Integrated Genomics and Transcriptomics Provide Insights into Salt Stress Response in Bacillus subtilis ACP81 from Moso Bamboo Shoot (Phyllostachys praecox) Processing Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Bacteria
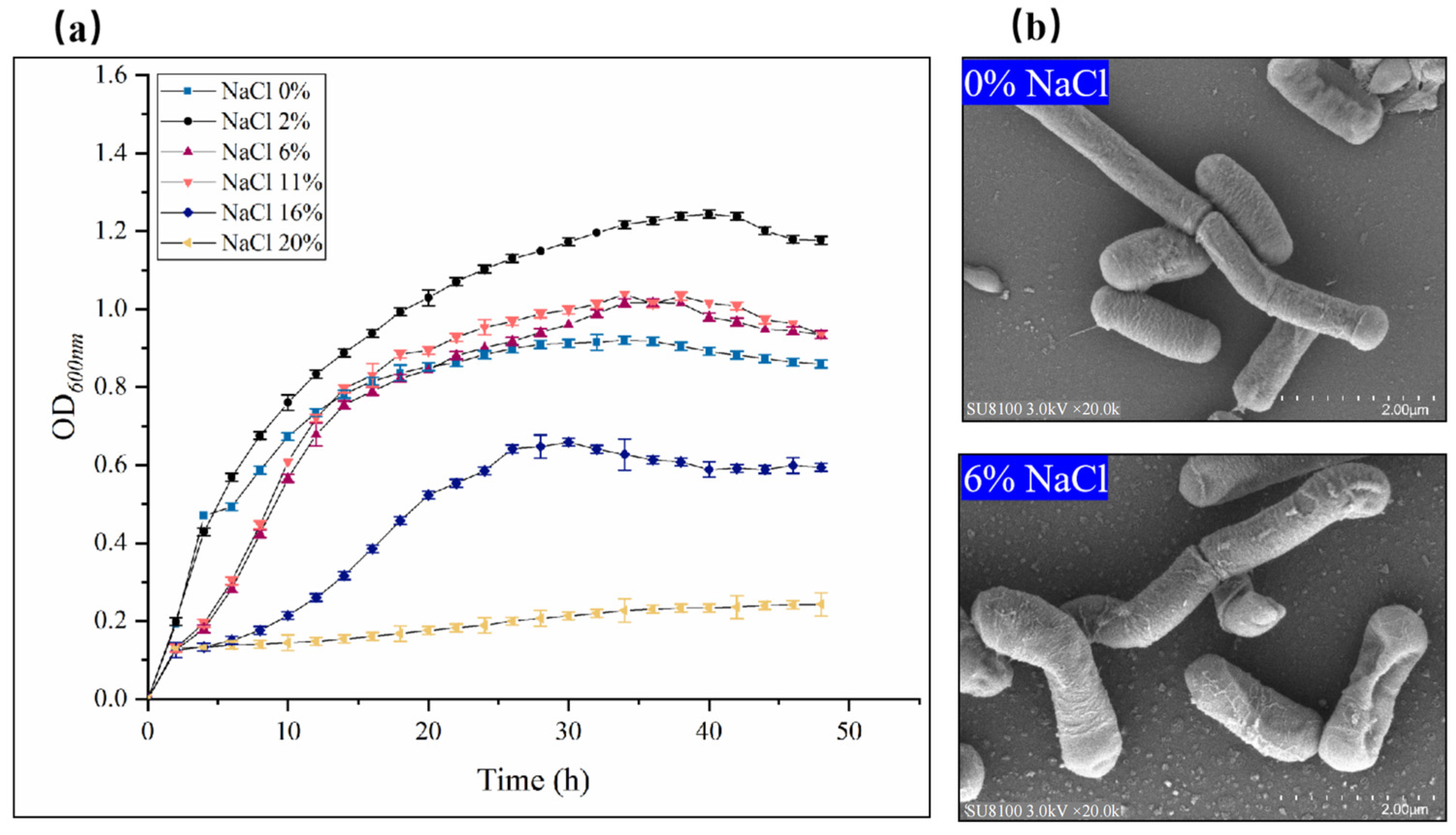
2.2. Effect of Salt Stress on the Growth, Hydrolytic Enzymes, and Antioxidant Enzyme of Bacillus subtilis ACP81
2.3. Genomic DNA and Whole-Transcriptome Sequencing
2.4. Transcriptome Information Analysis
2.5. Relative Gene Expression Analysis by Using Quantitative Reverse Transcription RT-qPCR
2.6. Data Analysis
3. Results
3.1. Isolation and Identification of Halotolerant Bacteria
3.2. Hydrolase Enzymes of B. subtilis ACP81
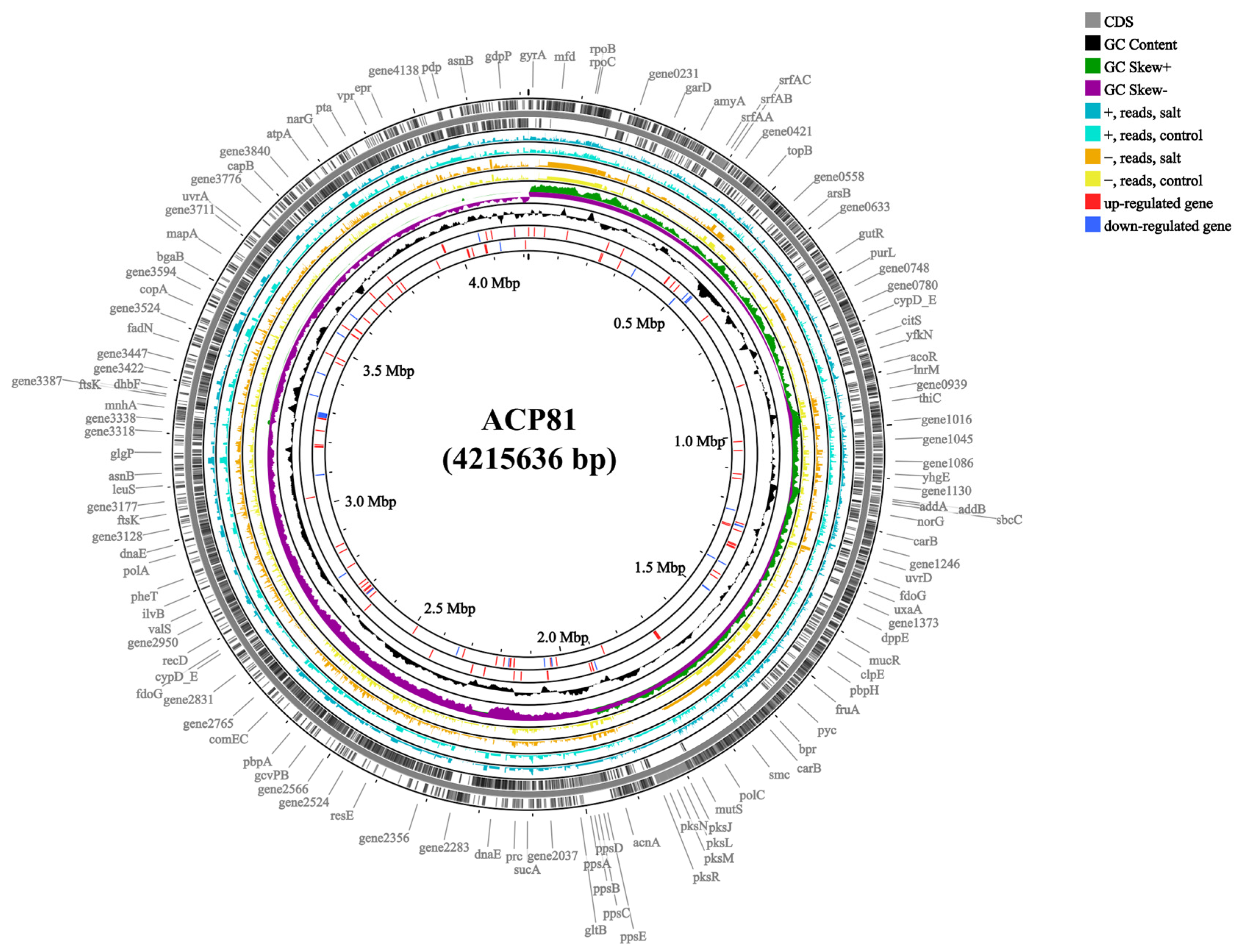
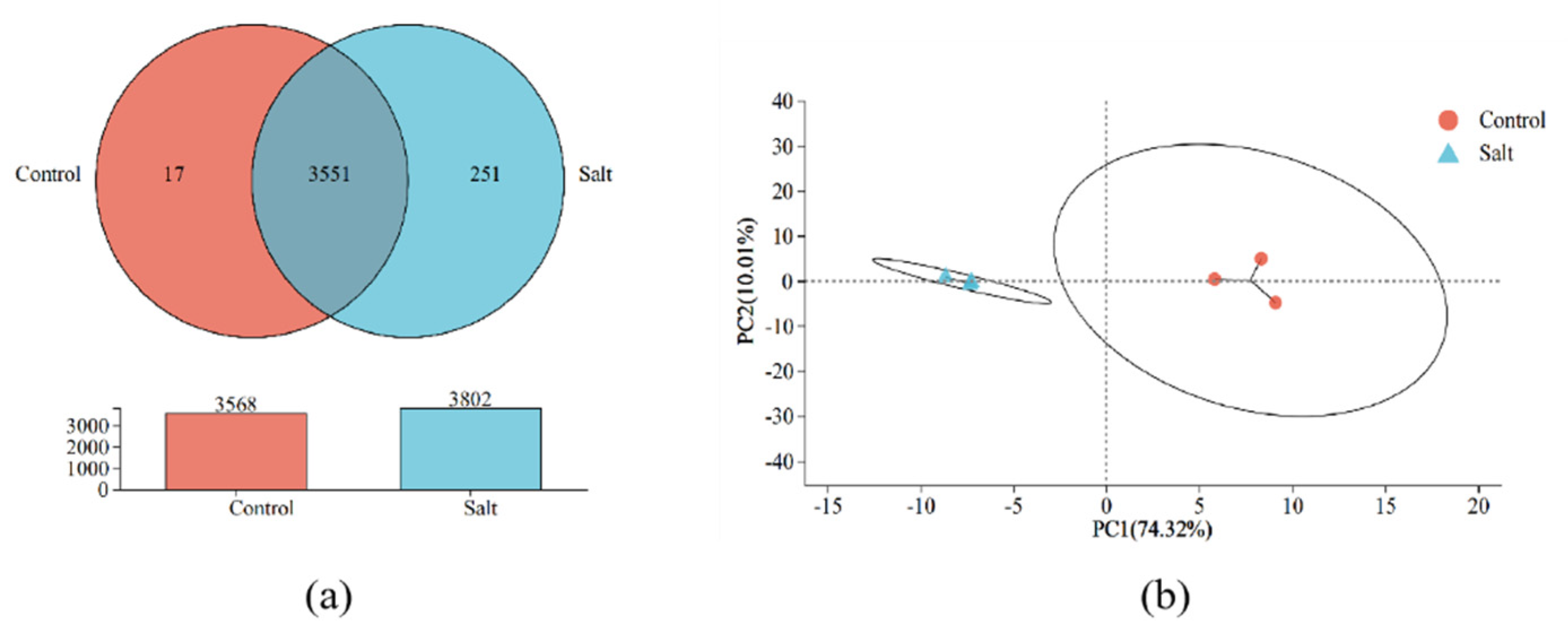
3.3. Genomic Features and Transcriptome Profiles of B. subtilis ACP81
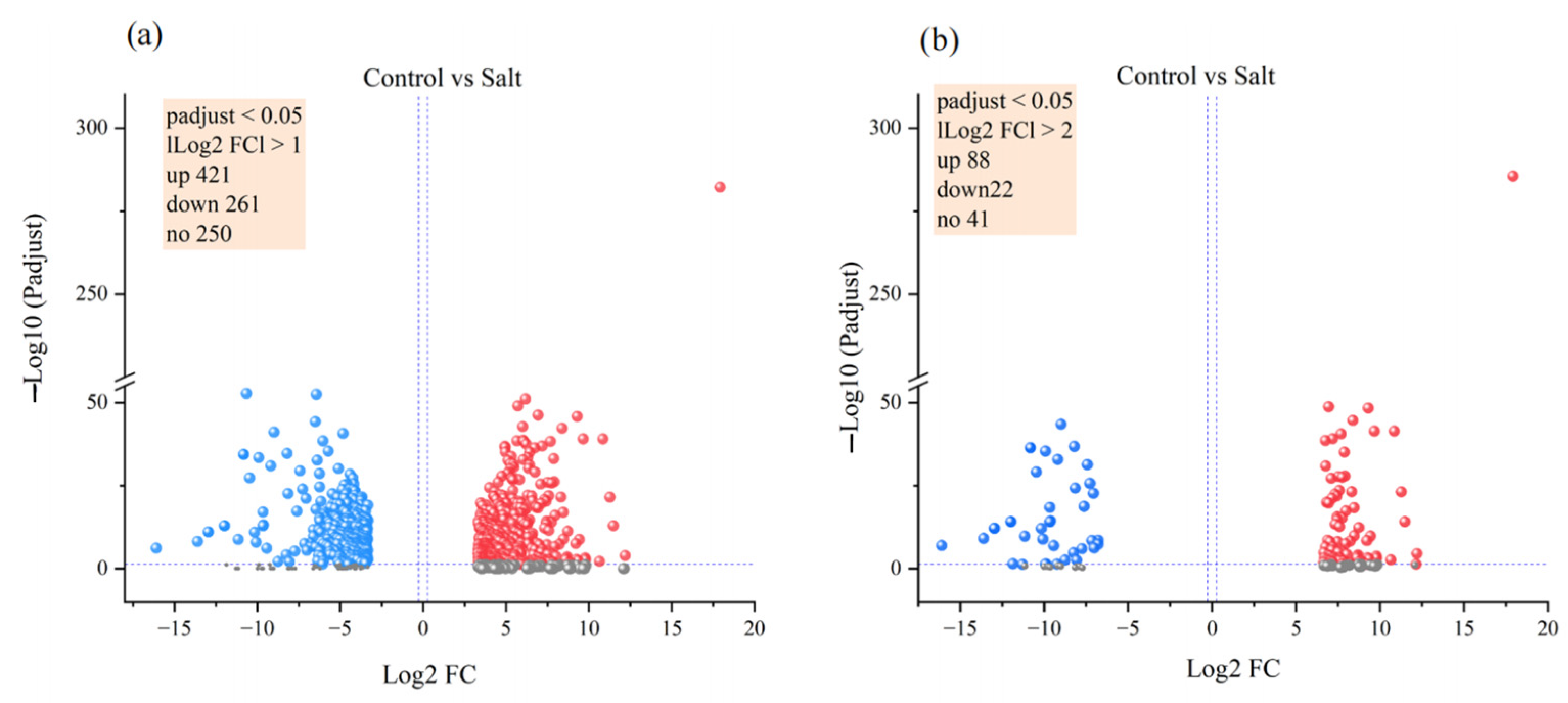
3.4. Gene Expression Analysis
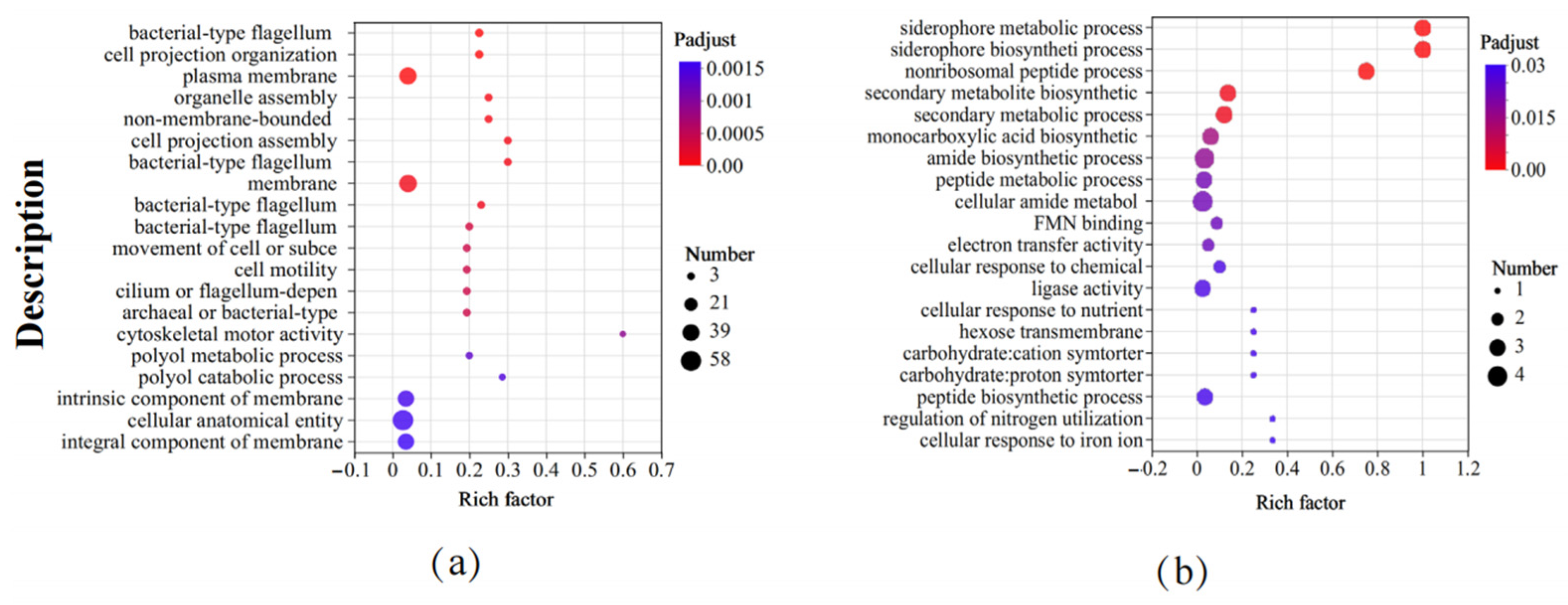
3.5. GO Functional Enrichment Analysis
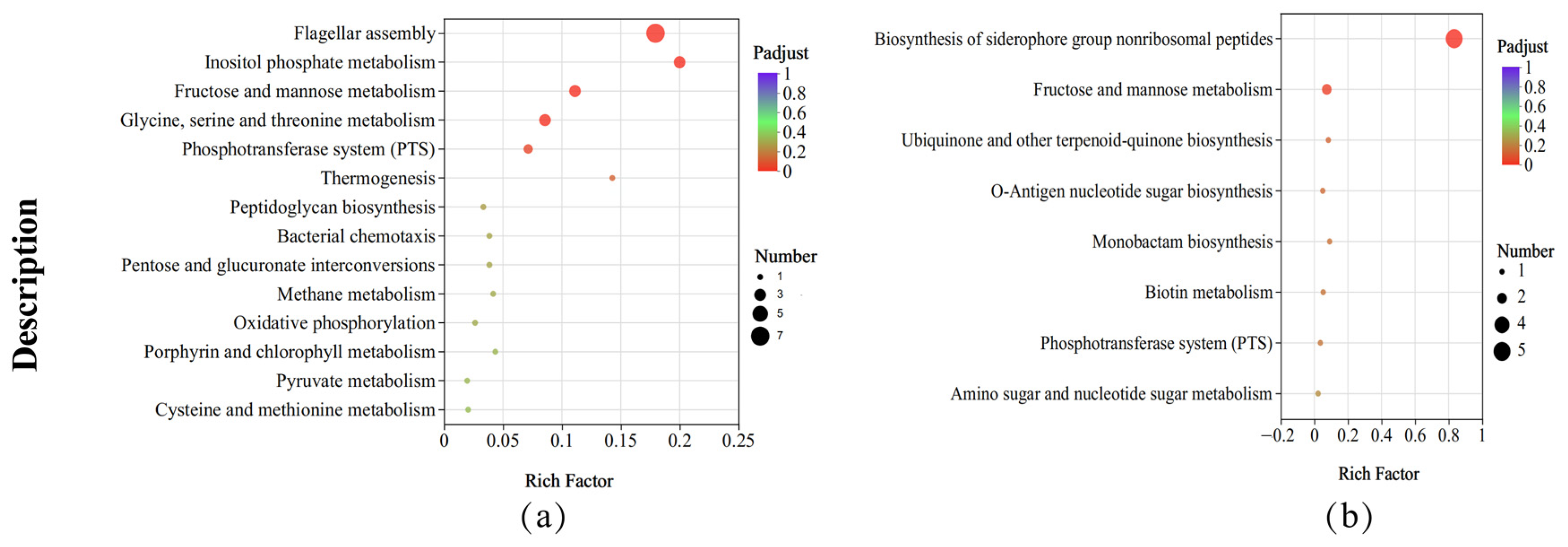
3.6. KEGG Pathway Enrichment Analysis
3.7. Analysis of Metabolic Pathways Analysis Related to Hydrolytic Enzymes
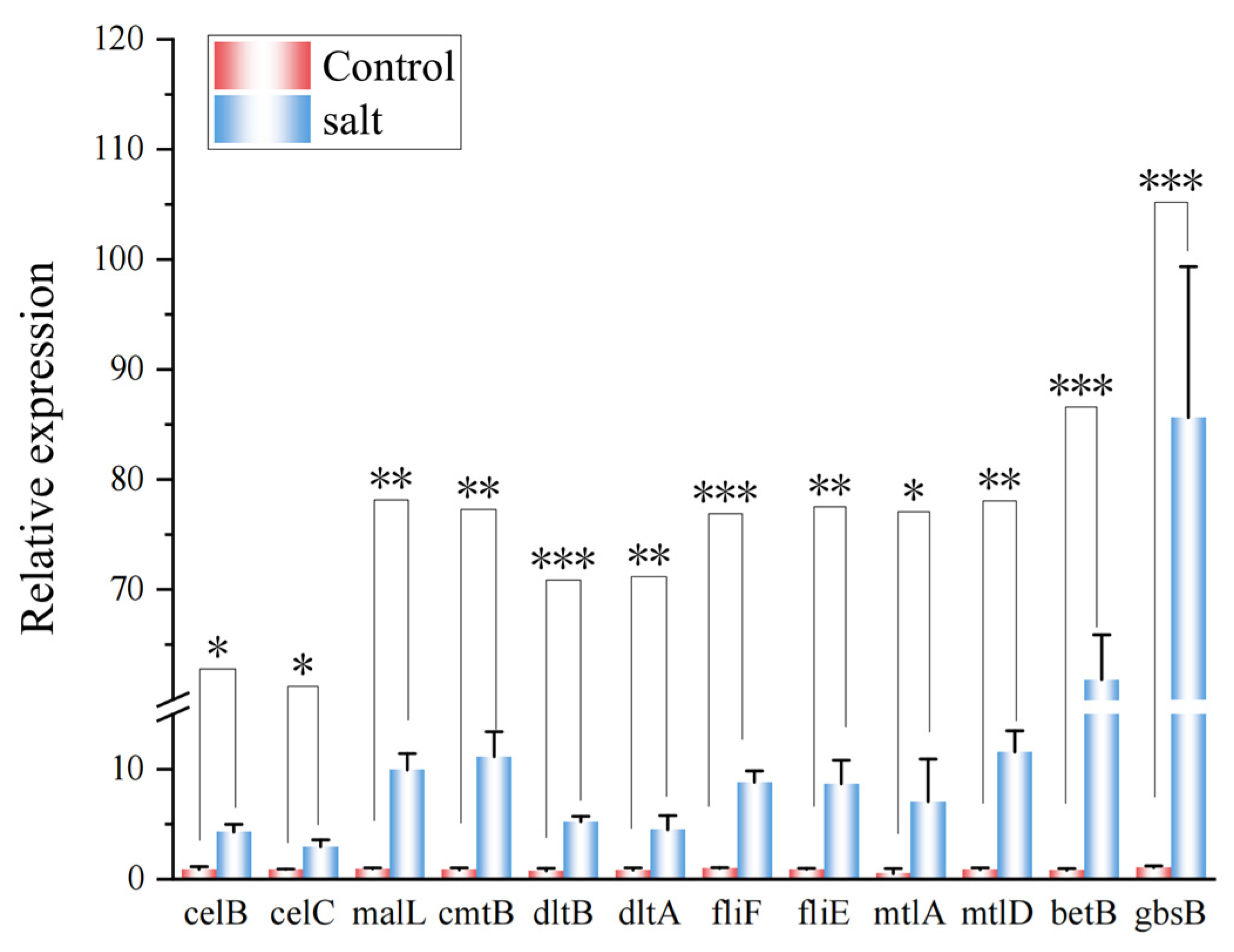
3.8. Experimental Validation by RT-qPCR
4. Discussion
4.1. Growth and Hydrolase Activities of B. subtilis ACP81 in Response to Salt Stress
4.2. Flagellar Assembly and Membrane Plays a Defensive Regulatory Role in ACP81
4.3. Compatible Solutes Play an Osmoregulatory Role in B. subtilis ACP81
4.4. Analysis of Metabolic Pathway Analyses Related to the Hydrolytic Enzyme of ACP81
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Upadhyay, S.K.; Chauhan, P.K. Optimization of eco-friendly amendments as sustainable asset for salt-tolerant plant growth-promoting bacteria mediated maize (Zea Mays L.) plant growth, Na uptake reduction and saline soil restoration. Environ. Res. 2022, 211, 113081. [Google Scholar] [CrossRef] [PubMed]
- Butcher, K.; Wick, A.F.; DeSutter, T.; Chatterjee, A.; Harmon, J. Soil Salinity: A Threat to Global Food Security. Agron. J. 2016, 108, 2189–2200. [Google Scholar] [CrossRef]
