 | |||
| 14 | 2 | 6 | 6 | 8 | 2 | 24 | |||
| 15 | 3 | 6 | 10 | 5 | 24 | ||||
| Total | 2 | 8 | 30 | 34 | 46 | 56 | 37 | 2 | 215 |
| Study Group | AMP a | TET | CIP | |||
|---|---|---|---|---|---|---|
| Reduced MIC | Not Affected | Reduced MIC | Not Affected | Reduced MIC | Not Affected | |
| 1 (n b = 7) | 6 | 1 | 2 | 5 | 2 | 5 |
| 2 (n = 9) | 6 | 3 | 9 | 0 | 1 | 8 |
| 3 (n = 9) | 8 | 1 | 5 | 4 | 5 | 4 |
| 4 (n = 10) | 10 | 0 | 8 | 2 | 4 | 6 |
| 5 (n = 6) | 6 | 0 | 5 | 1 | 2 | 4 |
| 6 (n = 13) | 10 | 3 | 8 | 5 | 1 | 12 |
| 7 (n = 12) | 12 | 0 | 6 | 6 | 1 | 11 |
| 8 (n = 12) | 11 | 1 | 9 | 3 | 6 | 6 |
| 9 (n = 16) | 15 | 1 | 14 | 2 | 11 | 5 |
| 10 (n = 14) | 10 | 4 | 10 | 4 | 5 | 9 |
| 11 (n = 17) | 5 | 12 | 10 | 7 | 3 | 14 |
| 12 (n = 22) | 12 | 10 | 10 | 12 | 11 | 11 |
| 13 (n = 20) | 13 | 7 | 8 | 12 | 6 | 14 |
| 14 (n = 24) | 14 | 10 | 13 | 11 | 15 | 9 |
| 15 (n = 24) | 13 | 11 | 13 | 11 | 17 | 7 |
| Total (n = 215) | 151 | 64 | 130 | 85 | 90 | 125 |
| Isolates with Species Specification | n (% of the Total 52 Resistant) | % (of the Total Isolates of Each Isolated Species, at 42 °C, as Incubation Temperature in Microbiological Analyses) |
|---|---|---|
| C. jejuni | 22 (42.31%) | 22.45% |
| C. coli | 10 (19.23%) | 15.63% |
| C. fetus | 4 (7.69%) | 28.57% |
| C. avium | 7 (13.46%) | 31.81% |
| C. lari | 3 (5.77%) | 13.04% |
| C. mucosalis | 1 (1.92%) | 100.00% |
| C. concisus | 1 (1.92%) | 50.00% |
| C. hepaticus | 2 (1.92%) | 20.00% |
| C. hyointestinalis | 1 (1.92%) | 25.00% |
| C. sputorum | 1 (1.92%) | 25.00% |
| Total | 52 |
| Study Group | Campylobacter Species | |||||
|---|---|---|---|---|---|---|
| C. coli | C. jejuni | C. lari | C. avium | C. fetus | C. hepaticus | |
| 1 | 0.071 a | - | - | 0.143 | - | - |
| 2 | 0.071 | 0.143 | - | 0.107 | - | 0.107 |
| 3 | 0.143 | 0.195 | - | - | - | - |
| 4 | 0.357 | 0.402 | - | - | 0.286 | - |
| 5 | 0.429 | 0.178 | 0.320 | - | 0.071 | - |
| 6 | 0.214 | 0.166 | - | 0.214 | - | 0.071 |
| 7 | 0.124 | 0.107 | 0 | 0.118 | - | - |
| 8 | 0.238 | 0.337 | - | - | 0.118 | - |
| 9 | 0.402 | 0.525 | 0.521 | 0.357 | 0.320 | - |
| 10 | 0.369 | 0.469 | 0.500 | - | 0.286 | - |
| 11 | 0.266 | - | 0.214 | 0.214 | - | 0.095 |
| 12 | 0.256 | 0.323 | 0.320 | 0.178 | - | 0.357 |
| 13 | 0.625 | 0.488 | 0.357 | - | 0.357 | - |
| 14 | 0.402 | 0.408 | 0.391 | - | 0.286 | - |
| 15 | 0.479 | 0.453 | 0.320 | 0.320 | - | 0.462 |
| Antibiotic Resistance-Encoded Genes/Mutations | Criteria with Their Individual Recombinations Shaped the Sampling Study Groups | |||||
|---|---|---|---|---|---|---|
| Small Size (the Size of the Flock: Up to 15 Birds) | Medium Size (the Size of the Flock: 15–40 Birds) | Large Size (the Size of the Flock: More than 40 Birds up to 60) | Presence of Other Poultry Species | Presence of Small Ruminants and/or Pigs | Feeding Approach: Mainly Administration of Concentrated Foods | |
| blaOxA-61 | NA a | NA | NA | NA | NA | NA |
| tet(O) | NA | NA | (+) | NA | NA | NA |
| tet(A) | (−) | NA | NA | NA | (+) | NA |
| gyrA(Thr-86-Ile mutation) | (−) | NA | (+) | NA | NA | NA |
| cmeA | (−) | NA | (+) | NA | NA | NA |
| cmeB | (−) | NA | (+) | (+) | (+) | NA |
| cmeC | (−) | NA | (+) | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dermatas, A.; Rozos, G.; Zaralis, K.; Dadamogia, A.; Fotou, K.; Bezirtzoglou, E.; Akrida-Demertzi, K.; Demertzis, P.; Voidarou, C. Overview of Ecology and Aspects of Antibiotic Resistance in Campylobacter spp. Isolated from Free-Grazing Chicken Tissues in Rural Households. Microorganisms 2024, 12, 368. https://doi.org/10.3390/microorganisms12020368
Dermatas A, Rozos G, Zaralis K, Dadamogia A, Fotou K, Bezirtzoglou E, Akrida-Demertzi K, Demertzis P, Voidarou C. Overview of Ecology and Aspects of Antibiotic Resistance in Campylobacter spp. Isolated from Free-Grazing Chicken Tissues in Rural Households. Microorganisms. 2024; 12(2):368. https://doi.org/10.3390/microorganisms12020368
Chicago/Turabian StyleDermatas, Argyrios, Georgios Rozos, Konstantinos Zaralis, Aikaterini Dadamogia, Konstantina Fotou, Eugenia Bezirtzoglou, Konstantoula Akrida-Demertzi, Panagiotis Demertzis, and Chrysoula (Chrysa) Voidarou. 2024. "Overview of Ecology and Aspects of Antibiotic Resistance in Campylobacter spp. Isolated from Free-Grazing Chicken Tissues in Rural Households" Microorganisms 12, no. 2: 368. https://doi.org/10.3390/microorganisms12020368
APA StyleDermatas, A., Rozos, G., Zaralis, K., Dadamogia, A., Fotou, K., Bezirtzoglou, E., Akrida-Demertzi, K., Demertzis, P., & Voidarou, C. (2024). Overview of Ecology and Aspects of Antibiotic Resistance in Campylobacter spp. Isolated from Free-Grazing Chicken Tissues in Rural Households. Microorganisms, 12(2), 368. https://doi.org/10.3390/microorganisms12020368







