Abstract
Clubroot (Plasmodiophora brassicae) is an important soilborne disease that causes severe damage to cruciferous crops in China. This study aims to compare the differences in chemical properties and microbiomes between healthy and clubroot-diseased soils. To reveal the difference, we measured soil chemical properties and microbial communities by sequencing 18S and 16S rRNA amplicons. The available potassium in the diseased soils was higher than in the healthy soils. The fungal diversity in the healthy soils was significantly higher than in the diseased soils. Ascomycota and Proteobacteria were the most dominant fungal phylum and bacteria phylum in all soil samples, respectively. Plant-beneficial microorganisms, such as Chaetomium and Sphingomonas, were more abundant in the healthy soils than in the diseased soils. Co-occurrence network analysis found that the healthy soil networks were more complex and stable than the diseased soils. The link number, network density, and clustering coefficient of the healthy soil networks were higher than those of the diseased soil networks. Our results indicate that the microbial community diversity and network structure of the clubroot-diseased soils were different from those of the healthy soils. This study is of great significance in exploring the biological control strategies of clubroot disease.
1. Introduction
Clubroot, caused by the obligate biotrophic soilborne pathogen Plasmodiophora brassicae, is considered a devastating disease of Brassica crops [1]. At present, clubroot is widespread in China and is becoming very problematic. The pathogen can survive in the soil in the form of resting spores for decades, and the number of spores in the soil increases rapidly in the continuous monoculture of Brassica crops [2]. Many control measures have been used for the management of clubroot, including the deployment of resistant cultivars, soil fumigation, and the application of fungicides [3]. However, successful management of the disease is difficult because of monetary and labor costs.
It is known that the occurrence of soilborne diseases correlates with soil properties, including the soil microbiome. The microorganisms in the rhizosphere are extremely abundant and are the first line of defense against pathogen invasion [4]. The destruction of this microbial defense can cause plants to be attacked by harmful microbes [5]. In addition, plants can recruit beneficial microbes to suppress pathogen invasion through the secretion of exudates [6]. Therefore, soil microorganisms play a vital role in regulating plant health and growth [7]. Using microorganisms to control soilborne diseases is considered a desirable approach. Microorganisms can protect plants from pathogens through many mechanisms, such as competing with pathogens for nutrients, producing antagonists that inhibit pathogens, or eliciting plant systemic defense [8,9]. However, the successful colonization of bioinoculants in plant rhizosphere depends on many factors, such as the composition and interactions with and within the locally adapted resident microbiota and soil attributes [10,11]. Therefore, a better knowledge of soil microbial community is essential for the prevention and control of plant diseases.
Many studies have suggested that the soil microbial community composition, diversity, structure, and function are related to the outbreak of plant soilborne diseases. For instance, a decrease in Firmicutes and Actinobacteria abundance in the tomato rhizosphere led to the outbreak of bacterial wilt disease [12]. Soil microbial communities containing high abundances of beneficial microorganisms can more effectively inhibit pathogens to maintain plant health [13]. Wei et al. [14] found that the initial soil microbiome composition and functioning can predetermine future plant health. High microbial diversity is likely related to the complexity of microbial interactions and, therefore, increases soil fungistasis and resistance to microbial invasion [15,16]. Previous studies have demonstrated that the disease severity of clubroot is associated with soil biotic factors [17]. However, knowledge of the differences in soil microbes between healthy and clubroot-diseased soils is limited.
Soil chemical properties also affect plant health and pathogen survival in soils [18,19]. Across a variety of crops, soil pH, organic matter content, extractable calcium, and boron contents are negatively correlated with disease severity [20]. In addition, the soil’s chemical properties can also indirectly influence the expression of soilborne diseases by affecting soil microbial communities [21]. For instance, soil acidification can largely impact bacterial communities and further reduce the capacity of soils to defend against fungal pathogens [22]. It has been reported that the occurrence of clubroot disease is negatively correlated with soil pH, boron, and calcium levels but positively corrected with bulk density and soil humidity [23,24,25,26]. However, different studies often show inconsistent results [27]. Hence, it is necessary to clarify the correlation between soil properties and plant health.
The present study aims to evaluate the characteristics and differences between clubroot-diseased and healthy soils, including the soil’s chemical properties and microbial properties. We hypothesized that (1) soil chemical properties and microbial community are correlated with clubroot disease and (2) that the soil microbial community varies in healthy and clubroot-diseased soils. We employed Illumina-MiSeq sequencing to compare the rhizosphere soil microbial communities of healthy and clubroot-diseased soils from different regions. As a result, this study provides a foundation for the biological control of clubroot disease in cruciferous crops.
2. Materials and Methods
2.1. Site Description and Sample Collection
All soil samples were collected from cruciferous vegetable fields designated as Licang (120.4267° E, 36.1511° N), Shandong Province; Youxian (108.6638° E, 30.3212° N), Sichuan Province; and Lichuan (109.7990° E, 30.7344° N) and Jianshi (31.5959° N, 104.8624° E), Hubei Province, China (Figure S1). The soils covered two different soil types (Cambisols and Luvisols), and other detailed information is listed in Table 1. Each field has a high clubroot disease incidence (over 50% over the last five years).

Table 1.
Description of the soil samples.
For each field, 3 random subplots (approximately 60 m2) were chosen, and soil samples from approximately 10 healthy (healthy soil) or 10 clubroot-diseased (diseased soil) plants from each subplot were collected using the checkerboard sampling method in August 2020. Briefly, each subplot was divided into 10 areas, and the rhizosphere soils of healthy and clubroot-diseased plants in the central point of each area were collected via manual shaking [28]. In each field, not only did the healthy plants have no root gall, but the aboveground plant growth was also good and consistent. Accordingly, the diseased plants showed noticeable wilting in the aboveground parts, and the root systems showed typical gall formation. The 10 healthy or 10 diseased soils were mixed to form one composite sample. The composite samples were placed into separate sterile bags and transported to the laboratory on ice. Each composite sample was ground and sieved through a 2 mm sieve and then divided into two subsamples: one portion was used for chemical property analysis after air-drying, and another was stored at −80 °C for subsequent DNA extraction.
2.2. Quantification of P. brassicae
For each composite soil, total DNA was extracted from 0.5 g of soil using the FastDNA® Spin kit (MO BIO Laboratories, Inc., Carlsbad, CA, USA), according to the manufacturer’s protocol. Each composite soil sample was extracted in triplicate, and the extracted DNA solutions were pooled. We quantified P. brassicae using quantitative polymerase chain reaction (qPCR) according to the methods described by Chai et al. [29]. Fluorescence was detected after each cycle and the Log of the resting spore numbers was calculated according to the method described in our previous work [29].
2.3. Soil Chemical Properties
The soil pH was determined using a pH meter (Shanghai Sanxin Instrumentation, Inc., Shanghai, China) in a 1:5 (m/m) soil:water solution. Soil organic matter (SOM) was determined using the potassium dichromate external heating method [30]. The total nitrogen (TN) level was determined using the semi-micro Kjeldahl method. Available nitrogen (AN) was determined using the alkaline-hydrolyzable diffusion method [31]. Available phosphorus (AP) was determined using a protocol according to Shen et al. [32]. Available potassium (AK) was extracted with ammonium acetate and determined via flame photometry using an FP640 Flame Photometer (Shanghai Instruments Group Co., Ltd., Shanghai, China). The cation exchange capacity (CEC) was measured according to the method of Mulvaney et al. [33]. Electrical conductivity (EC) was determined using a conductivity meter (Shanghai Leici Instrument Factory, Shanghai, China) in a soil water suspension (1:5 w/v) after being shaken at 200 r min−1 for 15 min at room temperature. The exchangeable calcium (Ca) and available boron (B) were measured using the methods of Bao [34] and Bhering et al. [35], respectively.