- Wang, J.; Ishfaq, M.; Miao, Y.; Liu, Z.; Hao, M.; Wang, C.; Wang, J.; Chen, X. Dietary administration of Bacillus subtilis KC1 improves growth performance, immune response, heat stress tolerance, and disease resistance of broiler chickens. Poult. Sci. 2022, 101, 101693. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zou, H.; Wang, B.; Yuan, F. Progress and Applications of Plant Growth-Promoting Bacteria in Salt Tolerance of Crops. Int. J. Mol. Sci. 2022, 23, 7036. [Google Scholar] [CrossRef]
- Foot, N.; Henshall, T.; Kumar, S. Ubiqutination and the regulation of membrane proteins. Physiol. Rev. 2017, 97, 253–281. [Google Scholar] [CrossRef]
- Pandey, S.K.; Yadav, S.; Goel, Y.; Singh, S.M. Cytotoxic action of acetate on tumor cells of thymic origin: Role of MCT-1, pH homeostasis and altered cell survival regulation. Biochimie 2019, 157, 1–9. [Google Scholar] [CrossRef]
- Priya, P.; Aneesh, B.; Harikrishnan, K. Genomics as a potential tool to unravel the rhizosphere microbiome interactions on plant health. J. Microbiol. Meth. 2021, 185, 106215. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, S.; Li, B.; Li, J.; Wang, X.; Zhang, J.; Ji, J. The combined use of a plant growth promoting Bacillus spp. strain and GABA promotes the growth of rice under salt stress by regulating antioxidant enzyme system, enhancing photosynthesis and improving soil enzyme activities. Microbiol. Res. 2023, 266, 127225. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Jia, R.; Chen, X.; Yang, L.; Duan, M.; Xiao, F.; Liang, C.; Zhou, D.; Li, W.; Liu, C. Impact of bacteria-nitrogen coupling on cotton growth and nitrogen utilization under different salt stress. Agric. Water Manag. 2023, 280, 108221. [Google Scholar] [CrossRef]
- Jia, P.; Tu, Y.; Liu, Z.; Li, F.; Yan, T.; Ma, S.; Dong, L.; Diao, Q. Diets supplementation with Bacillus subtilis and Macleaya cordata extract improve production performance and the metabolism of energy and nitrogen, while reduce enteric methane emissions in dairy cows. Anim. Feed. Sci. Technol. 2022, 294, 115481. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Singh, D.P. Effect of salt-tolerant plant growth-promoting rhizobacteria on wheat plants and soil health in a saline environment. Plant Biology 2015, 17, 288–293. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, X.; Su, M.; Li, J.; Man, T.; Wang, S.; Li, C.; Gao, S.; Zhang, R.; Zhang, M.; et al. Isolation of potassium solubilizing bacteria in soil and preparation of liquid bacteria fertilizer from food wastewater. Biochem. Eng. J. 2022, 181, 108378. [Google Scholar] [CrossRef]
