Verticillium dahliae VdPBP1 Transcription Factor Is Required for Hyphal Growth, Virulence, and Microsclerotia Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioinformatic Analysis of DNA and Protein Sequences
2.2. Microorganism Cultivation Condition
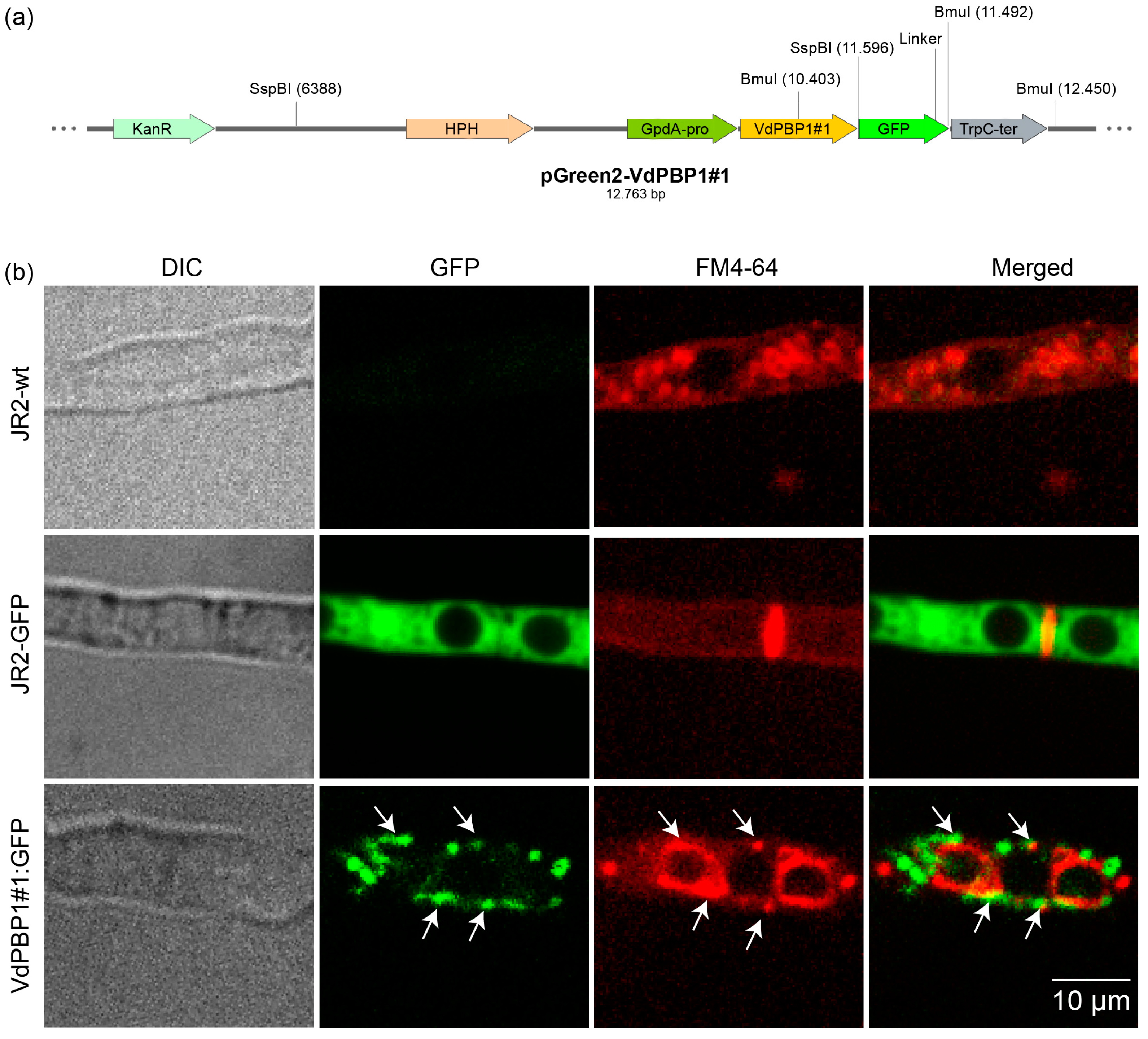
2.3. Localization Studies
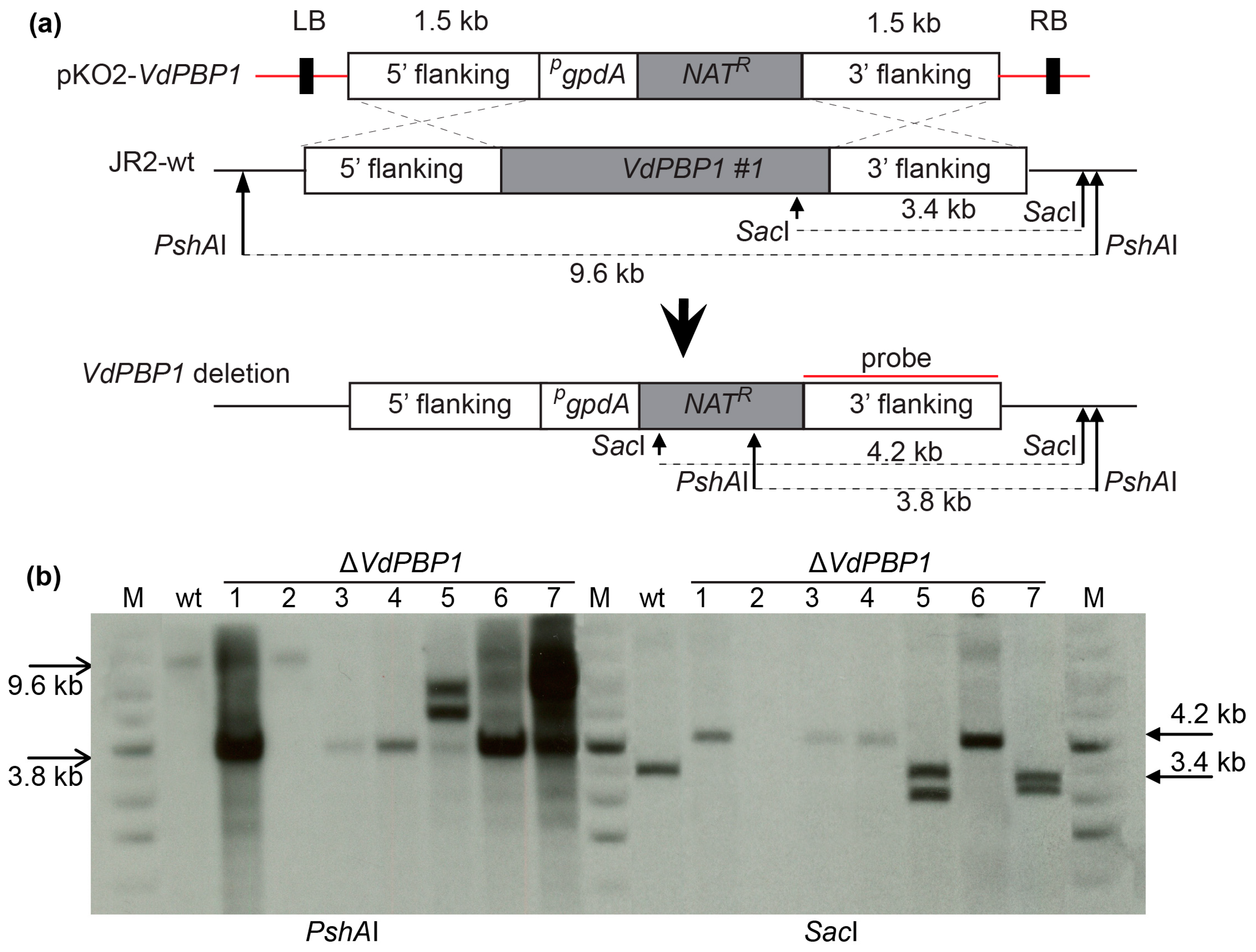
2.4. Gene Deletion
2.5. Tomato Infection Assay
2.6. Microsclerotia Counting
3. Results
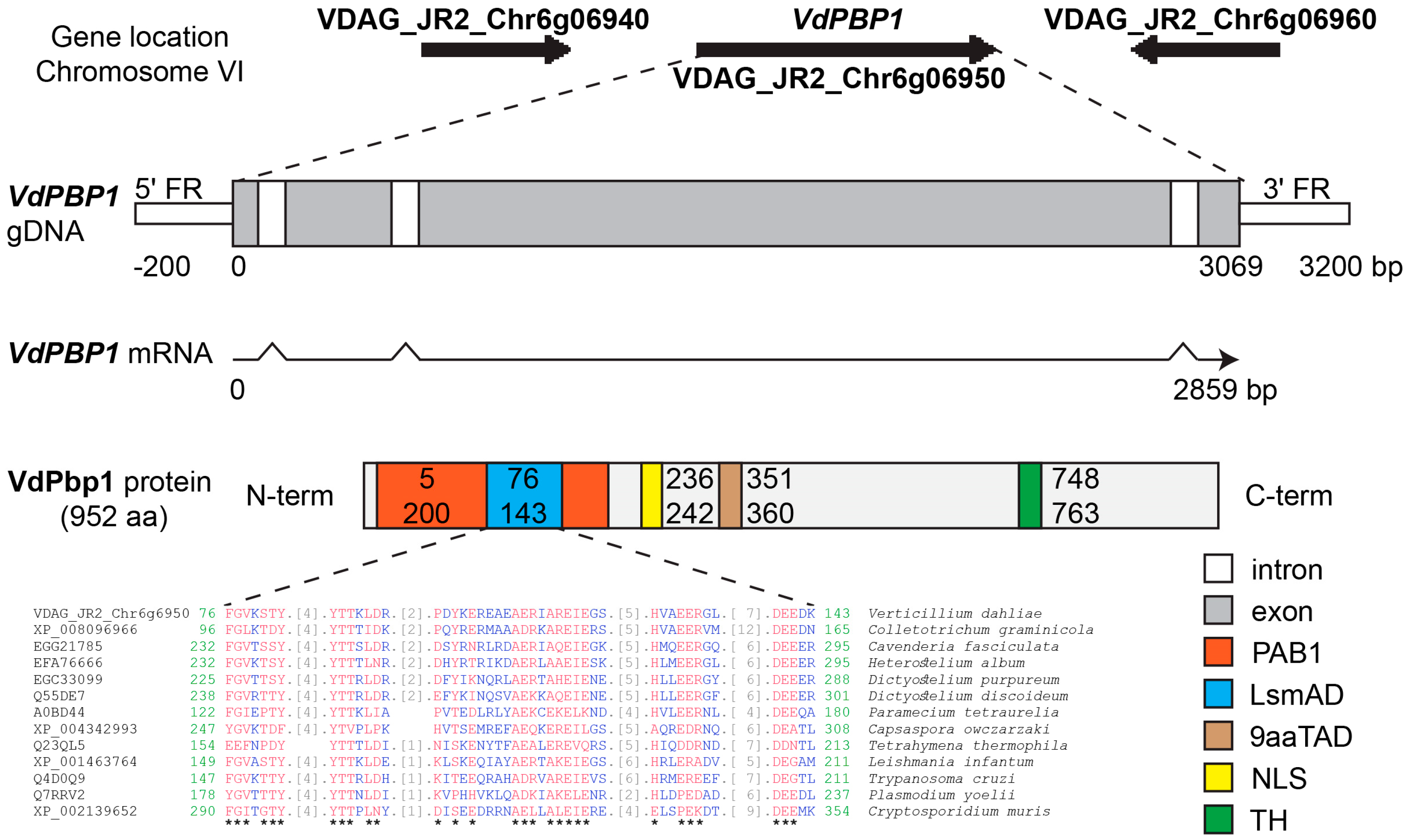
3.1. The Gene VdPBP1 Encodes Proteins Comprising Both a LsmAD Domain and a PAB-Binding Domain
3.2. VdPBP1 Is a Transmembrane Helix Protein
3.3. The Deletion of VdPBP1 in V. dahliae Was Confirmed
3.4. VdPBP1 Promote Microsclerotia Formation
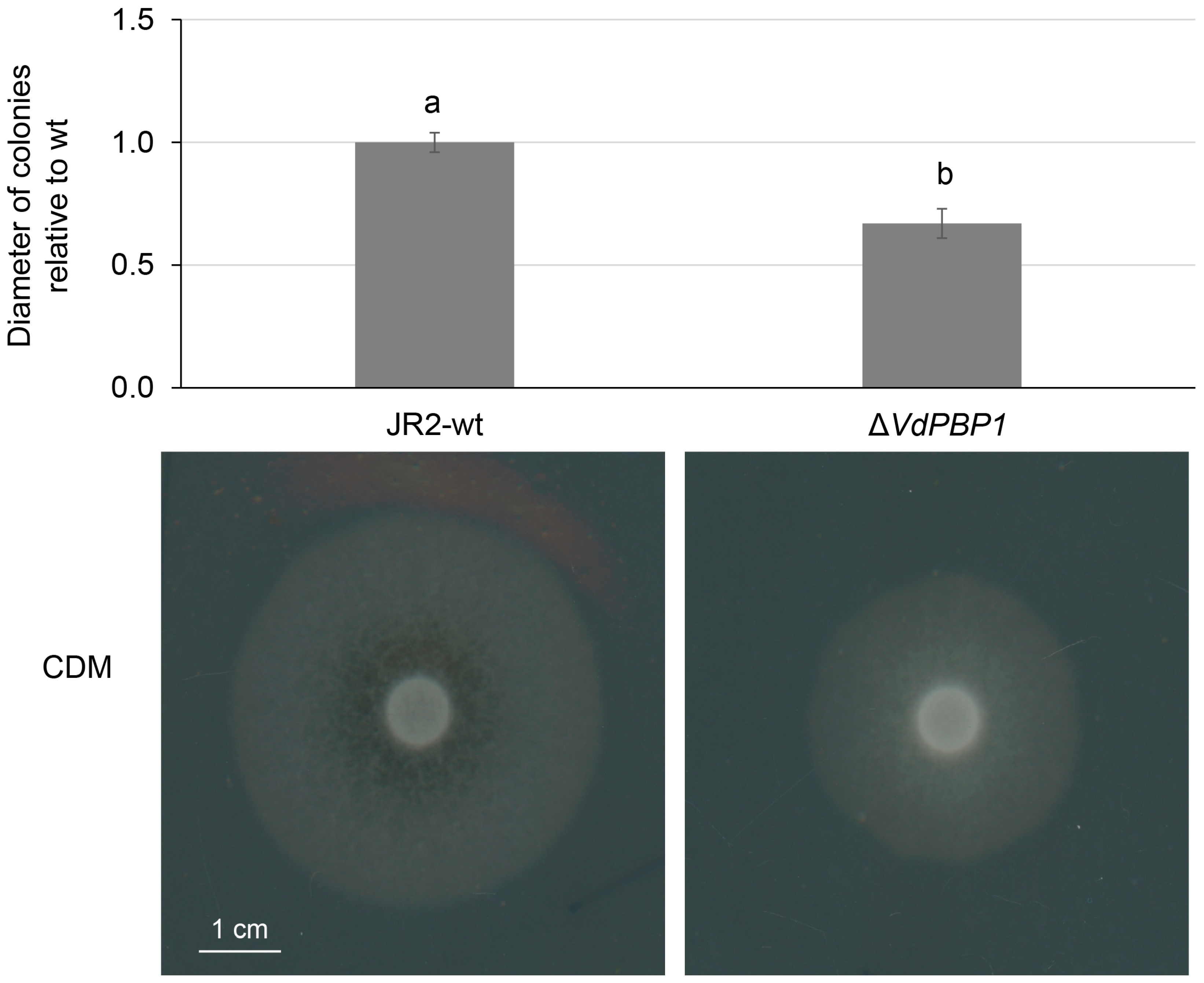
3.5. VdPBP1 Is Needed for Normal Hyphal Growth of V. dahliae on Agar Medium
3.6. VdPBP1 Is Necessary for the Full Development of Disease Symptoms in Tomatoes
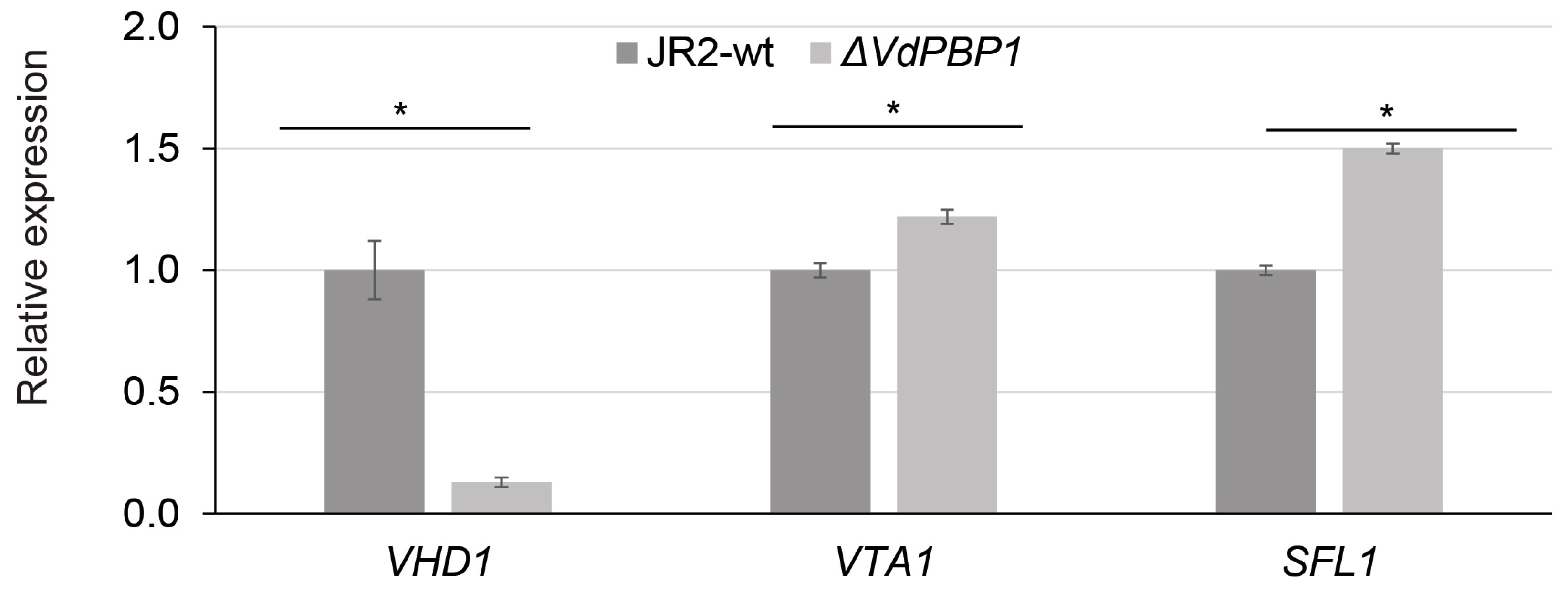
3.7. VdPBP1 Regulates the Expression of Putative Target Genes for Microsclerotia Formation
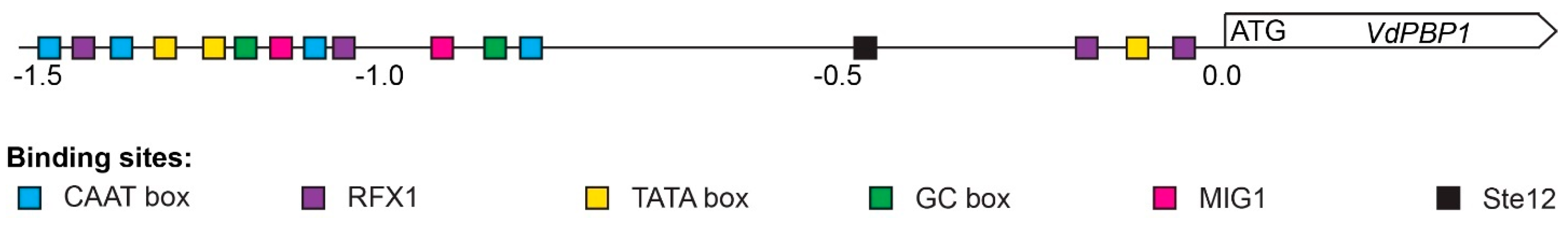
3.8. Protein Regulatory Elements in the Promoter Region of the VdPBP1 Gene
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berlanger, I.; Powelson, M.L. Verticillium wilt. Plant Health Instr. 2005. [Google Scholar] [CrossRef]