2.4. Amplicon Sequencing and Data Processing
Soil total DNA was extracted as described above. The primer set ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) [36] and ITS2 (5′-GCTGCGTTCTTC ATCGATGC-3′) were selected to target the fungal ITS1 region. 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACNNGGGTATC TAAT-3′) [37] were used to amplify the V4 hypervariable regions of the bacterial 16S rRNA gene. Amplicons were sequenced with the MiSeq platform at Allwegene CO., LIMITED (Beijing, China).
The raw data were first screened, and low-quality sequences with a quality score lower than 20 or a length shorter than 230 bp were discarded. Sequences with a 97% identity level were assigned to the same OTU using the Uparse algorithm in the Vsearch (v2.7.1) software. The chimeras were removed using Usearch (version 8.0.1623). The taxonomy of each 16S rRNA and ITS gene sequence was classified using the SILVA database and the UNITE database, respectively, with a confidence threshold of 80%. The alpha diversity indices (Chao1 index and Shannon index) were calculated using the QIIME software (v1.8.0). All raw sequences were uploaded to the NCBI Sequence Read Archive (SRA) database under the accession number PRJNA982587.
2.5. Network Analysis
To determine the complexity of the interactions between microbial taxa, co-occurrence networks were constructed using the rhizosphere microorganisms based on a Spearman correlation. In this study, the 200 most abundant fungal and bacterial OTUs from healthy and diseased soil samples were used for the network constructions (n = 12). The Benjamini–Hochberg FDR controlling procedure was used to correct the p-value via multiple tests. Correlation data were filtered with a cut-off at an absolute r-value of 0.6–0.93 and a p-value < 0.05. Nodes in the network represent gate-level classification units, and line-connecting nodes represent significant positive or negative correlations. The clustering coefficient (the clustering coefficient shows how well a node is connected with the neighbor nodes) and network density (the ratio of realized to possible edge numbers) were chosen to reflect the changes in soil microbiome associations [38]. Gephi (version 0.9.2) was used to visualize the co-occurrence networks.
The topological roles of nodes were determined using among-module connectivity (Pi) and within-module connectivity (Zi). All the nodes were classified into four categories, including module hubs (named generalists, Pi ≤ 0.62 and Zi > 2.5), network hubs (named supergeneralists, Zi > 2.5 and Pi > 0.62), connectors (also named generalists, Pi > 0.62 and Zi ≤ 2.5), and peripherals (named specialists, Pi ≤ 0.62 and Zi ≤ 2.5). Module hubs and connectors are considered putative keystone taxa [39,40]. The connectors, module hubs, and network hubs are core species that play important roles in maintaining network stability [41]. The topology property parameters of the network were calculated using the R (3.6.0) packages “Hmisc” and “igraph”. Zi–Pi plots were visualized using ggplot2 in the R platform [42].
2.6. Statistics Analysis
Differences in P. brassicae abundance, soil properties, and fungal and bacterial alpha diversity indices between the healthy and diseased soils were compared via one-way ANOVA (analysis of variance) followed by Tukey’s test (p < 0.05). The Wilcoxon rank-sum test was performed to identify differences in bacterial and fungal taxa between the healthy and diseased soils [43]. Pearson’s correlations between chemical parameters and disease incidence and between the abundance of P. brassicae and the abundant taxa were also performed. All the above statistical analyses were performed using the SPSS 22.0 software (SPSS Inc., Chicago, IL, USA). To compare microbial community structures across all samples, a hierarchical cluster tree was constructed using the unweighted paired-group method with arithmetic means (UPGMA) based on the Bray–Curtis distance matrix.
3. Results
3.1. P. brassicae Abundance in Different Fields
The abundance of P. brassicae was analyzed in all soil samples collected from four regions (Figure 1). The results showed that the population of P. brassicae ranged from 3.13 Log CFU/g to 8.04 Log CFU/g in the soil samples. Quantitative analysis showed that the colonization of P. brassicae was less than 3.92 Log CFU/g in the healthy soil samples from the four different fields, but more than 5.28 Log CFU/g of P. brassicae was detected in the diseased soils. The P. brassicae population in the healthy soil samples collected from Licang (3.69 Log CFU/g), Lichuan (3.92 Log CFU/g), and Jianshi (3.80 Log CFU/g) was significantly (Tukey’s test, p < 0.05) higher than that collected from Youxian (3.13 Log CFU/g). However, the P. brassicae population in the diseased soil samples collected from Youxian (8.04 Log CFU/g) was significantly higher than those collected from the other three fields. Among the four diseased soil samples, the lowest level (5.28 Log CFU/g) of resting spore infestation was detected in Lichuan. Within the same soil type, the abundance of P. brassicae in the healthy soils was significantly lower than that of the diseased soils. For the same field, the P. brassicae population in the diseased soil samples was significantly (Tukey’s test, p < 0.05) higher than in the healthy soil samples.
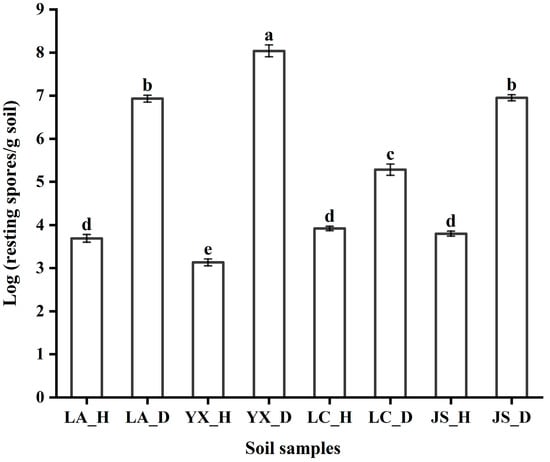
Figure 1.
Quantification of the resting spores of P. brassicae in different soil samples. LA_H, YX_H, LC_H, and JS_H represent the healthy soil samples collected from Licang, Youxian, Lichuan, and Jianshi, respectively. LA_D, YX_D, LC_D, and JS_D represent the clubroot-diseased soil samples collected from Licang, Youxian, Lichuan, and Jianshi, respectively. The error bars indicate the standard deviation of the means (n = 3). Different letters above the bars indicate statistically significant differences according to Tukey’s test (p < 0.05).
3.2. Soil Chemical Properties
The chemical properties of the soils are presented in Table 2. The pH, AN, AP, AK, and Ca contents in healthy soil samples were significantly (Tukey’s test, p < 0.05) different between the four regions. For diseased soil samples, AK, AP, and Ca levels in the four regions were significantly different. For the same field or each of the two soil types, compared with the healthy soils, the diseased soils had higher AK content but with no significant difference. There were significant differences in Ca levels between the healthy and diseased soils in the same field or each soil type. The pH values of the healthy and diseased soils were significantly different in Youxian, Lichuan, and Licang but did not show a consistent tendency in different fields. The CEC and B contents showed no difference between the healthy and diseased soils in the same field. However, other chemical properties (SOM, TN, AN, AP, and EC) showed different trends between the healthy and diseased soils in different regions.

