Unveiling the Probiotic Potential of Streptococcus thermophilus MCC0200: Insights from In Vitro Studies Corroborated with Genome Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Culture Conditions
2.2. Genome Sequencing and Annotation
2.3. Evolutionary Analysis
2.4. Nucleotide Sequence Accession Number
2.5. In Vitro Evaluation of Probiotic Properties of MCC0200
2.5.1. Resistance to Simulated Gastrointestinal Conditions
2.5.2. Adhesion Potential
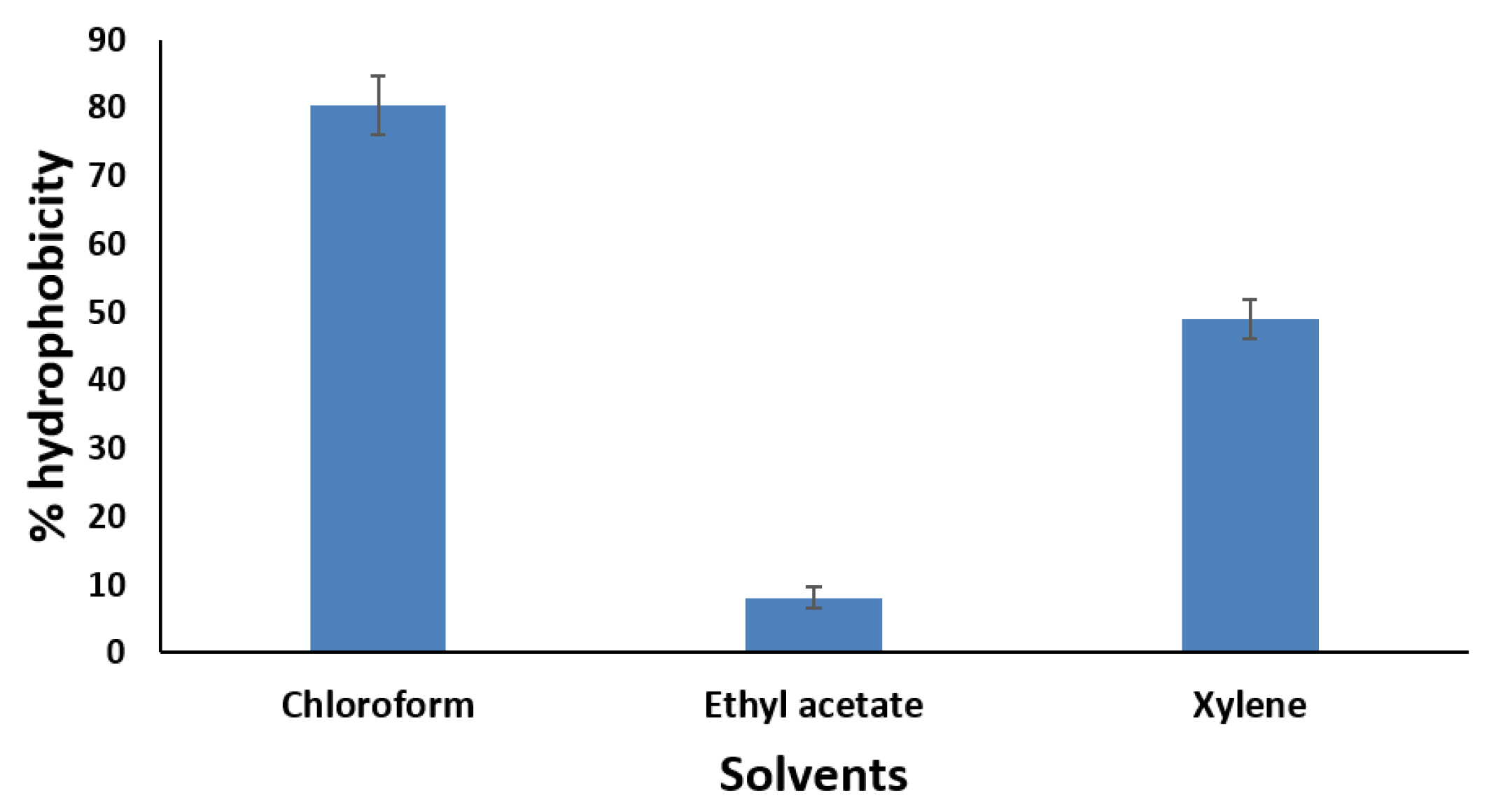
Cell Surface Hydrophobicity
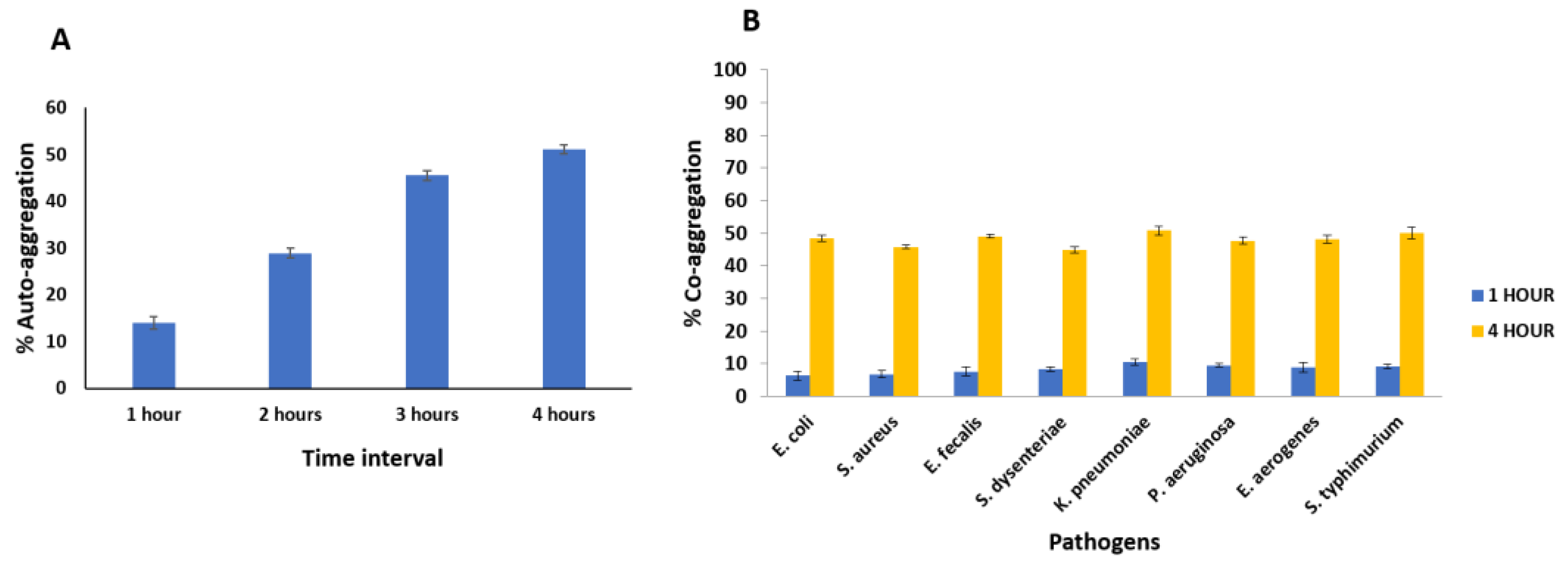
Aggregation Assay
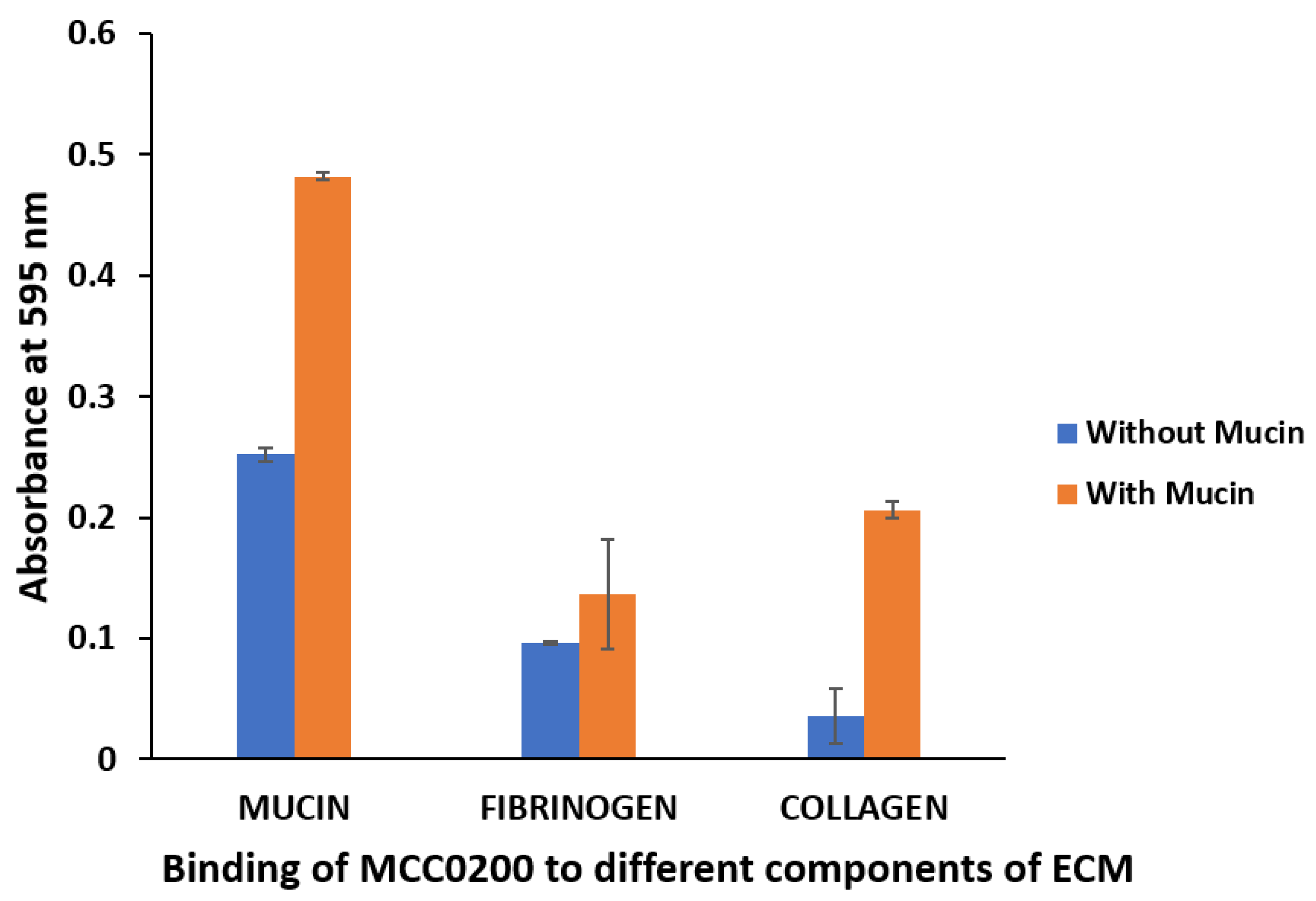
In Vitro Binding to Mucin, Fibrinogen and Collagen
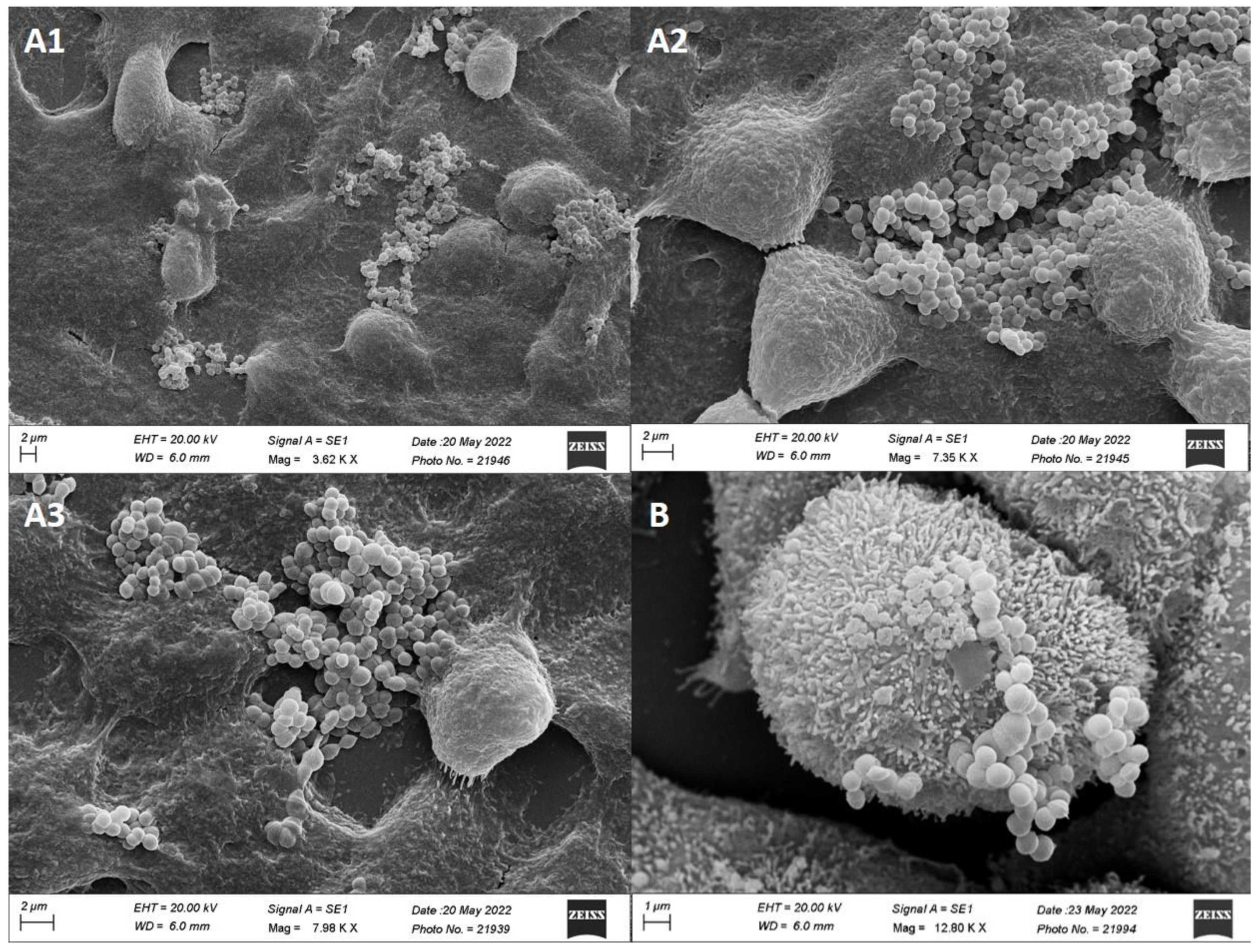
Adhesion of MCC0200 to HT-29
2.6. Antioxidant Activity
2.6.1. Scavenging Activity to 2,2-Diphenyl-1-Picrylhydrazyl Free Radical (DPPH)