- Jia, Y.F.; Shi, W.Y.; Wu, L.H.; Wang, H.L. Effects of ensilage on the preseravtion of bamboo shoots and them fibre characteristics. J. Trop. For. Sci. 2011, 23, 396–403. [Google Scholar]
- Priyadarshini, S.; Ray, P. Exploration of detergent-stable alkaline α-amylase AA7 from Bacillus sp. strain SP-CH7 isolated from Chilika Lake. Int. J. Biol. Macromol. 2019, 140, 825–832. [Google Scholar] [CrossRef]
- Su, L.; Li, Y.; Wu, J. Efficient secretory expression of Bacillus stearothermophilus α/β-cyclodextrin glycosyltransferase in Bacillus subtilis. J. Biotechnol. 2021, 331, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Li, J.X.; Zhang, F.; Jiang, D.D.; Li, J.; Wang, F.L.; Zhang, Z.; Wang, W.; Zhao, X.Q. Diversity of cellulase-producing filamentous fungi from tibet and transcriptomic analysis of a superior cellulase producer Trichoderma harzianum LZ117. Front. Microbiol. 2020, 11, 1617. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-Time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yin, L.; Xue, Y.; Ma, Y. Global microarray analysis of alkaliphilic halotolerant Bacterium Bacillus sp. N16-5 salt stress adaptation. PLoS ONE 2015, 10, e0128649. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nie, C.; Zhu, P.; Ma, S.; Wang, M.; Hu, Y. Purification, characterization and immunomodulatory activity of polysaccharides from stem lettuce. Carbohyd. Polym. 2018, 188, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, G.; Wang, F.; Zhao, H.; Wei, Y.; Liu, L.; Zhang, H. Extraction, characterization, antioxidant activity and rheological behavior of a polysaccharide produced by the extremely salt tolerant Bacillus subtilis LR-1. LWT-Food Sci. Technol. 2022, 162, 113413. [Google Scholar] [CrossRef]
- Wu, T.Y.; Wu, X.Q.; Xu, X.Q.; Kong, W.L.; Wu, F. Salt tolerance mechanism and species identification of the plant rhizosphere Bacterium JYZ-SD2. Curr. Microbiol. 2020, 77, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.; Eid, A.M.; Elsaied, A.; El-Belely, E.F.; Barghoth, M.G.; Azab, E.; Gobouri, A.; Hassan, S.E.D. Plant growth-promoting endophytic bacterial community inhabiting the leaves of Pulicaria incisa (Lam.) DC inherent to arid regions. Plants 2021, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, S.; Muneer, M.A.; Tahir, M.; Javed, M.T.; Mahmood, T.; Afridi, M.S.; Pakar, N.P.; Abbasi, H.A.; Munis, M.F.H. Deciphering distinct biological control and growth promoting potential of multi-stress tolerant Bacillus subtilis PM32 for potato stem canker. Physiol. Mol. Biol. Plants 2021, 27, 2101–2114. [Google Scholar] [CrossRef]