Table 2.
Chemical properties of the soil samples.
3.3. Microbial Community Diversity
Altogether, 3, 685, 142 ITS sequence reads and 1, 642, 542 16s RNA effective sequence reads were obtained across the 24 soil samples. In total, 3652 fungal OTUs and 9185 bacterial OTUs were identified from these reads. We used the OTU number, Chao 1 (richness) and Shannon indices to evaluate and compare the diversity and richness of microbial communities among different soil samples (Table 3). For each field, there was no significant difference in the number of fungal OTUs and richness between the healthy and diseased soils. The fungal Shannon indices were higher in the healthy soils than in the diseased soils but with no significant difference except Licang. For bacteria, the OTU number and Shannon indices of the healthy and diseased soils were significantly (Tukey’s test, p < 0.05) different in Licang, Youxian, and Lichuan. The Chao1 indices of the healthy and diseased soils were significantly different in Youxian and Lichuan. The results indicated that the overall effect of the diseased soils on microbial diversity and richness was not significant. The fungal Shannon diversity indices in the healthy soil groups were significantly (Tukey’s test, p < 0.05) higher than those in the diseased soil groups (Table 4). However, there was no significant difference in the fungal OTU number and Chao1 indices between the healthy soil and diseased soil groups. For bacteria, there was no significant difference in OTU number, richness, and Shannon diversity indices between the healthy soil and diseased soil groups.

Table 3.
OTU number, Chao1 indices, and Shannon diversity indices of fungi and bacteria from different fields.

Table 4.
OTU number, Chao1 indices, and Shannon diversity indices of the healthy and diseased soil groups.
Hierarchical cluster analysis revealed that the same field presented similar fungal and bacterial microbial community structures (Figure 2). The fungal and bacterial communities from the healthy soils were separated from the diseased soils collected from the same field site, suggesting contrasting microbial community structures according to disease status.
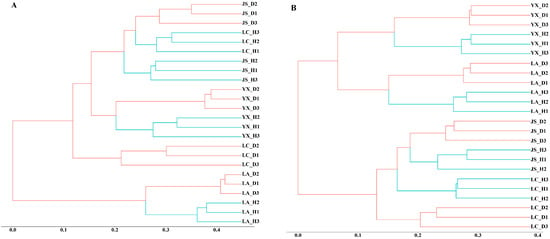
Figure 2.
Hierarchical cluster trees constructed based on the Bray–Curtis distance matrix for the healthy and diseased soil samples. (A) Hierarchical cluster tree of fungi. (B) Hierarchical cluster tree of bacteria. LA_H, YX_H, LC_H, and JS_H represent the healthy soil samples collected from Licang, Youxian, Lichuan, and Jianshi, respectively. LA_D, YX_D, LC_D, and JS_D represent the clubroot-diseased soil samples collected from Licang, Youxian, Lichuan, and Jianshi, respectively. Green lines represent healthy soil samples and red lines represent diseased soil samples.
3.4. Microbial Composition
A total of 16 fungal phyla were identified from all of the soil samples. Ascomycota, Mortierellomycota, Basidiomycota, and Chytridiomycota were the dominant phyla in all the samples, with Ascomycota being the most dominant phylum, accounting for 82.83% (Licang), 61.11% (Youxian), 64.33% (Lichuan), and 51.63% (Jianshi) in the healthy soils and 87.49% (Licang), 55.08% (Youxian), 84.34% (Lichuan), and 64.37% (Jianshi) in the diseased soils (Figure 3A). For the bacterial community, all OTUs were classified into 45 phyla. Proteobacteria, Acidobacteriota, Actinobacteriota, Chloroflexi, Gemmatimonadota, Bacteroidota, and Patescibacteria were the dominant phyla across all the samples (Figure 3B). The most abundant phylum in soils was Proteobacteria, which accounted for 28.21% (Licang), 35.21% (Youxian), 47.56% (Lichuan), and 37.08% (Jianshi) in the healthy soils and 38.05% (Licang), 36.41% (Youxian), 39.10% (Lichuan), and 31.08% (Jianshi) in the diseased soils. Among the abundant phyla (relative abundance > 0.1%), a higher abundance of Gemmatimonadota was in the healthy soil samples compared with the diseased soil samples within the same field. However, the other abundant phyla did not show a consistent tendency between the healthy and diseased soils in the same field. There was no significant difference in the fungi and bacteria at the phylum level between the healthy and diseased soils.
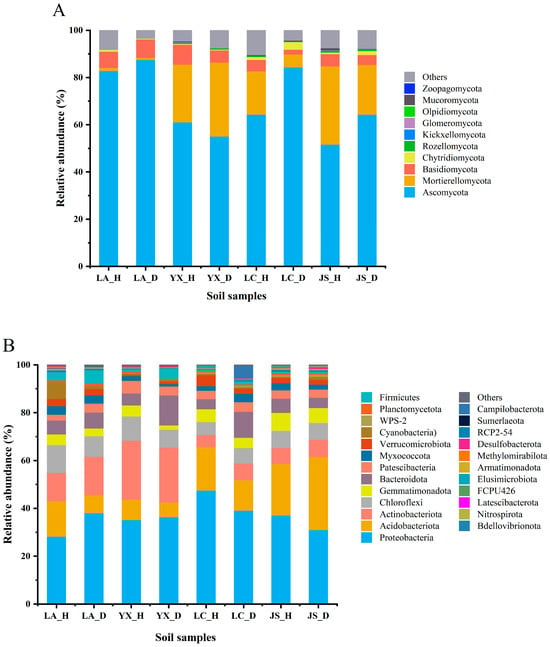
Figure 3.
Average relative abundance of fungal (A) and bacterial (B) phyla in soil samples. LA_H, YX_H, LC_H, and JS_H represent the healthy soil samples collected from Licang, Youxian, Lichuan, and Jianshi, respectively. LA_D, YX_D, LC_D, and JS_D represent the clubroot-diseased soil samples collected from Licang, Youxian, Lichuan, and Jianshi, respectively. Phyla below 0.1% of relative abundance and the unclassified phyla are classified as “Others”.
We further analyzed the association of the 15 most abundant fungal and bacterial genera with the healthy and diseased soils (Figure 4). The abundance of Chaetomium and Botryotrichum in the healthy soils was significantly higher than in the diseased soils (Wilcoxon rank-sum test, p < 0.05), but other genera showed no obvious difference between the diseased and healthy soil samples. For bacteria, Sphingomonas was more abundant in the healthy soils than in the diseased soils. There was no significant difference in the abundance of other bacterial genera between the healthy and diseased soils.
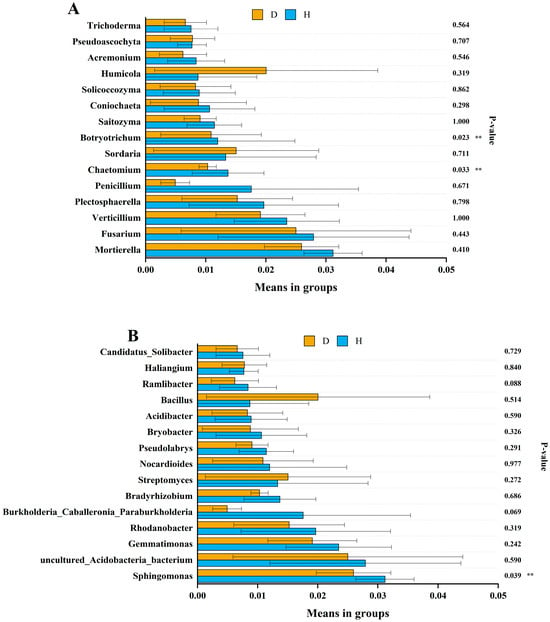
Figure 4.