- Pegg, G.F.; Brady, B.L. Verticillium Wilts; CABI Publishing: Wallingford, UK, 2002. [Google Scholar]
- Tran, V.T.; Braus-Stromeyer, S.A.; Kusch, H.; Reusche, M.; Kaever, A.; Kuhn, A.; Valerius, O.; Landesfeind, M.; Asshauer, K.; Tech, M.; et al. Verticillium transcription activator of adhesion Vta2 suppresses microsclerotia formation and is required for systemic infection of plant roots. New Phytol. 2014, 202, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Mangus, D.A.; Amrani, N.; Jacobson, A. Pbp1p, a factor interacting with Saccharomyces cerevisiae poly(A)-binding protein, regulates polyadenylation. Mol. Cell. Biol. 1998, 18, 7383–7396. [Google Scholar] [CrossRef] [PubMed]
- Tadauchi, T.; Inada, T.; Matsumoto, K.; Irie, K. Posttranscriptional regulation of HO expression by the Mkt1-Pbp1 complex. Mol. Cell. Biol. 2004, 24, 3670–3681. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Chow, E.W.; Fu, C.; Soderblom, E.J.; Moseley, M.A.; Heitman, J.; Cardenas, M.E. Calcineurin Targets Involved in Stress Survival and Fungal Virulence. PLoS Pathog. 2016, 12, e1005873. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.E.; Fu, C.; Jung, W.H.; Oh, S.H.; Kwak, J.H.; Cardenas, M.E.; Heitman, J.; Park, H.S. Pbp1-Interacting Protein Mkt1 Regulates Virulence and Sexual Reproduction in Cryptococcus neoformans. Front. Cell Infect. Microbiol. 