- Zanella, M.; Borghi, G.L.; Pirone, C.; Thalmann, M.; Pazmino, D.; Costa, A.; Santelia, D.; Trost, P.; Sparla, F. β-amylase 1 (BAM1) degrades transitory starch to sustain proline biosynthesis during drought stress. J. Exp. Bot. 2016, 67, 1819–1826. [Google Scholar] [CrossRef]
- Monroe, J.D.; Storm, A.R.; Badley, E.M.; Lehman, M.D.; Platt, S.M.; Saunders, L.K.; Schmitz, J.M.; Torres, C.E. β-amylase1 and β-amylase3 are plastidic starch hydrolases in arabidopsis that seem to be adapted for different thermal, pH, and stress conditions. Plant Physiol. 2014, 166, 1748–1763. [Google Scholar] [CrossRef]
- Toral, L.; Rodríguez, M.; Béjar, V.; Sampedro, I. Antifungal activity of lipopeptides from Bacillus XT1 CECT 8661 against botrytis cinerea. Front. Microbiol. 2018, 9, 1315. [Google Scholar] [CrossRef]
- Silva, C.F.D.; Vitorino, L.C.; Mendonça, M.A.C.; Araújo, W.L.; Dourado, M.N.; Albuquerque, L.C.; Soares, M.A.; Souchie, E.L. Screening of plant growth-promoting endophytic bacteria from the roots of the medicinal plant Aloe vera. S. Afr. J. Bot. 2020, 134, 3–16. [Google Scholar] [CrossRef]
- Shao, W.; Li, M.; Teng, Z.; Qiu, B.; Huo, Y.; Zhang, K. Effects of Pb(II) and Cr(VI) Stress on Phosphate-Solubilizing Bacteria (Bacillus sp. MRP-3): Oxidative Stress and Bioaccumulation Potential. Int. J. Environ. Res. Public Health 2019, 16, 2172. [Google Scholar] [CrossRef]
- Lin, X.; Xu, X.; Yang, C.; Zhao, Y.; Feng, Z.; Dong, Y. Activities of antioxidant enzymes in three bacteria exposed to bensulfuron-methyl. Ecotoxicol. Environ. Saf. 2009, 72, 1899–1904. [Google Scholar] [CrossRef]
- Rhee, S.G.; Yang, K.S.; Kang, S.W.; Woo, H.A.; Chang, T.S. Controlled elimination of intracellular H2O2: Regulation of peroxiredoxin, catalase, and glutathione peroxidase via post-translational modification. Antioxid. Redox Signal. 2005, 7, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Jin, Y.; Huang, J.; Wu, C. Effect of NaCl on nitrification performance and extracellular polymeric substance characteristic of Klebsiella sp. TN-10. Environ. Sci. Pollut. Res. Int. 2019, 26, 24900–24910. [Google Scholar] [CrossRef]