2.6.2. Scavenging Activity to ABTS (2,2′-Azino-Bis(3-Ethylbenzothiazoline-6-Sulfonic acid) Radical
2.7. In Vitro Evaluation of the Anti-Hypercholesterolemic Effect of MCC0200
2.8. Screening of MCC0200 for Beta-Galactosidase Production
2.9. Safety Assessment
2.9.1. Antibiotic Susceptibility/Resistance Testing
2.9.2. Pathogenicity and Virulence
2.9.3. Stability of the Genome
3. Results and Discussion
3.1. Genome Attributes of S. thermophilus MCC0200:
3.2. Evolutionary Analysis and Comparison of MCC0200 with Other S. thermophilus Strains
3.3. Assessment of Probiotic Properties
3.3.1. Resistance to Gastric Conditions
3.3.2. Adhesion Potential of MCC0200
Assays for Evaluating Bacterial Adhesion
- Cell surface Hydrophobicity of MCC0200
- 2.
- Aggregation ability of MCC0200
- 3.
- Adhesion to mucin, fibrinogen and collagen
Adhesion of MCC0200 to HT-29 Cell Line
3.3.3. Antioxidant Activity
The Redox System in MCC0200
3.3.4. MCC0200 as Nutrient Factory: Biosynthetic Capabilities
3.3.5. Beta Galactosidase Production
3.3.6. In Vitro Evaluation of the Anti-Hypercholesterolemic Effect of MCC0200
3.4. Safety Assessment of MCC0200 as a Probiotic
Genome Based Safety Evaluation of MCC0200
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horiuchi, H.; Sasaki, Y. Effect of oxygen on symbiosis between Lactobacillus bulgaricus and Streptococcus thermophilus. J. Dairy Sci. 2012, 95, 2904–2909. [Google Scholar] [CrossRef]
- Iyer, R.; Tomar, S.K.; Maheswari, T.U.; Singh, R. Streptococcus thermophilus strains: Multifunctional lactic acid bacteria. Int. Dairy J. 2010, 20, 133–141. [Google Scholar] [CrossRef]
- Food and Drug Administration [FDA]. 21 CFR Part 131: Microorganisms & Microbial-Derived Ingredients Used in Food (Partial List); FDA: Silver Spring, MA, USA, 2007.
- EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Álvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; Hilbert, F.; Lindqvist, R.; et al. Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 9: Suitability of taxonomic units notified to EFSA until September 2018. Efsa J. 2019, 17, e05555. [Google Scholar]
- Cui, Y.; Xu, T.; Qu, X.; Hu, T.; Jiang, X.; Zhao, C. New insights into various production characteristics of Streptococcus thermophilus strains. Int. J. Mol. Sci. 2016, 17, 1701. [Google Scholar] [CrossRef]
- Martinović, A.; Cocuzzi, R.; Arioli, S.; Mora, D. Streptococcus thermophilus: To survive, or not to survive the gastrointestinal tract, that is the question! Nutrients 2020, 12, 2175. [Google Scholar] [CrossRef]
- Uriot, O.; Denis, S.; Junjua, M.; Roussel, Y.; Dary-Mourot, A.; Blanquet-Diot, S. Streptococcus thermophilus: From yogurt starter to a new promising probiotic candidate? J. Funct. Foods 2017, 37, 74–89. [Google Scholar] [CrossRef]
- del Campo, R.; Bravo, D.; Cantón, R.; Ruiz-Garbajosa, P.; García-Albiach, R.; Montesi-Libois, A.; Yuste, F.J.; Abraira, V.; Baquero, F. Scarce evidence of yogurt lactic acid bacteria in human feces after daily yogurt consumption by healthy volunteers. Appl. Environ. Microbiol. 2005, 71, 547–549. [Google Scholar] [CrossRef]
- Ballesta, S.; Velasco, C.; Borobio, M.V.; Argueelles, F.; Perea, E.J. Fresh versus pasteurized yogurt: Comparative study of the effects on microbiological and immunological parameters, and gastrointestinal comfort. Enfermedades Infecc. Microbiol. Clin. 2008, 26, 552–557. [Google Scholar] [CrossRef][Green Version]
- Elli, M.; Callegari, M.L.; Ferrari, S.; Bessi, E.; Cattivelli, D.; Soldi, S.; Morelli, L.; Goupil Feuillerat, N.; Antoine, J.M. Survival of yogurt bacteria in the human gut. Appl. Environ. Microbiol. 2006, 72, 5113–5117. [Google Scholar] [CrossRef]
- Mater, D.D.; Bretigny, L.; Firmesse, O.; Flores, M.J.; Mogenet, A.; Bresson, J.L.; Corthier, G. Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus survive gastrointestinal transit of healthy volunteers consuming yogurt. FEMS Microbiol. Lett. 2005, 250, 185–187. [Google Scholar] [CrossRef]
- Hu, T.; Cui, Y.; Zhang, Y.; Qu, X.; Zhao, C. Genome analysis and physiological characterization of four Streptococcus thermophilus strains isolated from Chinese traditional fermented milk. Front. Microbiol. 2020, 11, 184. [Google Scholar] [CrossRef] [PubMed]
- Roux, E.; Nicolas, A.; Valence, F.; Siekaniec, G.; Chuat, V.; Nicolas, J.; Le Loir, Y.; Guédon, E. The genomic basis of the Streptococcus thermophilus health-promoting properties. BMC Genom. 2022, 23, 210. [Google Scholar] [CrossRef]
- Cui, Y.; Jiang, X.; Hao, M.; Qu, X.; Hu, T. New advances in exopolysaccharides production of Streptococcus thermophilus. Arch. Microbiol. 2017, 199, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.Q.; Kong, L.H.; Lai, P.F.H.; Xia, Y.J.; Liu, J.C.; Li, Q.Y.; Ai, L.Z. Genomic and phenotypic analyses of exopolysaccharide biosynthesis in Streptococcus thermophilus S-3. J. Dairy Sci. 2019, 102, 4925–4934. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Cui, Y.; Qu, X. Analysis of CRISPR-Cas system in Streptococcus thermophilus and its application. Front. Microbiol. 2018, 9, 257. [Google Scholar] [CrossRef]
- Tian, H.; Muhammad, Z.; Evivie, S.E.; Gu, C.T.; Huo, G.C. Exact identification of six starter-strain candidates of Streptococcus thermophilus by analysing genotypic and industrial properties. Int. J. Dairy Technol. 2018, 71, 11–21. [Google Scholar] [CrossRef]
- Prajapati, J.B.; Nathani, N.M.; Patel, A.K.; Senan, S.; Joshi, C.G. Genomic analysis of dairy starter culture Streptococcus thermophilus MTCC 5461. J. Microbiol. Biotechnol. 2013, 23, 459–466. [Google Scholar] [CrossRef]
- Rasmussen, T.B.; Danielsen, M.; Valina, O.; Garrigues, C.; Johansen, E.; Pedersen, M.B. Streptococcus thermophilus core genome: Comparative genome hybridization study of 47 strains. Appl. Environ. Microbiol. 2008, 74, 4703–4710. [Google Scholar] [CrossRef]
- Vendramin, V.; Treu, L.; Campanaro, S.; Lombardi, A.; Corich, V.; Giacomini, A. Genome comparison and physiological characterization of eight Streptococcus thermophilus strains isolated from Italian dairy products. Food Microbiol. 2017, 63, 47–57. [Google Scholar] [CrossRef]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 1–15. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Morishima, K. BlastKOALA and GhostKOALA: KEGG tools for functional characterization of genome and metagenome sequences. J. Mol. Biol. 2016, 428, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Vecchione, A.; Celandroni, F.; Mazzantini, D.; Senesi, S.; Lupetti, A.; Ghelardi, E. Compositional quality and potential gastrointestinal behavior of probiotic products commercialized in Italy. Front. Med. 2018, 5, 59. [Google Scholar] [CrossRef] [PubMed]
- Prokopovich, P.; Perni, S. An investigation of microbial adhesion to natural and synthetic polysaccharide-based films and its relationship with the surface energy components. J. Mater. Sci. Mater. Med. 2009, 20, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Farniya, F.; Jamalli, A.; Dadgar, T. Physicochemical surface characteristics in different pathogenic bacteria. Cogent Biol. 2019, 5, 1638572. [Google Scholar] [CrossRef]