2019, 9, 355. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Lee, S.B.; Kang, H.S.; Oh, G.T.; Kim, T. Two distinct domains of Flo8 activator mediates its role in transcriptional activation and the physical interaction with Mss11. Biochem. Biophys. Res. Commun. 2014, 449, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.T.; Chen, K.Z.; Liu, J.Y.; Li, W.H.; Wang, Y.Z.; Wang, L.L.; Xiang, M.J. FLO8 deletion leads to decreased adhesion and virulence with downregulated expression of EPA1, EPA6, and EPA7 in Candida glabrata. Braz. J. Microbiol. 2022, 53, 727–738. [Google Scholar] [CrossRef]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef]
- Finn, R.D.; Tate, J.; Mistry, J.; Coggill, P.C.; Sammut, S.J.; Hotz, H.R.; Ceric, G.; Forslund, K.; Eddy, S.R.; Sonnhammer, E.L.; et al. The Pfam protein families database. Nucleic Acids Res. 2008, 36, D281–D288. [Google Scholar] [CrossRef]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef] [PubMed]
- Piskacek, S.; Gregor, M.; Nemethova, M.; Grabner, M.; Kovarik, P.; Piskacek, M. Nine-amino-acid transactivation domain: Establishment and prediction utilities. Genomics 2007, 89, 756–768. [Google Scholar] [CrossRef]