- Ali, B.; Wang, X.; Saleem, M.H.; Sumaira Hafeez, A.; Afridi, M.S.; Khan, S.; Ullah, I.; do Amaral Júnior, A.T.; Alatawi, A.; Ali, S. PGPR-mediated salt tolerance in maize by modulating plant physiology, antioxidant defense, compatible solutes accumulation and bio-surfactant producing genes. Plants 2022, 11, 345. [Google Scholar] [CrossRef]
- Rajkovic, A.; Hummels, K.R.; Witzky, A.; Erickson, S.; Gafken, P.R.; Whitelegge, J.P.; Faull, K.F.; Kearns, D.B.; Ibba, M. Translation control of swarming proficiency in Bacillus subtil is by 5-amino-pentanolylated elongation factor P. J. Biol. Chem. 2016, 291, 10976–10985. [Google Scholar] [CrossRef]
- Courtney, C.R.; Cozy, L.M.; Kearns, D.B. Molecular characterization of the flagellar hook in Bacillus subtilis. J. Bacteriol. 2012, 194, 4619–4629. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Hu, C.; Ou, L.; Kuramitsu, Y.; Masuda, Y.; Honjoh, K.I.; Miyamoto, T. Transcriptional analysis on heat resistance and recovery from thermal damage in Salmonella under high salt condition. LWT-Food Sci. Technol. 2019, 106, 194–200. [Google Scholar] [CrossRef]
- Woods, E.C.; Nawrocki, K.L.; Suárez, J.M.; McBride, S.M. The clostridium difficile Dlt Pathway is controlled by the extracytoplasmic function sigma factor sigma(V) in response to lysozyme. Infect. Imm. 2016, 84, 1902–1916. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cai, D.; He, P.; Mo, F.; Zhang, Q.; Ma, X.; Chen, S. Enhanced production of heterologous proteins by Bacillus licheniformis with defective d-alanylation of lipoteichoic acid. World J. Microb. Biot. 2018, 34, 135. [Google Scholar] [CrossRef]
- Maria, J.J.P.S.; Sadaka, A.; Moussa, S.H.; Brown, S.; Zhang, Y.J.; Rubin, E.J.; Gilmore, M.S.; Walker, S. Compound-gene interaction mapping reveals distinct roles for Staphylococcus aureus teichoic acids. Proc. Natl. Acad. Sci. USA 2014, 111, 12510–12515. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria. Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Maisey, H.C.; Doran, K.S.; Nizet, V. Recent advances in understanding the molecular basis of group B Streptococcus virulence. Expert. Rev. Mol. Med. 2008, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Shai, Y. Mode of action of membrane active antimicrobial peptides. Biopolymers 2002, 66, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Saar-Dover, R.; Bitler, A.; Nezer, R.; Shmuel-Galia, L.; Firon, A.; Shimoni, E.; Trieu-Cuot, P.; Shai, Y. D-alanylation of lipoteichoic acids confers resistance to cationic peptides in group B Streptococcus by increasing the cell wall density. PLoS Pathog. 2012, 8, 1002891. [Google Scholar] [CrossRef]
- Bonis, M.; Ecobichon, C.; Guadagnini, S.; Prévost, M.-C.; Boneca, I.G. A M23B family metallopeptidase of Helicobacter pylori required for cell shape, pole formation and virulence. Mol. Microbiol. 2010, 78, 809–819. [Google Scholar] [CrossRef]
- Ventosa, A.; Nieto, J.J.; Oren, A. Biology of moderately halophilic aerobic bacteria. Microbiol. Mol. Biol. Rev. 1998, 62, 504–544. [Google Scholar] [CrossRef]
- Peddie, B.A.; Lever, M.; Hayman, C.M.; Randall, K.; Chambers, S.T. Relationship between osmoprotection and the structure and intracellular accumulation of betaines by Escherichia coli. FEMS. Microbiol. Lett. 1994, 120, 125–131. [Google Scholar] [CrossRef]
- Oshone, R.; Ngom, M.; Chu, F.; Mansour, S.; Sy, M.O.; Champion, A.; Tisa, L.S. Genomic, transcriptomic, and proteomic approaches towards understanding the molecular mechanisms of salt tolerance in Frankia strains isolated from Casuarina trees. BMC. Genomics 2017, 18, 633. [Google Scholar] [CrossRef]
- Tang, D.; Wang, X.; Wang, J.; Wang, M.; Wang, Y.; Wang, W. Choline-betaine pathway contributes to hyperosmotic stress and subsequent lethal stress resistance in Pseudomonas protegens SN15-2. J. Biosci. 2020, 45, 85. [Google Scholar] [CrossRef]
- Steil, L.; Hoffmann, T.; Budde, I.; Völker, U.; Bremer, E. Genome-wide transcriptional profiling analysis of adaptation of Bacillus subtilis to high salinity. J. Bacteriol. 2003, 185, 6358–6370. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Srivastava, R.; Sharma, A.; Bharati, A.P.; Yadav, J.; Singh, A.K.; Tiwari, P.K.; Srivatava, A.K.; Chakdar, H.; Saxena, A.K. Transcriptome analysis to understand salt stress regulation mechanism of Chromohalobacter salexigens ANJ207. Front. Microbiol. 2022, 13, 909276. [Google Scholar] [CrossRef]