- Sharma, S.; Kanwar, S.S. Adherence potential of indigenous lactic acid bacterial isolates obtained from fermented foods of Western Himalayas to intestinal epithelial Caco-2 and HT-29 cell lines. J. Food Sci. Technol. 2017, 54, 3504–3511. [Google Scholar] [CrossRef] [PubMed]
- Inturri, R.; Stivala, A.; Sinatra, F.; Morrone, R.; Blandino, G. Scanning electron microscopy observation of adhesion properties of Bifidobacterium longum W11 and chromatographic analysis of its exopolysaccaride. Food Nutr. Sci. 2014, 5, 1787. [Google Scholar]
- Mu, G.; Gao, Y.; Tuo, Y.; Li, H.; Zhang, Y.; Qian, F.; Jiang, S. Assessing and comparing antioxidant activities of lactobacilli strains by using different chemical and cellular antioxidant methods. J. Dairy Sci. 2018, 101, 10792–10806. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Yu, X.; Jing, Y. Optimized preparation, characterization, and antioxidant activity of chitooligosaccharide–glycine Maillard reaction products. J. Food Sci. Technol. 2018, 55, 712–720. [Google Scholar] [CrossRef]
- Tomaro-Duchesneau, C.; Jones, M.L.; Shah, D.; Jain, P.; Saha, S.; Prakash, S. Cholesterol assimilation by Lactobacillus probiotic bacteria: An in vitro investigation. BioMed Res. Int. 2014, 2014, 380316. [Google Scholar] [CrossRef]
- Rudel, L.L.; Morris, M.D. Determination of cholesterol using o-phthalaldehyde. J. Lipid Res. 1973, 14, 364–366. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ); Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernández Escámez, P.S.; Girones, R.; Koutsoumanis, K.; Lindqvist, R.; et al. Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 8: Suitability of taxonomic units notified to EFSA until March 2018. EFSA J. 2018, 16, e05315. [Google Scholar]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.L.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020, 48, D517–D525. [Google Scholar] [CrossRef] [PubMed]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef] [PubMed]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016, 44, W16–W21. [Google Scholar] [CrossRef]
- Couvin, D.; Bernheim, A.; Toffano-Nioche, C.; Touchon, M.; Michalik, J.; Néron, B.; Rocha, E.P.; Vergnaud, G.; Gautheret, D.; Pourcel, C. CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrates search for Cas proteins. Nucleic Acids Res. 2018, 46, W246–W251. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A.; Zankari, E.; García-Fernández, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Møller Aarestrup, F.; Hasman, H. In-silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef]
- Alexandraki, V.; Kazou, M.; Blom, J.; Pot, B.; Papadimitriou, K.; Tsakalidou, E. Comparative genomics of Streptococcus thermophilus support important traits concerning the evolution, biology and technological properties of the species. Front. Microbiol. 2019, 10, 2916. [Google Scholar] [CrossRef]
- Auch, A.F.; von Jan, M.; Klenk, H.P.; Göker, M. Digital DNA-DNA hybridization for microbial species delineation by means of genome-to-genome sequence comparison. Stand. Genom. Sci. 2010, 2, 117–134. [Google Scholar] [CrossRef]
- Lee, I.; Ouk Kim, Y.; Park, S.C.; Chun, J. OrthoANI: An improved algorithm and software for calculating average nucleotide identity. Int. J. Syst. Evol. Microbiol. 2016, 66, 1100–1103. [Google Scholar] [CrossRef]
- Alikhan, N.F.; Petty, N.K.; Ben Zakour, N.L.; Beatson, S.A. BLAST Ring Image Generator (BRIG): Simple prokaryote genome comparisons. BMC Genom. 2011, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, M.; Xu, J.; Qi, Y.; Zhao, N.; Fan, M. First insight into the probiotic properties of ten Streptococcus thermophilus strains based on in vitro conditions. Curr. Microbiol. 2020, 77, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.H.; Lai, Y.J.; Chou, C.C. The susceptibility of Streptococcus thermophilus 14085 to organic acid, simulated gastric juice, bile salt and disinfectant as influenced by cold shock treatment. Food Microbiol. 2013, 33, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.J.; Goin, C.; O’Flaherty, S.; Altermann, E.; Hutkins, R. Specialized adaptation of a lactic acid bacterium to the milk environment: The comparative genomics of Streptococcus thermophilus LMD-9. Microb. Cell Factories 2011, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Padan, E. The enlightening encounter between structure and function in the NhaA Na+–H+ antiporter. Trends Biochem. Sci. 2008, 33, 435–443. [Google Scholar] [CrossRef]
- Mora, D.; Monnet, C.; Parini, C.; Guglielmetti, S.; Mariani, A.; Pintus, P.; Molinari, F.; Daffonchio, D.; Manachini, P.L. Urease biogenesis in Streptococcus thermophilus. Res. Microbiol. 2005, 156, 897–903. [Google Scholar] [CrossRef]
- Bustos, A.Y.; de Valdez, G.F.; Fadda, S.; Taranto, M.P. New insights into bacterial bile resistance mechanisms: The role of bile salt hydrolase and its impact on human health. Food Res. Int. 2018, 112, 250–262. [Google Scholar] [CrossRef]