Analysis of abundance differences between the healthy and clubroot-diseased soils at the fungal (A) and bacterial (B) genus levels. D represents the diseased soils. H represents the healthy soils. The error bars indicate the standard deviation of three replicates (n = 12), and double asterisks indicate statistical significance (Wilcoxon rank-sum test, p < 0.05).
3.5. Effects of Soil Variables and Microbial Species on Clubroot Disease
Relationships between chemical properties and disease incidence and between the abundance of P. brassicae and the 15 most abundant taxa were analyzed using Pearson’s correlation coefficient analysis. Disease incidence was only significantly positively correlated with CEC (Table 5). There was no correlation between disease incidence and other chemical properties. For fungal genera, the abundance of P. brassicae was significantly negatively correlated with Chaetomium, Botryotrichum, and Acremonium. With to bacteria, only Bacillus was significantly positively correlated with the abundance of P. brassicae (Table 6).

Table 5.
Pearson’s correlation between incidence of clubroot disease and chemical properties.

Table 6.
Pearson’s correlation between the abundance of P. brassicae and the relative abundance of the 15 most abundant bacterial and fungal genera.
3.6. Network Analysis
To investigate the relationship between the soil microbe–microbe interactions and clubroot disease, we performed a microbial co-occurrence network analysis (Figure 5). For fungal networks, the healthy soils (3657 links) had higher link numbers than the diseased soils (2980 links) (Table 7). Both the healthy and diseased soils had higher positive link numbers than negative link numbers. The positive link/negative link ratio in diseased soils (3.02) was higher than that in healthy soils (2.15), demonstrating that the diseased soils contained more positive co-occurrence relationships than the healthy soils. The clustering coefficient of the healthy soils (0.57) was higher than that of the diseased soils (0.53). The network density of microbiome networks in the healthy soils was 0.19 higher than 0.15 in the diseased soils. The modularity value of the healthy (0.39) and diseased (0.40) soils was similar. In the fungal networks, 22 fungal nodes out of the top 200 nodes were sunk into “connectors” (Figure 6A and Table S1). Members from Ascomycota, Basidiomycota, Chytridiomycota, and Glomeromycota were identified as keystone fungal taxa. No network hubs and module hubs were found in the fungal networks.
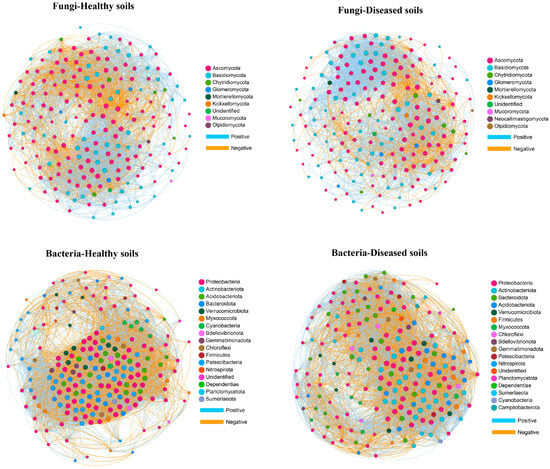
Figure 5.
Co-occurrence networks of the 200 most abundant fungal and bacterial genera. The size of the node is equivalent to its relative abundance. Blue and yellow edges represent positive and negative interactions, respectively.

Table 7.
Topological indices of each network.
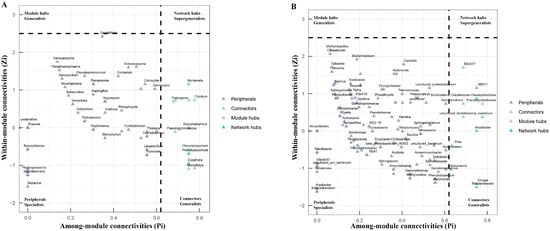
Figure 6.
Topological roles of nodes in fungal (A) and bacterial (B) networks. The topological roles of nodes were determined using among-module connectivity (Pi) and within-module connectivity (Zi). All the nodes were classified into four categories, including module hubs (named generalists, Pi ≤ 0.62 and Zi > 2.5), network hubs (named supergeneralists, Zi > 2.5 and Pi > 0.62), connectors (also named generalists, Pi > 0.62 and Zi ≤ 2.5), and peripherals (named specialists, Pi ≤ 0.62 and Zi ≤ 2.5).
For the bacteria networks of the healthy and diseased soils, the number of links was 8789 and 6750, respectively. The diseased soils (1.33) had a higher positive link/negative link ratio than the healthy soils (1.16). The network density and the clustering coefficient in networks of the healthy and diseased soils were 0.44 and 0.34 and 0.75 and 0.68, respectively. The healthy (0.31) soils had higher modularity than the diseased (0.18) soils. We found that 13% of the top 200 nodes were connectors (26 nodes), while 87% were peripherals (Figure 6B and Table S2). Similar to the fungal networks, there were no network hubs or module hubs detected in the bacterial networks. Members from Proteobacteria, Acidobacteriota, Actinobacteriota, Bacteroidota, Chloroflexi, Myxococcota, Nitrospirota, and Verrucomicrobiota were identified as keystone bacterial taxa.
4. Discussion
Plant health is closely related to soil properties, including physical, chemical, and microbial attributes. Characterizing the differences in soil properties between healthy and diseased soils is an important first step to understanding the pathogenesis of soilborne disease. In this study, we compared the soil chemical properties and microbial communities of healthy and clubroot-diseased Cruciferae crops in four regions of China. We found that the fungal diversity in the healthy soils was higher than in the diseased soils, and the networks of healthy soils were more complex than those of clubroot-diseased soils.
Clubroot severity in field conditions has been suggested to be related to soil pH value [44,45]. The soil pH in the clubroot-diseased cruciferous crops ranged from 5.13 to 7.54 across the four regions. This result is in line with previous findings showing that severe clubroot developed at pH 5.0–8.0 in field conditions [46]. The soil pH did not show a consistent tendency between healthy and diseased soils in four different regions, suggesting that pH may not be an important factor affecting clubroot severity in this study. Similarly, an assessment of soil pH in commercial canola fields infested with clubroot showed that there was only a weak correlation between soil pH and clubroot level [46]. It is noteworthy that the soil pH values of Lichuan and Jianshi were lower than those of Licang and Youxian, while the corresponding bacterial Chao1 indices of healthy and diseased soils in the two regions were, overall, at low levels among the four regions. Thus, soil pH may be one of the factors affecting bacterial diversity, which is in line with previous studies [47,48]. A high calcium concentration can reduce the rate of maturation of P. brassicae in root hairs and clubroot severity in Brassica crops [49,50]. In this study, the calcium levels of the healthy and diseased soils were significantly different in the same region, but the changing trend was inconsistent in different regions. Our results indicated that there was no significant correlation between calcium levels and healthy plant status, which is in agreement with previous findings [45,51]. Previous studies have shown that boron reduces the development of clubroot in Brassica crops. Nevertheless, the boron levels were not significantly different for the healthy and diseased soils in the same region. This may be because other conditions were conducive to infection. At present, there are few reports on the variation in CEC in soil during the infection process of P. brassicae in cruciferous crops. In the present study, CEC was significantly positively related to disease incidence. Our results may indicate that CEC could be an indicator of the severity of clubroot disease.