- Arias, S.; Del Moral, A.; Ferrer, M.R.; Tallon, R.; Quesada, E.; Bejar, V. Mauran, an exopolysaccharide produced by the halophilic bacterium Halomonas maura, with a novel composition and interesting properties for biotechnology. Extremophiles 2003, 7, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Nicolaus, B.; Kambourova, M.; Oner, E.T. Exopolysaccharides from extremophiles: From fundamentals to biotechnology. Environ. Technol. 2010, 31, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xie, J.; Zhang, Z.; Yan, R.; Wu, Z. RNA-Seq transcriptomic analysis of green tea polyphenols modulation of differently expressed genes in Enterococcus faecalis under bile salt stress. Curr. Microbiol. 2022, 79, 157. [Google Scholar] [CrossRef]
- Pfluger-Grau, K.; Gorke, B. Regulatory roles of the bacterial nitrogen-related phosphotransferase system. Trends Micobiol. 2010, 18, 205–214. [Google Scholar] [CrossRef]
- Cases, I.; Velazquez, F.; de Lorenzo, V. The ancestral role of the phosphoenolpyruvate-carbohydrate phosphotransferase system (PTS) as exposed by comparative genomics. Res. Microbiol. 2007, 158, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Wang, S.; Xu, F.; Xiao, G.; Bai, J.; Wang, J.; Sun, X. Integrative transcriptome and proteome analyses of Trichoderma longibrachiatum LC and its cellulase hyper-producing mutants generated by heavy ion mutagenesis reveal the key genes involved in cellulolytic enzymes regulation. Biotechnol. Biofuels Bioprod. 2022, 15, 63. [Google Scholar] [CrossRef] [PubMed]
- Galinier, A. Carbon catabolite repression or how bacteria choose their favorite sugars. Med. Sci. 2018, 34, 531–539. [Google Scholar]
- Bien, T.L.T.; Tsuji, S.; Tanaka, K.; Takenaka, S.; Yoshida, K.-I. Secretion of heterologous thermostable cellulases in Bacillus subtilis. J. Gen. Appl. Microbiol. 2014, 60, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Mokashe, N.; Chaudhari, B.; Patil, U. Operative utility of salt-stable proteases of halophilic and halotolerant bacteria in the biotechnology sector. Int. J. Biol. Macromol. 2018, 117, 493–522. [Google Scholar] [CrossRef] [PubMed]
- Uma, G.; Babu, M.M.; Prakash VS, G.; Nisha, S.J.; Citarasu, T. Nature and bioprospecting of haloalkaliphilics: A review. World. J. Microbiol. Biotechnol. 2020, 36, 66. [Google Scholar] [CrossRef]