- Tuncer, B.O.; Tuncer, Y. Exopolysaccharide producer Streptococcus thermophilus ST8. 01 strain; a potential probiotic culture. GIDA 2014, 39, 195–202. [Google Scholar]
- Arias, C.A.; Murray, B.E. Antibiotic-resistant bugs in the 21st century—A clinical super-challenge. N. Engl. J. Med. 2009, 360, 439–443. [Google Scholar] [CrossRef]
- Ruiz, L.; Margolles, A.; Sánchez, B. Bile resistance mechanisms in Lactobacillus and Bifidobacterium. Front. Microbiol. 2013, 4, 396. [Google Scholar] [CrossRef]
- Kebouchi, M.; Galia, W.; Genay, M.; Soligot, C.; Lecomte, X.; Awussi, A.A.; Perrin, C.; Roux, E.; Dary-Mourot, A.; Le Roux, Y. Implication of sortase-dependent proteins of Streptococcus thermophilus in adhesion to human intestinal epithelial cell lines and bile salt tolerance. Appl. Microbiol. Biotechnol. 2016, 100, 3667–3679. [Google Scholar] [CrossRef]
- Pfeiler, E.A.; Azcarate-Peril, M.A.; Klaenhammer, T.R. Characterization of a novel bile-inducible operon encoding a two-component regulatory system in Lactobacillus acidophilus. J. Bacteriol. 2007, 189, 4624–4634. [Google Scholar] [CrossRef]
- Weiss, G.; Jespersen, L. Transcriptional analysis of genes associated with stress and adhesion in Lactobacillus acidophilus NCFM during the passage through an in vitro gastrointestinal tract model. Microb. Physiol. 2010, 18, 206–214. [Google Scholar] [CrossRef]
- Duary, R.K.; Bhausaheb, M.A.; Batish, V.K.; Grover, S. Anti-inflammatory and immunomodulatory efficacy of indigenous probiotic Lactobacillus plantarum Lp91 in colitis mouse model. Mol. Biol. Rep. 2012, 39, 4765–4775. [Google Scholar] [CrossRef]
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C. Genes and molecules of lactobacilli supporting probiotic action. Microbiol. Mol. Biol. Rev. 2008, 72, 728–764. [Google Scholar] [CrossRef]
- Tuo, Y.; Yu, H.; Ai, L.; Wu, Z.; Guo, B.; Chen, W. Aggregation and adhesion properties of 22 Lactobacillus strains. J. Dairy Sci. 2013, 96, 4252–4257. [Google Scholar] [CrossRef]
- Wang, L.Q.; Meng, X.C.; Zhang, B.R.; Wang, Y.; Shang, Y.L. Influence of cell surface properties on adhesion ability of bifidobacteria. World J. Microbiol. Biotechnol. 2010, 26, 1999–2007. [Google Scholar] [CrossRef]
- Taj, R.; Masud, T.; Sohail, A.; Sammi, S.; Naz, R.; Sharma Khanal, B.K.; Nawaz, M.A. In vitro screening of EPS-producing Streptococcus thermophilus strains for their probiotic potential from Dahi. Food Sci. Nutr. 2022, 10, 2347–2359. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Isolauri, E.; Salminen, S. Specific probiotic strains and their combinations counteract adhesion of Enterobacter sakazakii to intestinal mucus. FEMS Microbiol. Lett. 2008, 285, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Ayyash, M.; Abushelaibi, A.; Al-Mahadin, S.; Enan, M.; El-Tarabily, K.; Shah, N. In vitro investigation into probiotic characterisation of Streptococcus and Enterococcus isolated from camel milk. LWT 2018, 87, 478–487. [Google Scholar] [CrossRef]
- Nishiyama, K.; Sugiyama, M.; Mukai, T. Adhesion properties of lactic acid bacteria on intestinal mucin. Microorganisms 2016, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, H.; Ström, E.; Roos, S. Addition of mucin to the growth medium triggers mucus-binding activity in different strains of Lactobacillus reuteri in vitro. FEMS Microbiol. Lett. 2001, 204, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, N.; Wrzosek, L.; Radziwill-Bienkowska, J.M.; Ringot-Destrez, B.; Duviau, M.P.; Noordine, M.L.; Laroute, V.; Robert, V.; Cherbuy, C.; Daveran-Mingot, M.L.; et al. Characterization of mucus-related properties of Streptococcus thermophilus: From adhesion to induction. Front. Physiol. 2018, 9, 980. [Google Scholar] [CrossRef] [PubMed]
- Kirjavainen, P.V.; Ouwehand, A.C.; Isolauri, E.; Salminen, S.J. The ability of probiotic bacteria to bind to human intestinal mucus. FEMS Microbiol. Lett. 1998, 167, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Ferraretto, A.; Bottani, M.; De Luca, P.; Cornaghi, L.; Arnaboldi, F.; Maggioni, M.; Fiorilli, A.; Donetti, E. Morphofunctional properties of a differentiated Caco2/HT-29 co-culture as an in vitro model of human intestinal epithelium. Biosci. Rep. 2018, 38, BSR20171497. [Google Scholar] [CrossRef]
- Haeri, A.; Khodaii, Z.; Ghaderian, S.M.H.; Tabatabaei Panah, A.S.; Akbarzadeh Najar, R. Comparison of adherence patterns of a selection of probiotic bacteria to Caco-2, HEp-2, and T84 cell lines. Ann. Microbiol. 2012, 62, 339–344. [Google Scholar] [CrossRef]
- Kerneis, S.; Bernet, M.F.; Coconnier, M.H.; Servin, A.L. Adhesion of human enterotoxigenic Escherichia coli to human mucus secreting HT-29 cell subpopulations in culture. Gut 1994, 35, 1449. [Google Scholar] [CrossRef]
- Kapczynski, D.R.; Meinersmann, R.J.; Lee, M.D. Adherence of Lactobacillus to intestinal 407 cells in culture correlates with fibronectin binding. Curr. Microbiol. 2000, 41, 136–141. [Google Scholar] [CrossRef]
- Kainulainen, V.; Korhonen, T.K. Dancing to another tune—Adhesive moonlighting proteins in bacteria. Biology 2014, 3, 178–204. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant properties of probiotic bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Lobo, R.E.; Gómez, M.I.; de Valdez, G.F.; Torino, M.I. Physicochemical and antioxidant properties of a gastroprotective exopolysaccharide produced by Streptococcus thermophilus CRL1190. Food Hydrocoll. 2019, 96, 625–633. [Google Scholar] [CrossRef]