- Lin, J.R.; Hu, J. SeqNLS: Nuclear localization signal prediction based on frequent pattern mining and linear motif scoring. PLoS ONE 2013, 8, e76864. [Google Scholar] [CrossRef] [PubMed]
- Yates, A.D.; Allen, J.; Amode, R.M.; Azov, A.G.; Barba, M.; Becerra, A.; Bhai, J.; Campbell, L.I.; Carbajo Martinez, M.; Chakiachvili, M.; et al. Ensembl Genomes 2022: An expanding genome resource for non-vertebrates. Nucleic Acids Res. 2022, 50, D996–D1003. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Higgins, D.G. Clustal Omega, accurate alignment of very large numbers of sequences. Methods Mol. Biol. 2014, 1079, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Nugent, T.; Jones, D.T. Detecting pore-lining regions in transmembrane proteinsequences. BMC Bioinfom. 2012, 13, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Lazo, G.R.; Stein, P.A.; Ludwig, R.A. A DNA transformation-competent Arabidopsis genomic library in Agrobacterium. Biotechnology 1991, 9, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Fradin, E.F.; Zhang, Z.; Juarez Ayala, J.C.; Castroverde, C.D.; Nazar, R.N.; Robb, J.; Liu, C.M.; Thomma, B.P. Genetic dissection of Verticillium wilt resistance mediated by tomato Ve1. Plant Physiol. 2009, 150, 320–332. [Google Scholar] [CrossRef]