- Liang, G.; Ma, Z.; Lu, S.; Ma, W.; Feng, L.; Mao, J.; Chen, B. Temperature-phase transcriptomics reveals that hormones and sugars in the phloem of grape participate in tolerance during cold acclimation. Plant Cell Rep. 2022, 41, 1357–1373. [Google Scholar] [CrossRef]
- Desai, T.A.; Rao, C.V. Regulation of arabinose and xylose metabolism in Escherichia coli. Appl. Environ. Microbiol. 2010, 76, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
| Cellulases (U/mL) | Neutral Proteases (U/mL) | α-Amylase (U/mL) | β-Amylase (U/mL) | |
|---|---|---|---|---|
| Control | 65.88 ± 2.75 b | 7.13 ± 0.93 a | 0.25 ± 0.01 a | 0.72 ± 0.05 b |
| Salt | 72.38 ± 1.10 a | 3.20 ± 0.52 b | 0.23 ± 0.01 a | 1.16 ± 0.23 a |
| Superoxide Dismutase (U/g) | Peroxidase (nmol/min/g) | Malondialdehyde (nmol/min/g) | Catalase (nmol/min/g) | Oxygen Free Radical (nmol/g) | |
|---|---|---|---|---|---|
| Control | 96.39 ± 12.27 a | 401.86 ± 17.83 b | 5.64 ± 0.91 a | 98.42 ± 8.44 b | 31.17 ± 5.68 a |
| Salt | 40.12 ± 7.11 b | 579.38 ± 91.36 a | 7.02 ± 0.71 a | 126.11 ± 12.38 a | 17.66 ± 5.11 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Huang, Z.; Zhong, Z.; Bian, F.; Zhang, X. Integrated Genomics and Transcriptomics Provide Insights into Salt Stress Response in Bacillus subtilis ACP81 from Moso Bamboo Shoot (Phyllostachys praecox) Processing Waste. Microorganisms 2024, 12, 285. https://doi.org/10.3390/microorganisms12020285
Li Q, Huang Z, Zhong Z, Bian F, Zhang X. Integrated Genomics and Transcriptomics Provide Insights into Salt Stress Response in Bacillus subtilis ACP81 from Moso Bamboo Shoot (Phyllostachys praecox) Processing Waste. Microorganisms. 2024; 12(2):285. https://doi.org/10.3390/microorganisms12020285
Chicago/Turabian StyleLi, Qiaoling, Zhiyuan Huang, Zheke Zhong, Fangyuan Bian, and Xiaoping Zhang. 2024. "Integrated Genomics and Transcriptomics Provide Insights into Salt Stress Response in Bacillus subtilis ACP81 from Moso Bamboo Shoot (Phyllostachys praecox) Processing Waste" Microorganisms 12, no. 2: 285. https://doi.org/10.3390/microorganisms12020285
APA StyleLi, Q., Huang, Z., Zhong, Z., Bian, F., & Zhang, X. (2024). Integrated Genomics and Transcriptomics Provide Insights into Salt Stress Response in Bacillus subtilis ACP81 from Moso Bamboo Shoot (Phyllostachys praecox) Processing Waste. Microorganisms, 12(2), 285. https://doi.org/10.3390/microorganisms12020285