The results showed that the microbial diversity of the healthy and diseased soils differed between regions. Some areas were the same, while others showed different microbial diversity. The Chao1 indices of the fungi were not significantly different for the healthy and diseased soils in all four regions, which is in line with previous findings [52,53]. For bacteria, the Chao1 indices of the healthy and diseased soils were significantly different in Youxian and Lichuan but not significantly different in Licang and Jianshi. The results indicated that the diseased soils in Youxian and Lichuan may have a significant effect on bacterial diversity. Taken together, the field site location had a large effect in determining microbial community diversity in the present study, which is similar to previous studies that illustrated the importance of spatial influences [47,54,55].
The composition and structure of soil microbial communities have an important impact on soil and plant health [7,56]. In the present study, the same region presented a similar microbial composition, but there were differences between different sites. For instance, Ascomycota and Basidiomycota were the most abundant fungal phyla identified in Licang, while Ascomycota and Mortierellomycota were the two most prevalent fungal phyla in Youxian, Lichuan, and Jianshi. Gemmatimonadota was relatively more abundant in the healthy soils compared with the diseased soils in each of the four regions, which is in agreement with previous results [57,58,59]. Thus, Gemmatimonadota may be an indicator of the soil supporting plant health. A recent investigation reported that members of Gemmatimonadota can encode diverse polyketide and nonribosomal peptide biosynthetic gene clusters [60]. Non-ribosomal peptides are one of the largest groups of natural microbial secondary metabolites, which are important for the producer in terms of defense responses, chemical communication, and nutrient acquisition [61,62]. The microbial community structure collected from the same region was more similar when compared with different regions, which is in agreement with previous studies [53,55].
We compared the microbial differences between groups for the 15 most abundant genera from different regions. The results showed that the relative abundance of Chaetomium in the healthy soils was significantly higher than in the diseased soils. Previous studies have shown that some species of Chaetomium can inhibit the plant pathogen through competition, mycoparasitism, antibiosis, and a combination of various mechanisms [63,64,65]. This taxon may serve as an important indicator of clubroot disease suppression in the cruciferous crop system. For bacteria, the relative abundance of Sphingomonas in the healthy soils was significantly higher than in the diseased soils, which is in line with previous studies [66]. Sphingomonas species play an important role in plant growth promotion, abiotic stress tolerance, and environmental remediation [67,68,69,70,71]. In a recent study, Sphingomonas was identified as one of the keystone genera associated with pathogen suppression in healthy plant microbiomes [14]. The relative abundance of Chaetomium, Botryotrichum, and Acremonium was significantly negatively correlated with that of P. brassicae (Table 6). We speculate that these species may play an important role in reducing the development of clubroot disease.
Many interactions between different microbial species in soil microbial communities formed a complex microbial network, which is vital to ecosystem stability [72,73,74]. In the present study, the co-occurrence networks revealed different patterns within the microbial communities of the healthy and clubroot-diseased soils. The healthy soils showed a higher number of co-occurrence relationships than the clubroot-diseased soils for both fungal and bacterial networks. A higher number of links means a more complex network, suggesting that the microbial networks of the healthy soils were more complex than those of the diseased soils, which followed the results of Xiong et al. [13] but contrasted with the results of Hu et al. [8]. This may be because the healthy soils had higher diversity than the clubroot-diseased soils [75]. Strikingly, the number of positive links was higher than that of the negative links for fungal and bacterial networks in the healthy and clubroot-diseased soils. More positive interactions may suggest more cooperation in soil microbial community ecosystems, which may be linked to a higher community function [76,77]. In addition, the healthy soils exhibited higher complexity, reflected by increased network density and clustering coefficients [78]. Overall, the healthy networks were more complex and stable, therefore making them better able to exclude the pathogen P. brassicae from the plant environment.
Connectors are essential microorganisms that act as regulators, mediators, and adaptors in a network [79]. The members of Ascomycota and Proteobacteria accounted for the largest proportion of connectors in the fungal and bacterial networks, respectively, which may be related to their high relative abundance in the soils. It is worth noting that species of Acremonium have been investigated as potential antagonistic agents against clubroot disease (Table S1) [80]. Future studies would be required to investigate the control effect of the species on clubroot disease. Among connectors in the bacterial networks, some beneficial microbes, such as Alcaligenes, Klebsiella, Nitrosospira, and Nitrospira, were identified, which function in the nitrogen cycle (Table S2) [81,82,83].
In conclusion, the bacterial and fungal communities of healthy and clubroot-diseased soils were different. The fungal diversity in the healthy soils was significantly higher than in the diseased soils. The healthy soils exhibited significantly higher relative abundances of Chaetomium and Sphingomonas than the diseased soils. The healthy soils had a higher link number, network density, and clustering coefficient for both fungal and bacterial networks and, thus, were more complex and stable than the diseased network. In addition, healthy soils had higher AK content than diseased soils. Together, these findings provide some clues into potential methods of clubroot disease management through the manipulation of microbial communities.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/microorganisms12020251/s1. Figure S1: Sampling sites. For each field, 3 random subplots (approximately 60 m2) were chosen, and soil samples from approximately 10 healthy (healthy soil) or 10 clubroot-diseased (diseased soil) plants from each subplot were collected using the checkerboard sampling method during August 2020. Briefly, each subplot was divided into 10 areas, and rhizosphere soils of healthy and clubroot-diseased plants in the central point of each area were collected by manual shaking. At each field, the healthy plants not only referred to having no root gall but also the above-ground plant growth being good and consistent. Accordingly, the diseased plants referred to the noticeable wilting of the aboveground parts, and the root system showed typical gall formation. The 10 healthy and 10 diseased soils were mixed to form one composite sample, respectively. The composite samples were placed into separate sterile bags and transported to the laboratory on ice. LA, Licang, Shandong Province; YX, Youxian, Sichuan Province; LC, Lichuan, Hubei Province; JS, Jianshi, Hubei Province; Table S1: The keystone taxa identified as connectors in the soil fungal networks; Table S2: The keystone taxa identified as connectors in the soil bacterial networks.
Author Contributions
Conceptualization, H.K., B.L. and A.C.; methodology, H.K. and A.C.; data curation, H.K., Z.L. and A.C.; investigation, H.K., A.C., Z.L. and X.X.; writing—original draft, H.K.; writing—review and editing, A.C., J.X. and B.L.; supervision, A.C., Y.S., T.F., L.L. and J.X.; resources, X.X., T.F., S.X., L.L. and B.L.; funding acquisition, B.L.; project administration, B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Key R&D Program of China (2022YFD1401200), the Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences (CAAS-ASTIP-IVFCAAS), and the China Agriculture Research System of MOF and MARA (CARS-23).
Data Availability Statement
The sequencing data are available upon request.