- Smith, G. The effect of adding trace elements to Czapek-Dox medium. Trans. Br. Mycol. Soc. 1948, 32, 280–283. [Google Scholar] [CrossRef]
- Neumann, M.J.; Dobinson, K.F. Sequence tag analysis of gene expression during pathogenic growth and microsclerotia development in the vascular wilt pathogen Verticillium dahliae. Fungal Genet. Biol. 2003, 38, 54–62. [Google Scholar] [CrossRef]
- Timpner, C.; Braus-Stromeyer, S.A.; Tran, V.T.; Braus, G.H. The Cpc1 regulator of the cross-pathway control of amino acid biosynthesis is required for pathogenicity of the vascular pathogen Verticillium longisporum. Mol. Plant-Microbe Interact. 2013, 26, 1312–1324. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bui, T.T.; Harting, R.; Braus-Stromeyer, S.A.; Tran, V.T.; Leonard, M.; Hofer, A.; Abelmann, A.; Bakti, F.; Valerius, O.; Schluter, R.; et al. Verticillium dahliae transcription factors Som1 and Vta3 control microsclerotia formation and sequential steps of plant root penetration and colonisation to induce disease. New Phytol. 2019, 221, 2138–2159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Reese, J.C. Ssn6-Tup1 requires the ISW2 complex to position nucleosomes in Saccharomyces cerevisiae. EMBO J. 2004, 23, 2246–2257. [Google Scholar] [CrossRef] [PubMed]
