Identification of Cellulose-Degrading Bacteria and Assessment of Their Potential Value for the Production of Bioethanol from Coconut Oil Cake Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Cellulolytic Bacteria
2.2. Qualitative Screening
2.3. Quantitative Screening
- F: blank glucose amount (μg);
- f: Glucose weight of the sample (μg);
- 30: The reaction time (min) between the enzyme and the substrate.
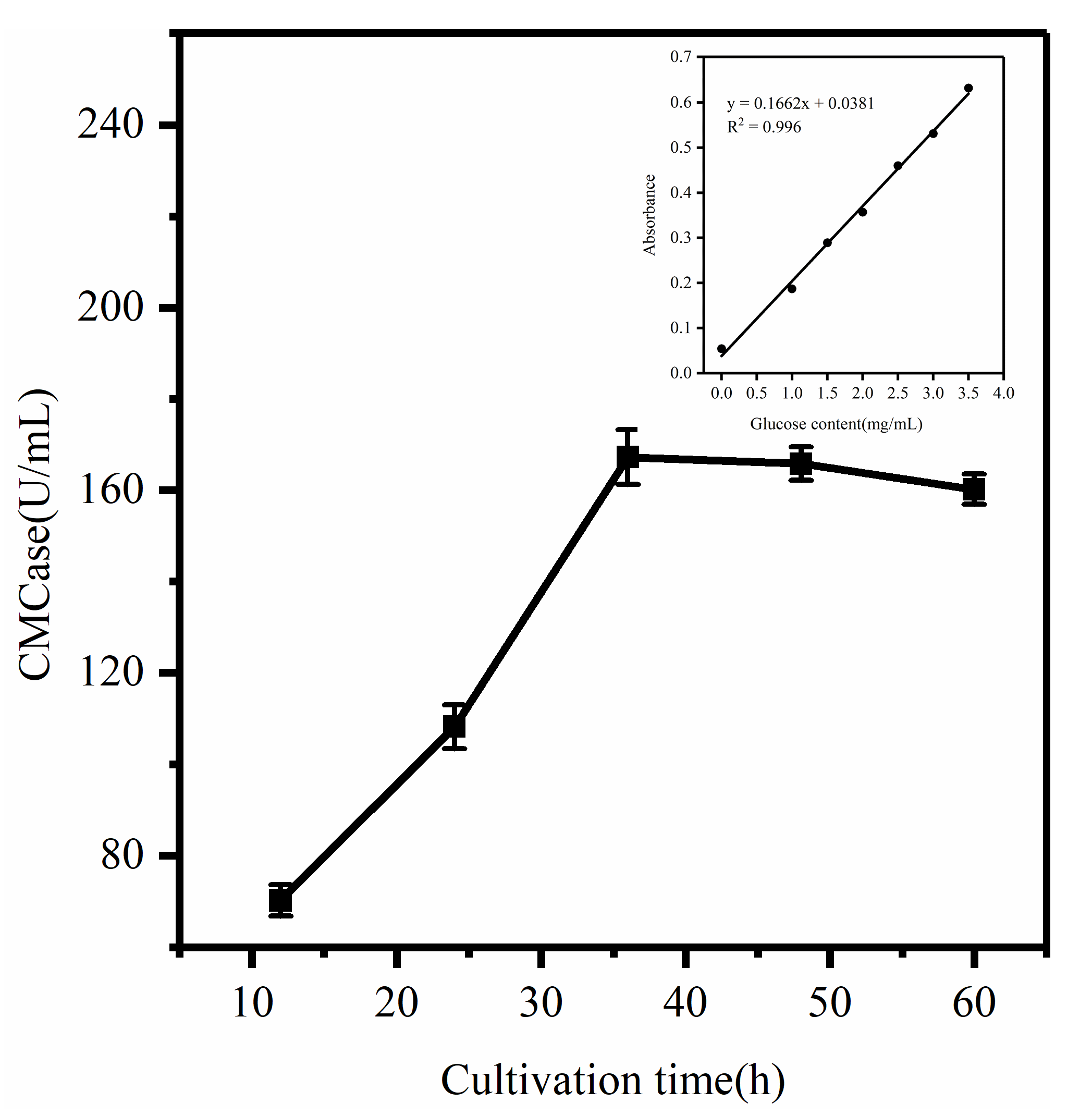
2.4. Fermentation Enzyme Production Curve
2.5. Bacterial Identification and Phylogenetic Analysis