- Kang, C.H.; Kim, J.S.; Park, H.M.; Kim, S.; Paek, N.S. Antioxidant activity and short-chain fatty acid production of lactic acid bacteria isolated from Korean individuals and fermented foods. 3 Biotech 2021, 11, 217. [Google Scholar] [CrossRef]
- Feng, T.; Wang, J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut Microbes 2020, 12, 1801944. [Google Scholar] [CrossRef] [PubMed]
- Agbas, A.; Moskovitz, J. The role of methionine oxidation/reduction in the regulation of immune response. Curr. Signal Transduct. Ther. 2009, 4, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Saubade, F.; Hemery, Y.M.; Guyot, J.P.; Humblot, C. Lactic acid fermentation as a tool for increasing the folate content of foods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3894–3910. [Google Scholar] [CrossRef] [PubMed]
- Juers, D.H.; Matthews, B.W.; Huber, R.E. LacZ β-galactosidase: Structure and function of an enzyme of historical and molecular biological importance. Protein Sci. 2012, 21, 1792–1807. [Google Scholar] [CrossRef] [PubMed]
- Yañez-Ñeco, C.V.; Cervantes, F.V.; Amaya-Delgado, L.; Ballesteros, A.O.; Plou, F.J.; Arrizon, J. Synthesis of β (1→ 3) and β (1→ 6) galactooligosaccharides from lactose and whey using a recombinant β-galactosidase from Pantoea anthophila. Electron. J. Biotechnol. 2021, 49, 14–21. [Google Scholar] [CrossRef]
- Kumar, M.; Nagpal, R.; Kumar, R.; Hemalatha, R.; Verma, V.; Kumar, A.; Chakraborty, C.; Singh, B.; Marotta, F.; Jain, S.; et al. Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. J. Diabetes Res. 2012, 2012, 902917. [Google Scholar] [CrossRef] [PubMed]
- Ziarno, M. Viability and cholesterol uptake by Streptococcus thermophilus cultures in artificial GIT fluids. Acta Sci. Pol. Technol. Aliment. 2010, 9, 83–94. [Google Scholar]
- Noh, D.O.; Kim, S.H.; Gilliland, S.E. Incorporation of cholesterol into the cellular membrane of Lactobacillus acidophilus ATCC 43121. J. Dairy Sci. 1997, 80, 3107–3113. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.; Yun, H.S.; Kim, J.G.; Oh, S.; Kim, S.H. Genetic and proteomic analysis of factors affecting serum cholesterol reduction by Lactobacillus acidophilus A4. Appl. Environ. Microbiol. 2010, 76, 4829–4835. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Tomar, S.K.; Goswami, P.; Sangwan, V.; Singh, R. Antibiotic resistance among commercially available probiotics. Food Res. Int. 2014, 57, 176–195. [Google Scholar] [CrossRef]

| Genome Attributes | Values |
|---|---|
| Genome size (bp) | 1,855,815 |
| GC content % | 39.1 |
| Number of contigs | 6 |
| Protein coding genes (CDS) | 2239 |
| Subsystems | 219 |
| RNA encoding genes | 83 |
| Reference Strain | % ANI | % DDH |
|---|---|---|
| Streptococcus thermophilus LMD-9 | 99.93 | 99.7 |
| Streptococcus thermophilus TH1477 | 98.96 | 91.7 |
| Streptococcus thermophilus MTH17CL396 | 99.01 | 92.2 |
| Streptococcus thermophilus TH1436 | 99.25 | 94 |
| Streptococcus thermophilus TH1435 | 99.31 | 93.9 |
| Genes Detected in MCC0200 | FigFam No. | Predicted Function |
|---|---|---|
| ATP synthase subunit a | fig|6666666.935801.peg.921 | |
| ATP synthase subunit b | fig|6666666.935801.peg.922 | Acid tolerance by maintaining pH homeostasis |
| ATP synthase subunit c | fig|6666666.935801.peg.920 | |
| ATP synthase alpha chain | fig|6666666.935801.peg.924 | |
| ATP synthase Beta chain | fig|6666666.935801.peg.926 | |
| ATP synthase Gamma chain | fig|6666666.935801.peg.925 | |
| ATP synthase Epsilon chain | fig|6666666.935801.peg.927 | |
| ATP synthase delta chain | fig|6666666.935801.peg.923 | |
| Na+/H+ antiporter | fig|6666666.935801.peg.2147 | |
| Urease system Urease cluster protein | fig|6666666.935801.peg.706 | Acid tolerance by Alkali production |
| Alpha | fig|6666666.935801.peg.707 | |
| Beta | fig|6666666.935801.peg.711 | |
| Gamma | fig|6666666.935801.peg.710 | |
| Accessory proteins: | fig|6666666.935801.peg.709 | |
| Urease accessory protein UreD | fig|6666666.935801.peg.715 | |
| Urease accessory protein UreE | fig|6666666.935801.peg.712 | |
| Urease accessory protein UreF | fig|6666666.935801.peg.713 | |
| Urease accessory protein UreG | fig|6666666.935801.peg.714 | |
| Ffh | fig|6666666.935801.peg.1370 | Proteins involved in protection and repair of molecules under acid stress |
| DnaK | fig|6666666.935801.peg.487 | |
| DnaJ | fig|6666666.935801.peg.488 | |
| GrpE | fig|6666666.935801.peg.485 | |
| HrcA | fig|6666666.935801.peg.484 | |
| GroEL | fig|6666666.935801.peg.603 | |
| GroES | fig|6666666.935801.peg.601 | |
| Clp proteases | fig|6666666.935801.peg.801 | |
| EF-Tu | fig|6666666.935801.peg.929 | |
| recA | fig|6666666.935801.peg.410 | |
| recN | fig|6666666.935801.peg.1668 | |
| Exonuclease V | fig|6666666.935801.peg.1681 | |
| UvrABCD | fig|6666666.935801.peg.31 | |
| fig|6666666.935801.peg.1985 | ||
| fig|6666666.935801.peg.1783 | ||
| fig|6666666.935801.peg.1458 | ||
| DNA polymerase | fig|6666666.935801.peg.46 | |