Acknowledgments
We acknowledge the support of the Key Laboratory of Biology and Genetic Improvement of Horticultural Crops, Ministry of Agriculture and Rural Affairs, China, and the China Agriculture Research System of MOF and MARA (CARS-23).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- Kowata-Dresch, L.S.; May-De Mio, L.L. Clubroot management of highly infested soils. Crop Prot. 2012, 35, 47–52. [Google Scholar] [CrossRef]
- Donald, C.; Porter, I. Integrated control of clubroot. J. Plant Growth Regul. 2009, 28, 289–303. [Google Scholar] [CrossRef]
- Struck, C.; Rüsch, S.; Strehlow, B. Control strategies of clubroot disease caused by Plasmodiophora brassicae. Microorganisms 2022, 10, 620. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Riera, N.; Jin, T.; Li, J.; Wang, N. Huanglongbing impairs the rhizosphere-to-rhizoplane enrichment process of the citrus root-associated microbiome. Microbiome 2017, 5, 97. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, R.L.; Vismans, G.; Yu, K.; Song, Y.; de Jonge, R.; Burgman, W.P.; Burmølle, M.; Herschend, J.; Bakker, P.A.H.M.; Pieterse, C.M.J. Disease-induced assemblage of a plant-beneficial bacterial consortium. ISME J. 2018, 12, 1496–1507. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; van der Heijden, M.G. Soil microbiomes and one health. Nat. Rev. Microbiol. 2023, 21, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Tan, L.; Gu, S.; Xiao, Y.; Xiong, X.; Zeng, W.; Feng, K.; Wei, Z.; Deng, Y. Network analysis infers the wilt pathogen invasion associated with non-detrimental bacteria. NPJ Biofilms Microb. 2020, 6, 8. [Google Scholar] [CrossRef]
- Sun, X.; Xu, Z.; Xie, J.; Hesselberg-Thomsen, V.; Tan, T.; Zheng, D.; Strube, M.L.; Dragoš, A.; Shen, Q.; Zhang, R.; et al. Bacillus velezensis stimulates resident rhizosphere Pseudomonas stutzeri for plant health through metabolic interactions. ISME J. 2022, 16, 774–787. [Google Scholar] [CrossRef]
- Poppeliers, S.W.M.; Sánchez-Gil, J.J.; de Jonge, R. Microbes to support plant health: Understanding bioinoculant success in complex conditions. Curr. Opin. Microbiol. 2023, 73, 102286. [Google Scholar] [CrossRef]
- Qian, J.J.; Akçay, E. The balance of interaction types determines the assembly and stability of ecological communities. Nat. Ecol. Evol. 2020, 4, 356–365. [Google Scholar] [CrossRef]
- Lee, S.M.; Kong, H.G.; Song, G.C.; Ryu, C.M. Disruption of Firmicutes and Actinobacteria abundance in tomato rhizosphere causes the incidence of bacterial wilt disease. ISME J. 2021, 15, 330–347. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Li, R.; Ren, Y.; Liu, C.; Zhao, Q.; Wu, H.; Jousset, A.; Shen, Q. Distinct roles for soil fungal and bacterial communities associated with the suppression of vanilla Fusarium wilt disease. Soil Biol. Biochem. 2017, 107, 198–207. [Google Scholar] [CrossRef]
- Wei, Z.; Gu, Y.; Friman, V.P.; Kowalchuk, G.A.; Xu, Y.; Shen, Q.; Jousset, A. Initial soil microbiome composition and functioning predetermine future plant health. Sci. Adv. 2019, 5, eaaw0759. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, G.; Capodilupo, M.; Incerti, G.; Gaglione, S.A.; Scala, F. Fungal diversity increases soil fungistasis and resistance to microbial invasion by a nonresident species. Biol. Control 2014, 72, 38–45. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, T.; Friman, V.P.; Xu, Y.; Shen, Q.; Jousset, A. Trophic network architecture of root-associated bacterial communities determines pathogen invasion and plant health. Nat. Commun. 2015, 6, 8413. [Google Scholar] [CrossRef] [PubMed]
- Murakami, H.; Tsushima, S.; Shishido, Y. Soil suppressiveness to clubroot disease of Chinese cabbage caused by Plasmodiophora brassicae. Soil Biol. Biochem. 2000, 32, 1637–1642. [Google Scholar] [CrossRef]
- Li, J.; Wan, X.; Liu, X.; Chen, Y.; Slaughter, L.C.; Weindorf, D.C.; Dong, Y. Changes in soil physical and chemical characteristics in intensively cultivated greenhouse vegetable fields in North China. Soil Till. Res. 2019, 195, 104366. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, H.; Sun, L.; Qi, G.; Chen, S.; Zhao, X. Microbial community composition is related to soil biological and chemical properties and bacterial wilt outbreak. Sci. Rep. 2017, 7, 343. [Google Scholar] [CrossRef]
- Orr, R.; Nelson, P.N. Impacts of soil abiotic attributes on Fusarium wilt, focusing on bananas. Appl. Soil Ecol. 2018, 132, 20–33. [Google Scholar] [CrossRef]
- Meng, T.; Wang, Q.; Abbasi, P.; Ma, Y. Deciphering differences in the chemical and microbial characteristics of healthy and Fusarium wilt-infected watermelon rhizosphere soils. Appl. Microbiol. Biotechnol. 2019, 103, 1497–1509. [Google Scholar] [CrossRef]
- Li, X.; Chen, D.; Carrión, V.J.; Revillini, D.; Yin, S.; Dong, Y.; Zhang, T.; Wang, X.; Delgado-Baquerizo, M. Acidification suppresses the natural capacity of soil microbiome to fight pathogenic Fusarium infections. Nat. Commun. 2023, 14, 5090. [Google Scholar] [CrossRef]
- Kasinathan, H. Influence of pH, Temperature, and Biofungicides on Clubroot of Canola. Master’s Thesis, University of Guelph, Guelph, ON, Canada, 2012. [Google Scholar]
- Gludovacz, T.V. Clubroot in Canola and Cabbage in Relation to Soil Temperature, Plant Growth and Host Resistance. Master’s Thesis, University of Guelph, Guelph, ON, Canada, 2013. [Google Scholar]
- Deora, A.; Gossen, B.D.; Walley, F.; McDonald, M.R. Boron reduces development of clubroot in canola. Can. J. Plant Pathol. 2011, 33, 475–484. [Google Scholar] [CrossRef]
- Webster, M.A.; Dixon, G.R. Calcium, pH and inoculum concentration influencing colonization by Plasmodiophora brassicae. Mycol. Res. 1991, 95, 64–73. [Google Scholar] [CrossRef]
- Gossen, B.D.; Deora, A.; Peng, G.; Hwang, S.F.; McDonald, M.R. Effect of environmental parameters on clubroot development and the risk of pathogen spread. Can. J. Plant Pathol. 2014, 36, 37–48. [Google Scholar] [CrossRef]
- Niu, J.; Chao, J.; Xiao, Y.; Chen, W.; Zhang, C.; Liu, X.; Rang, Z.; Yin, H.; Dai, L. Insight into the effects of different cropping systems on soil bacterial community and tobacco bacterial wilt rate. J. Basic Microbiol. 2007, 57, 3–11. [Google Scholar] [CrossRef]