2.6. Field Emission Scanning Electron Microscopy (FESEM)
2.7. Fourier-Transformed Infrared (FTIR)
2.8. X-ray Diffraction (XRD)
2.9. Color Analysis
2.10. Enzymatic Hydrolysis and Ethanol Production
3. Results and Discussion
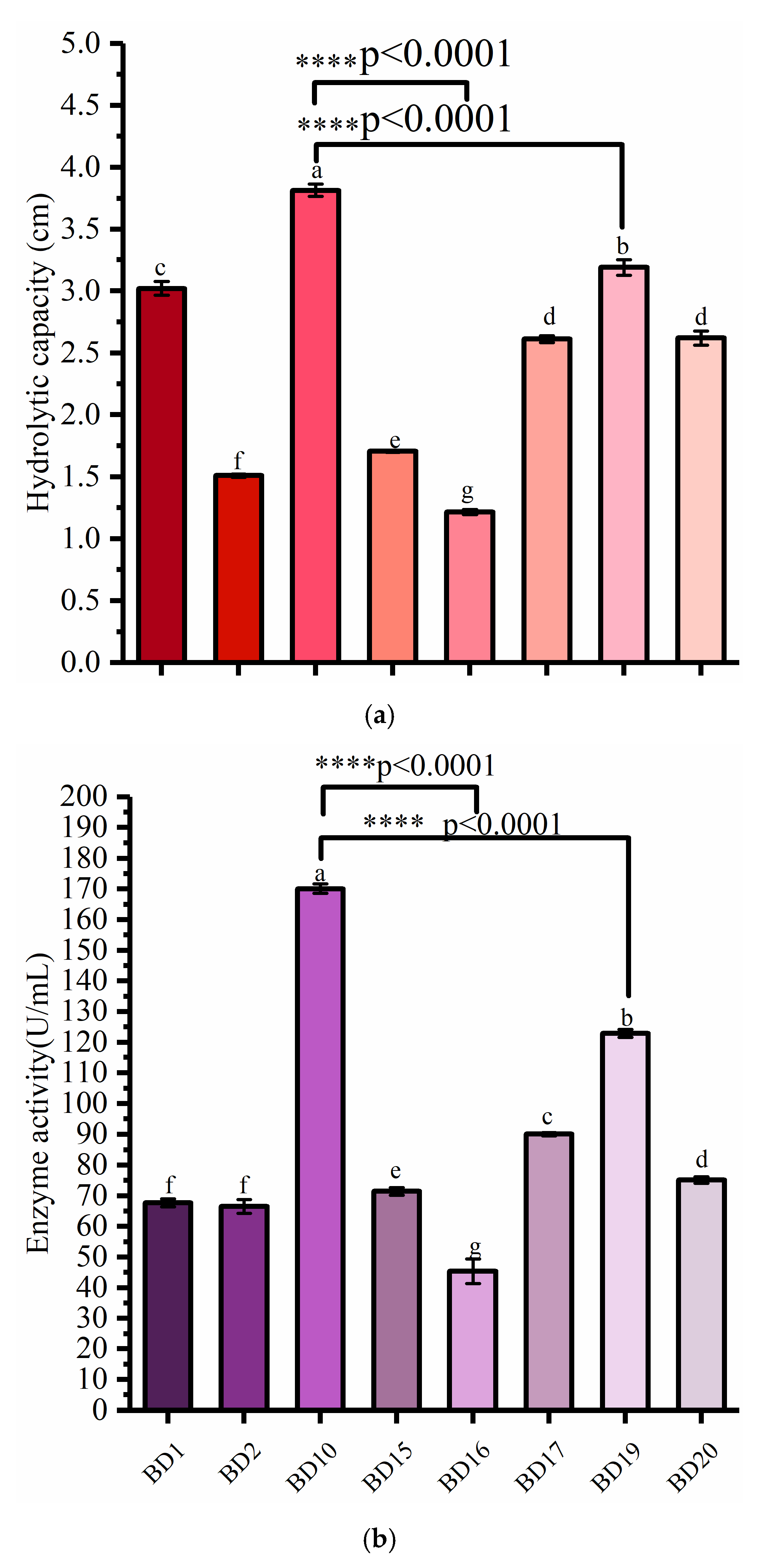
3.1. Screening of Cellulose-Degrading Bacteria