- Froger, A.; Hall, J.E. Transformation of Plasmid DNA into E. coli Using the Heat Shock Method. J. Vis. Exp. 2007, 6, e253. [Google Scholar] [CrossRef]
- Zhu, H.Q.; Feng, Z.L.; Li, Z.F.; Shi, Y.Q.; Zhao, L.H.; Yang, J.R. Characterization of two fungal isolates from cotton and evaluation of their potential for biocontrol of Verticillium wilt of cotton. J. Phytopathol. 2013, 161, 70–77. [Google Scholar] [CrossRef]
- Wang, Z.P.; Xu, H.M.; Wang, G.Y.; Chi, Z.; Chi, Z.M. Disruption of the MIG1 gene enhances lipid biosynthesis in the oleaginous yeast Yarrowia lipolytica ACA-DC 50109. Biochim. Biophys. Acta 2013, 1831, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Al-Jaradi, A.; Al-Mahmooli, I.; Janke, R.; Maharachchikumbura, S.; Al-Saady, N.; Al-Sadi, A.M. Isolation and identification of pathogenic fungi and oomycetes associated with beans and cowpea root diseases in Oman. PeerJ 2018, 6, e6064. [Google Scholar] [CrossRef] [PubMed]
- Farré, D.; Roset, R.; Huerta, M.; Adsuara, J.E.; Roselló, L.; Albà, M.M.; Messeguer, X. Identification of patterns in biological sequences at the ALGGEN server: PROMO and MALGEN. Nucleic Acids Res. 2003, 31, 3651–3653. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, C.Y.; Bai, X.W.; Song, H.Y.; Xiao, D.G. Effects of MIG1, TUP1 and SSN6 deletion on maltose metabolism and leavening ability of baker’s yeast in lean dough. Microb. Cell Factories 2014, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; Kim, H.Y.; Yoon, J.H.; Kang, H.S. Recruitment of the Swi/Snf complex by Ste12-Tec1 promotes Flo8-Mss11-mediated activation of STA1 expression. Mol. Cell. Biol. 2004, 24, 9542–9556. [Google Scholar] [CrossRef][Green Version]


| V. dahliae Strain Name | Description | Reference |
|---|---|---|
| JR2-wt | JR2-wt | [19] |
| ∆VdPBP1 | ∆VdPBP1:NAT1R | This study |
| Plasmid Name | Description | Reference |
|---|---|---|
| PKO2 | p trpC:NAT1 R | [23] |
| pKO2-VdPBP1 | p VdPBP1:p trpC:NAT1 R: VdPBP1 t | This study |
| pGreen2-VdPBP1#1 | p GpdA:VdPBP1#1::GFP:trpC t | This study |
| Primer Names | 5′-3′ Direction | Reference |
|---|---|---|
| gdpA-NAT-F | CAACTGATATTGAAGGAGCATTT | This study |
| gdpA-NAT-R | TCAGGGGCAGGGCATGC | This study |
| VdPBP1-P1 (PacI) | TACGAATTCTTAATTAACTCGACCCGTGAAAACAAGT | This study |
| VdPBP1-P2 (SpeI) | GTACCACTAGTGAGCTCACGCCACGTTGGTATCGTAT | This study |
| VdPBP1-P3 (XbaI) | TCGAGAGGCCTTCTAGAGGTGGTTCTAGGCTGCATGT | This study |
| VdPBP1-P4 (SbfI) | GCTTGCATGCCTGCAGGAATCACCTCGTCGCAGTCAC | This study |
| OE-VdPBP1-F | ACCCTGACATCACCCTCGAGATGCGCCTGACATACAAAGAA | This study |