- Chai, A.L.; Li, J.P.; Xie, X.W.; Shi, Y.X.; Li, B.J. Dissemination of Plasmodiophora brassicae in livestock manure detected by qPCR. Plant Pathol. 2016, 65, 137–144. [Google Scholar] [CrossRef]
- Ciavatta, C.; Govi, M.; Antisari, L.V.; Sequi, P. Determination of organic carbon in aqueous extracts of soils and fertilizers. Commun. Soil Sci. Plant Anal. 1991, 22, 795–807. [Google Scholar] [CrossRef]
- Li, J.G.; Ren, G.D.; Jia, Z.J.; Dong, Y.H. Composition and activity of rhizosphere microbial communities associated with healthy and diseased greenhouse tomatoes. Plant Soil 2004, 380, 337–347. [Google Scholar] [CrossRef]
- Shen, Z.Q.; Zhang, Q.; Liu, L.J.; Qiu, Y. Determination of available phosphorus in soil by sodium bicarbonate extraction Mo-Sb anti-spectrophotometry method. Environ. Monit. Forew. 2001, 3, 12–15. [Google Scholar]
- Mulvaney, R.L.; Yaremych, S.A.; Khan, S.A.; Swiader, J.M.; Horgan, B.P. Use of diffusion to determine soil cation-exchange capacity by ammonium saturation. Commun. Soil Sci. Plant Anal. 2004, 35, 51–67. [Google Scholar] [CrossRef]
- Bao, S.D. Analysis of water soluble salts in soil. In Soil and Agricultural Chemistry Analysis, 3rd ed.; Chinese Agricultural Press: Beijing, China, 2000; pp. 189–191. [Google Scholar]
- Bhering, A.D.S.; do Carmo, M.G.; Matos, T.D.S.; Lima, E.S.; do Amaral Sobrinho, N.M. Soil factors related to the severity of clubroot in Rio de Janeiro, Brazil. Plant Dis. 2007, 101, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Ma, G.; Chen, S.; Lin, C.; Zhao, X. Microbial network and soil properties are changed in bacterial wilt-susceptible soil. Appl. Environ. Microbiol. 2019, 85, e00162-19. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.B.; He, J.Z.; Geisen, S.; Han, L.L.; Wang, J.T.; Shen, J.P.; Wei, W.X.; Fang, Y.T.; Li, P.P.; Zhang, L.M. Protist communities are more sensitive to nitrogen fertilization than other microorganisms in diverse agricultural soils. Microbiome 2009, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Jiang, Y.H.; Yang, Y.; He, Z.; Luo, F.; Zhou, J. Molecular ecological network analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef]
- Ling, N.; Wang, T.; Kuzyakov, Y. Rhizosphere bacteriome structure and functions. Nat. Commun. 2022, 13, 836. [Google Scholar] [CrossRef]
- Banerjee, S.; Kirkby, C.A.; Schmutter, D.; Bissett, A.; Kirkegaard, J.A.; Richardson, A.E. Network analysis reveals functional redundancy and keystone taxa amongst bacterial and fungal communities during organic matter decomposition in an arable soil. Soil Biol. Biochem. 2016, 97, 188–198. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing, 3.6.0 edn.; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Shi, W.; Li, M.; Wei, G.; Tian, R.; Li, C.; Wang, B.; Lin, R.; Shi, C.; Chi, X.; Zhou, B.; et al. The occurrence of potato common scab correlates with the community composition and function of the geocaulosphere soil microbiome. Microbiome 2019, 7, 14. [Google Scholar] [CrossRef]
- Niwa, R.; Nomura, Y.; Osaki, M.; Ezawa, T. Suppression of clubroot disease under neutral pH caused by inhibition of spore germination of Plasmodiophora brassicae in the rhizosphere. Plant Pathol. 2008, 5, 445–452. [Google Scholar] [CrossRef]
- Bhering, A.S.; Carmo, M.G.F.; Coelho, I.S.; Lima, E.S.A.; de Carvalho, C.F.; Saraiva, A.L.R.F.; Passos, S.R.; Sobrinho, N.M.B.A. Soil management in a mountain agroecosystem and clubroot disease. Plant Pathol. 2020, 69, 302–309. [Google Scholar] [CrossRef]
- Gossen, B.D.; Kasinathan, H.; Cao, T.; Manolii, V.P.; Strelkov, S.E.; Hwang, S.F.; McDonald, M.R. Interaction of pH and temperature affect infection and symptom development of Plasmodiophora brassicae in canola. Can. J. Plant Pathol. 2013, 35, 294–303. [Google Scholar] [CrossRef]
- Shi, H.; Xu, P.; Wu, S.; Yu, W.; Cheng, Y.; Chen, Z.; Yang, X.; Yu, X.; Li, B.; Ding, A.; et al. Analysis of rhizosphere bacterial communities of tobacco resistant and non-resistant to bacterial wilt in different regions. Sci. Rep. 2022, 12, 18309. [Google Scholar] [CrossRef] [PubMed]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Fox, N.M.; Hwang, S.F.; Manolii, V.P.; Turnbull, G.; Strelkov, S.E. Evaluation of lime products for clubroot (Plasmodiophora brassicae) management in canola (Brassica napus) croping systems. Can. J. Plant Pathol. 2022, 44, 21–38. [Google Scholar] [CrossRef]
- Dixon, G.R. Managing clubroot disease (caused by Plasmodiophora brassicae Wor.) by exploiting the interactions between calcium cyanamide fertilizer and soil microorganisms. J. Agric. Sci. 2017, 155, 527–543. [Google Scholar] [CrossRef]
- Saraiva, A.L.R.F.; Bhering, A.S.; do Carmo, M.G.F.; Andreote, F.D.; Dias, A.C.F.; Coelho, I.D.S. Bacterial composition in brassica-cultivated soils with low and high severity of clubroot. J. Phytopathol. 2020, 168, 613–619. [Google Scholar] [CrossRef]
- Jia, M.; Sun, X.; Chen, M.; Liu, S.; Zhou, J.; Peng, X. Deciphering the microbial diversity associated with healthy and wilted Paeonia suffruticosa rhizosphere soil. Front. Microbiol. 2022, 13, 967601. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Jing, T.; Chen, Y.; Wang, F.; Qi, D.; Feng, R.; Xie, J.; Li, H. Deciphering microbial diversity associated with Fusarium wilt-diseased and disease-free banana rhizosphere soil. BMC Microbiol. 2019, 19, 161. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, R.; Xue, C.; Xun, W.; Sun, L.; Xu, Y.; Shen, Q. Pyrosequencing reveals contrasting soil bacterial diversity and community structure of two main winter wheat cropping systems in China. Microb. Ecol. 2004, 67, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Ruan, Y.; Xue, C.; Zhong, S.; Li, R.; Shen, Q. Soils naturally suppressive to banana Fusarium wilt disease harbor unique bacterial communities. Plant Soil 2015, 393, 21–33. [Google Scholar] [CrossRef]
- Van Elsas, J.D.; Chiurazzi, M.; Mallon, C.A.; Elhottovā, D.; Krištůfek, V.; Salles, J.F. Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc. Natl. Acad. Sci. USA 2022, 109, 1159–1164. [Google Scholar] [CrossRef]
- Liu, L.; Yan, Y.; Ding, H.; Zhao, J.; Cai, Z.; Dai, C.; Huang, X. The fungal community outperforms the bacterial community in predicting plant health status. Appl. Microbiol. Biotechnol. 2021, 105, 6499–6513. [Google Scholar] [CrossRef]