| DNA ligase | fig|6666666.935801.peg.2048 | |
| Sortase A | fig|6666666.935801.peg.1755 | Proteins involved in bile salt tolerance |
| Sortase-dependent proteins | fig|6666666.935801.peg.992 | |
| fig|6666666.935801.peg.1848 | ||
| HtrA | fig|6666666.935801.peg.349 | |
| DnaJ | fig|6666666.935801.peg.488 | |
| GroEL | fig|6666666.935801.peg.603 |
| Genes Detected in MCC0200 | Predicted Function | FigFam No. |
|---|---|---|
| Fibronectin/fibrinogen-binding protein | Binds to fibronectin | fig|6666666.935801.peg.1423 |
| Sortase A, LPXTG specific | Cell surface localization and peptidoglycan interaction | fig|6666666.935801.peg.1755 |
| Moonlighting proteins | ||
| Enolase | Binding to plasmin(ogen), fibronectin, laminin, albumin, collagen, salivary mucin, intestinal epithelial cells, | fig|6666666.935801.peg.1108 |
| EF-Tu | Binding to plasmin(ogen), plasma Factor H and Factor H-related protein 1 (FHR-1), intestinal epithelial cells and HT-MTX-derived mucus, salivary mucin, fibronectin | fig|6666666.935801.peg.929 |
| EF-G | Binding to salivary mucin | fig|6666666.935801.peg.75 |
| Triosephosphate isomerase | Binding to plasmin(ogen), intestinal epithelial cells, | fig|6666666.935801.peg.930 |
| GroEL | Binding to intestinal HT-29 cells and mucus | fig|6666666.935801.peg.603 |
| DnaK | Binding to plasmin(ogen) | fig|6666666.935801.peg.486 |
| Pyruvate kinase | Binding to salivary mucin | fig|6666666.935801.peg.1651 |
| Inosine 5′-monophosphate dehydrogenase (IMPDH) | Binding to plasmin(ogen) | fig|6666666.935801.peg.342 |
| Glutamine synthetase | Binding to plasmin(ogen), laminin, collagen I, fibronectin | fig|6666666.935801.peg.61 |
| Glucose-6-phosphate isomerase (GPI) | Binding to collagen | fig|6666666.935801.peg.585 |
| Gene Detected in MCC0200 Genome | FigFam No. | Predicted Function |
|---|---|---|
| Thiol peroxidase, Tpx-type (EC 1.11.1.15) | fig|6666666.935801.peg.1462 | H2O2-degrading enzymes |
| NADH peroxidase | fig|6666666.935801.peg.1758 | |
| Superoxide dismutase [Mn] (EC 1.15.1.1) | fig|6666666.935801.peg.1192 | Hydroperoxide radical detoxification |
| Thioredoxin reductase (EC 1.8.1.9) | fig|6666666.935801.peg.1905 | Redox homeostasis |
| Thioredoxin | fig|6666666.935801.peg.88 | |
| Peptide-methionine (S)-S-oxide reductase MsrA/MrsB | fig|6666666.935801.peg.1824 | Resistance to oxidative stress |
| fig|6666666.935801.peg.2133 | ||
| recA | fig|6666666.935801.peg.410 | Induces DNA repair mechanism |
| GroES/EL, clp proteases, CtsR, HrcA | fig|6666666.935801.peg.602 fig|6666666.935801.peg.603 fig|6666666.935801.peg.801 fig|6666666.935801.peg.425 | Targeting and degradation of misfolded proteins. |
| fig|6666666.935801.peg.484 | ||
| HtrA | fig|6666666.935801.peg.349 | Proteolysis of abnormal proteins |
| GrpE | fig|6666666.935801.peg.485 | Proper protein folding |
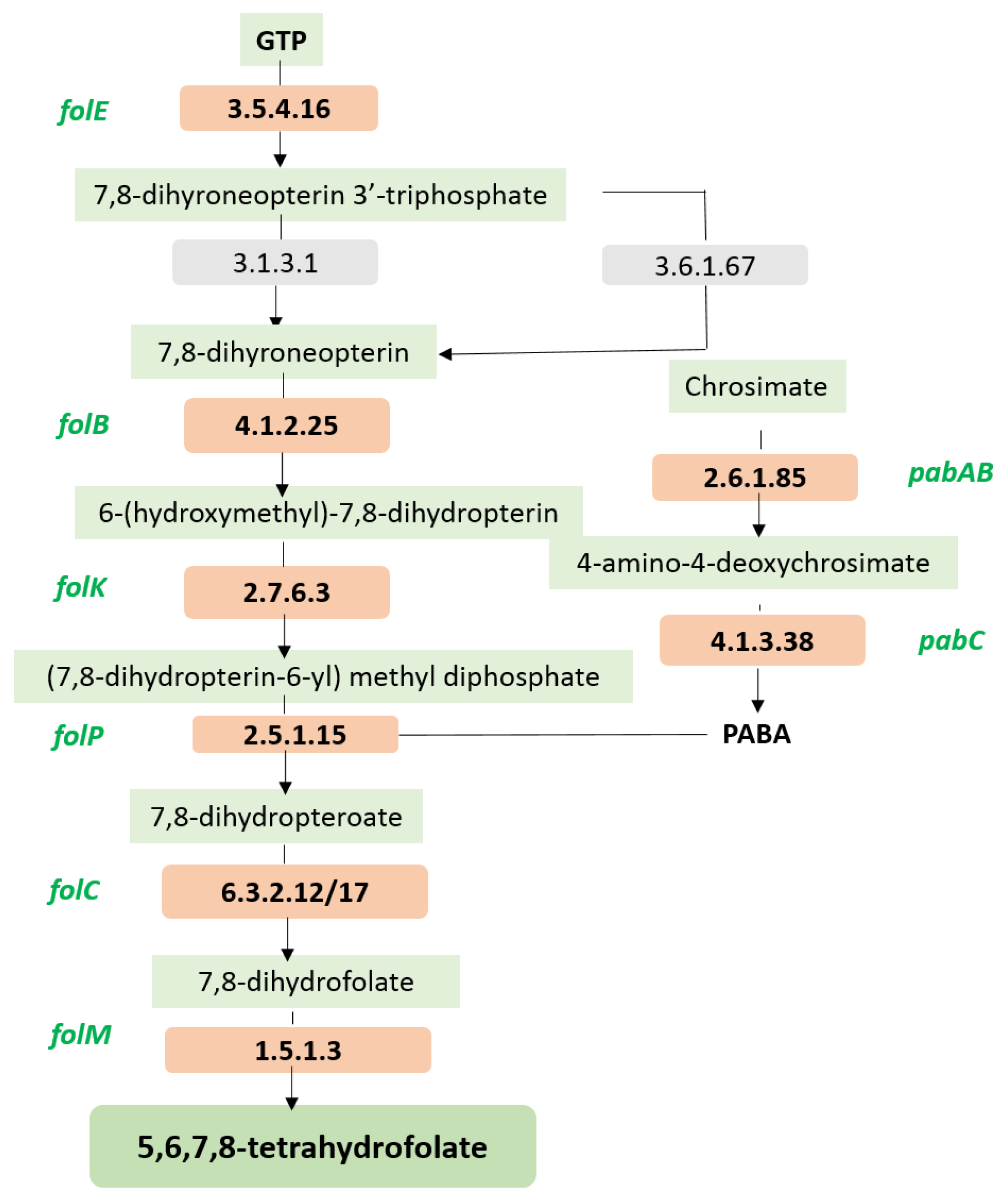
| Folate Biosynthesis Protein/Gene/System Detected in the MCC0200 | FigFam No. |
|---|---|
| FolE, GTP cyclohydrolase I (EC 3.5.4.16) type 1 | fig|6666666.935801.peg.2035 |
| FolB, dihydroneopterin aldolase | fig|6666666.935801.peg.2032 |
| FolK,2-amino-4-hydroxy-6-hydroxymethyldihydropteridine pyrophosphokinase (EC 2.7.6.3) | fig|6666666.935801.peg.2031 |
| FolP, Dihydropteroate synthase (EC 2.5.1.15) | fig|6666666.935801.peg.2034 |
| FolC1, Dihydrofolate synthase (EC 6.3.2.12) | fig|6666666.935801.peg.846 |
| FolC2, Dihydrofolate synthase (EC 6.3.2.12) | fig|6666666.935801.peg.2038 |
| FolM, FolA, Dihydrofolate reductase (EC 1.5.1.3) | fig|6666666.935801.peg.1044 |
| PabC, Aminodeoxychorismate lyase | fig|6666666.935801.peg.1232 |
| PabAB, Para-aminobenzoate synthase, aminase component (EC 2.6.1.85) | fig|6666666.935801.peg.1232 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapse, N.; Pisu, V.; Dhakephalkar, T.; Margale, P.; Shetty, D.; Wagh, S.; Dagar, S.; Dhakephalkar, P.K. Unveiling the Probiotic Potential of Streptococcus thermophilus MCC0200: Insights from In Vitro Studies Corroborated with Genome Analysis. Microorganisms 2024, 12, 347. https://doi.org/10.3390/microorganisms12020347
Kapse N, Pisu V, Dhakephalkar T, Margale P, Shetty D, Wagh S, Dagar S, Dhakephalkar PK. Unveiling the Probiotic Potential of Streptococcus thermophilus MCC0200: Insights from In Vitro Studies Corroborated with Genome Analysis. Microorganisms. 2024; 12(2):347. https://doi.org/10.3390/microorganisms12020347
Chicago/Turabian StyleKapse, Neelam, Vaidehi Pisu, Tanisha Dhakephalkar, Prajakta Margale, Deepa Shetty, Shilpa Wagh, Sumit Dagar, and Prashant K. Dhakephalkar. 2024. "Unveiling the Probiotic Potential of Streptococcus thermophilus MCC0200: Insights from In Vitro Studies Corroborated with Genome Analysis" Microorganisms 12, no. 2: 347. https://doi.org/10.3390/microorganisms12020347
APA StyleKapse, N., Pisu, V., Dhakephalkar, T., Margale, P., Shetty, D., Wagh, S., Dagar, S., & Dhakephalkar, P. K. (2024). Unveiling the Probiotic Potential of Streptococcus thermophilus MCC0200: Insights from In Vitro Studies Corroborated with Genome Analysis. Microorganisms, 12(2), 347. https://doi.org/10.3390/microorganisms12020347


_Di_Marco.png)