| OE-VdPBP1-R | CCTCGCCCTTGCTCACCATACCACCGCTACCACCCTTGGCCTCGGCAGGCC | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.T.; Duong, T.T.; Nguyen, V.X.; Nguyen, T.-D.; Bui, T.T.; Pham, D.T.N. Verticillium dahliae VdPBP1 Transcription Factor Is Required for Hyphal Growth, Virulence, and Microsclerotia Formation. Microorganisms 2024, 12, 265. https://doi.org/10.3390/microorganisms12020265
Nguyen HT, Duong TT, Nguyen VX, Nguyen T-D, Bui TT, Pham DTN. Verticillium dahliae VdPBP1 Transcription Factor Is Required for Hyphal Growth, Virulence, and Microsclerotia Formation. Microorganisms. 2024; 12(2):265. https://doi.org/10.3390/microorganisms12020265
Chicago/Turabian StyleNguyen, Huong Thi, Thanh Thi Duong, Vu Xuan Nguyen, Tien-Dung Nguyen, Thuc Tri Bui, and Dung Thuy Nguyen Pham. 2024. "Verticillium dahliae VdPBP1 Transcription Factor Is Required for Hyphal Growth, Virulence, and Microsclerotia Formation" Microorganisms 12, no. 2: 265. https://doi.org/10.3390/microorganisms12020265
APA StyleNguyen, H. T., Duong, T. T., Nguyen, V. X., Nguyen, T.-D., Bui, T. T., & Pham, D. T. N. (2024). Verticillium dahliae VdPBP1 Transcription Factor Is Required for Hyphal Growth, Virulence, and Microsclerotia Formation. Microorganisms, 12(2), 265. https://doi.org/10.3390/microorganisms12020265