- Ou, Y.; Penton, C.R.; Geisen, S.; Shen, Z.; Sun, Y.; Lv, N.; Wang, B.; Ruan, Y.; Xiong, W.; Li, R.; et al. Deciphering underlying drivers of disease suppressiveness against pathogenic Fusarium oxysporum. Front. Microbiol. 2019, 10, 2535. [Google Scholar] [CrossRef]
- Cha, J.Y.; Han, S.; Hong, H.J.; Cho, H.; Kim, D.; Kwon, Y.; Crüsemann, M.; Lee, Y.B.; Kim, J.F.; Giaever, G.; et al. Microbial and biochemical basis of a Fusarium wilt-suppressive soil. ISME J. 2016, 10, 119–129. [Google Scholar] [CrossRef]
- Crits-Christoph, A.; Diamond, S.; Butterfield, C.N.; Thomas, B.C.; Banfield, J.F. Novel soil bacteria possess diverse genes for secondary metabolite biosynthesis. Nature 2018, 558, 440–444. [Google Scholar] [CrossRef]
- Prieto, C. Characterization of nonribosomal peptide synthetases with NRPSsp. In Nonribosomal Peptide and Polyketide Biosynthesis; Bradley, S.E., Ed.; Springer: New York, NY, USA, 2016; Volume 1401, pp. 273–278. [Google Scholar]
- Raaijmakers, J.M.; De Bruijn, I.; Nybroe, O.; Ongena, M. Natural functions of lipopeptides from Bacillus and Pseudomonas: More than surfactants and antibiotics. FEMS Microbiol. Rev. 2010, 34, 1037–1062. [Google Scholar] [CrossRef] [PubMed]
- Soytong, K.; Kanokmedhakul, S.; Kukongviriyapa, V.; Isobe, M. Application of Chaetomium species (Ketomium) as a new broad spectrum biological fungicide for plant disease control. Fungal Divers. 2001, 7, 1–15. [Google Scholar]
- Pothiraj, G.; Hussain, Z.; Singh, A.K.; Solanke, A.U.; Aggarwal, R.; Ramesh, R.; Shanmugam, V. Characterization of Fusarium spp. inciting vascular wilt of tomato and its management by a Chaetomium-based biocontrol consortium. Front. Plant Sci. 2021, 12, 748013. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Y.; Wei, F.; Zhao, L.; Zhou, J.; Qi, G.; Ma, Z.; Zhu, H.; Feng, H.; Feng, Z. Applications of Chaetomium globosum CEF-082 improve soil health and mitigate the continuous croping obstacles for Gossypium hirsutum. Ind. Crops Prod. 2023, 197, 116586. [Google Scholar] [CrossRef]
- Nerva, L.; Zanzotto, A.; Gardiman, M.; Gaiotti, F.; Chitarra, W. Soil microbiome analysis in an ESCA diseased vineyard. Soil Biol. Biochem. 2019, 135, 60–70. [Google Scholar] [CrossRef]
- Chu, C.; Liu, B.; Liu, J.; He, J.; Lv, L.; Wang, H.; Xie, X.; Tao, Q.; Chen, Q. Phytoremediation of acetochlor residue by transgenic Arabidopsis expressing the acetochlor N-dealkylase from Sphingomonas wittichii DC-6. Sci. Total Environ. 2020, 728, 138687. [Google Scholar] [CrossRef] [PubMed]
- Lombardino, J.; Bijlani, S.; Singh, N.K.; Wood, J.M.; Barker, R.; Gilroy, S.; Wang, C.C.C.; Venkateswaran, K. Genomic characterization of potential plant growth-promoting features of Sphingomonas strains isolated from the International Space Station. Microbiol. Spectr. 2022, 10, e01994-21. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wei, Y.; Yan, T.; Wang, C.; Chao, Y.; Jia, M.; An, L.; Sheng, H. Sphingomonas sp. Hbc-6 alters physiological metabolism and recruits beneficial rhizosphere bacteria to improve plant growth and drought tolerance. Front. Plant Sci. 2022, 13, 1002772. [Google Scholar] [CrossRef] [PubMed]
- Mazoyon, C.; Hirel, B.; Pecourt, A.; Catterou, M.; Gutierrez, L.; Sarazin, V.; Dubois, F.; Duclercq, J. Sphingomonas sediminicola is an endosymbiotic bacterium able to induce the formation of root nodules in Pea (Pisum sativum L.) and to enhance plant biomass production. Microorganisms 2023, 11, 199. [Google Scholar] [CrossRef]
- Asaf, S.; Numan, M.; Khan, A.L.; Al-Harrasi, A. Sphingomonas: From diversity and genomics to functional role in environmental remediation and plant growth. Crit. Rev. Biotechnol. 2020, 40, 138–152. [Google Scholar] [CrossRef]
- Morrien, E.; Hannula, S.E.; Snoek, L.B.; Helmsing, N.R.; Zweers, H.; de Hollander, M.; Soto, R.L.; Bouffaud, M.L.; Buée, M.; Dimmers, W.; et al. Soil networks become more connected and take up more carbon as nature restoration progresses. Nat. Commun. 2017, 8, 14349. [Google Scholar] [CrossRef]
- Ratzke, C.; Barrere, J.; Gore, J. Strength of species interactions determines biodiversity and stability in microbial communities. Nat. Ecol. Evol. 2020, 4, 376–383. [Google Scholar] [CrossRef]
- Schmitt, S.; Tsai, P.; Bell, J.; Fromont, J.; Ilan, M.; Lindquist, N.; Perez, T.; Rodrigo, A.; Schupp, P.J.; Vacelet, J.; et al. Assessing the complex sponge microbiota: Core, variable and species-specific bacterial communities in marine sponges. ISME J. 2012, 6, 564–576. [Google Scholar] [CrossRef]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; van der Heijden, M.G. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 2019, 10, 4841. [Google Scholar] [CrossRef]
- Coyte, K.Z.; Schluter, J.; Foster, K.R. The ecology of the microbiome: Networks, competition, and stability. Science 2015, 350, 663–666. [Google Scholar] [CrossRef]
- Palmer, J.D.; Foster, K.R. Bacterial species rarely work together. Science 2022, 376, 581–582. [Google Scholar] [CrossRef]
- Price, G.W.; Langille, M.G.; Yurgel, S.N. Microbial co-occurrence network analysis of soils receiving short-and long-term applications of alkaline treated biosolids. Sci. Total Environ. 2021, 751, 141687. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Weisenhorn, P.; Gilbert, J.A.; Chu, H. Wheat rhizosphere harbors a less complex and more stable microbial co-occurrence pattern than bulk soil. Soil Biol. Biochem. 2018, 125, 251–260. [Google Scholar] [CrossRef]
- Jäschke, D.; Dugassa-Gobena, D.; Karlovsky, P.; Vidal, S.; Ludwig-Müller, J. Suppression of clubroot (Plasmodiophora brassicae) development in Arabidopsis thaliana by the endophytic fungus Acremonium alternatum. Plant Pathol. 2010, 59, 100–111. [Google Scholar] [CrossRef]
- Koch, H.; Lücker, S.; Albertsen, M.; Kitzinger, K.; Herbold, C.; Spieck, E.; Nielsen, P.H.; Wagner, M.; Daims, H. Expanded metabolic versatility of ubiquitous nitrite-oxidizing bacteria from the genus Nitrospira. Proc. Natl. Acad. Sci. USA 2015, 112, 11371–11376. [Google Scholar] [CrossRef] [PubMed]
- Martikainen, P.J. Heterotrophic nitrification-An eternal mystery in the nitrogen cycle. Soil Biol. Biochem. 2022, 168, 108611. [Google Scholar] [CrossRef]
- Kuypers, M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).