3.2. Determination of Enzyme Production Efficiency in the Degradation of COC
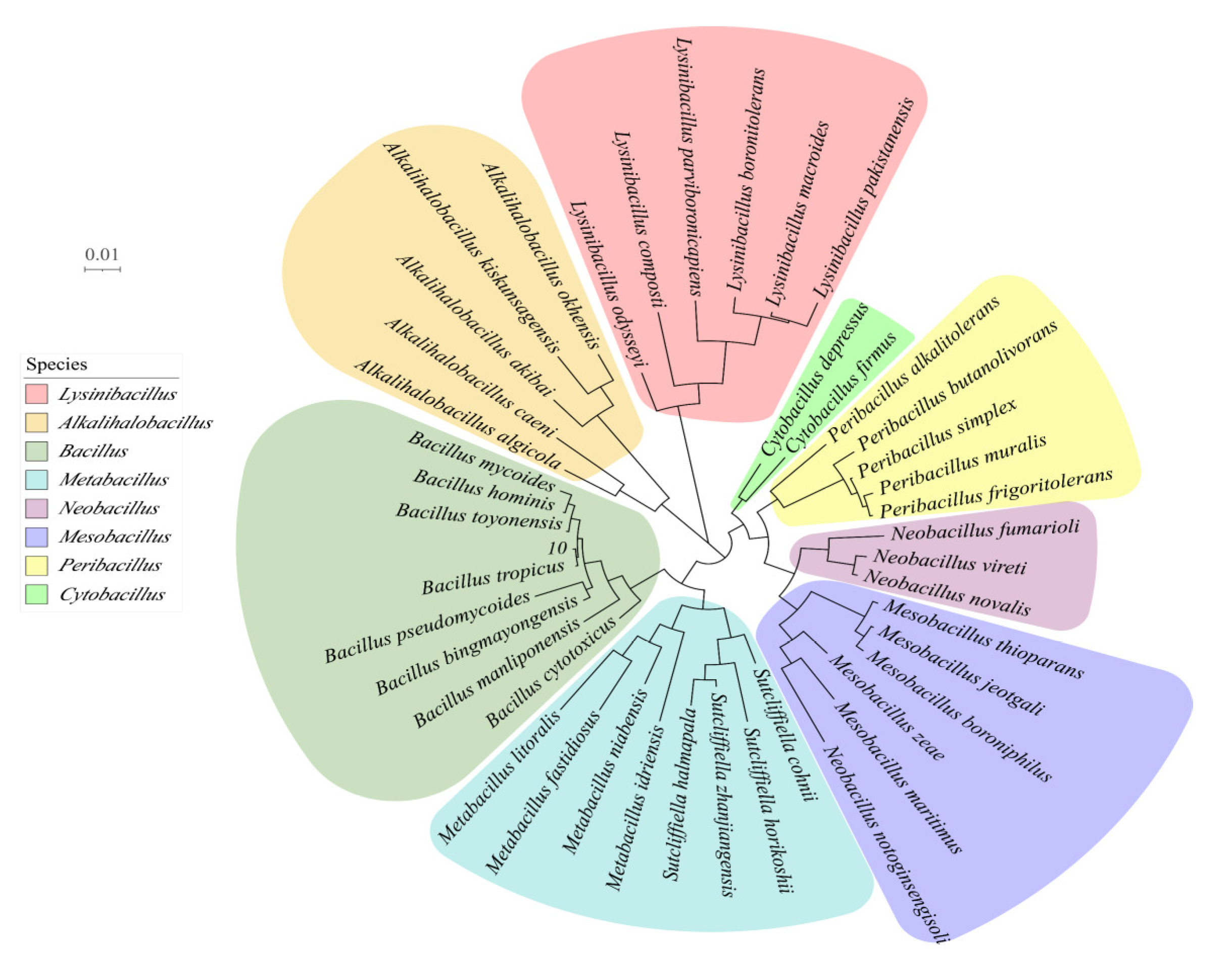
3.3. Molecular Biology of Strains
3.4. Characterization of COC before and after Degradation
3.4.1. FESEM Analysis
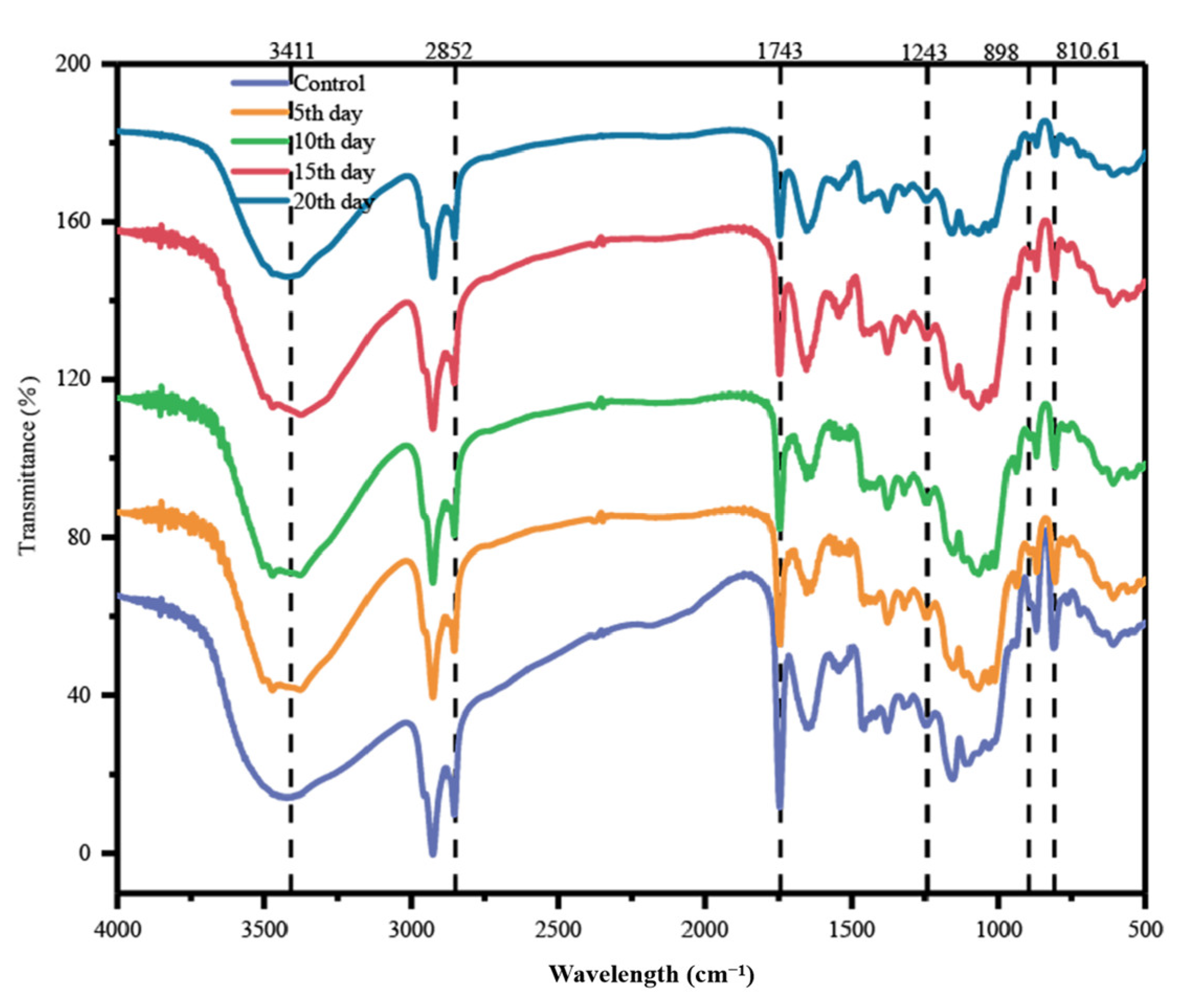
3.4.2. FTIR Analysis
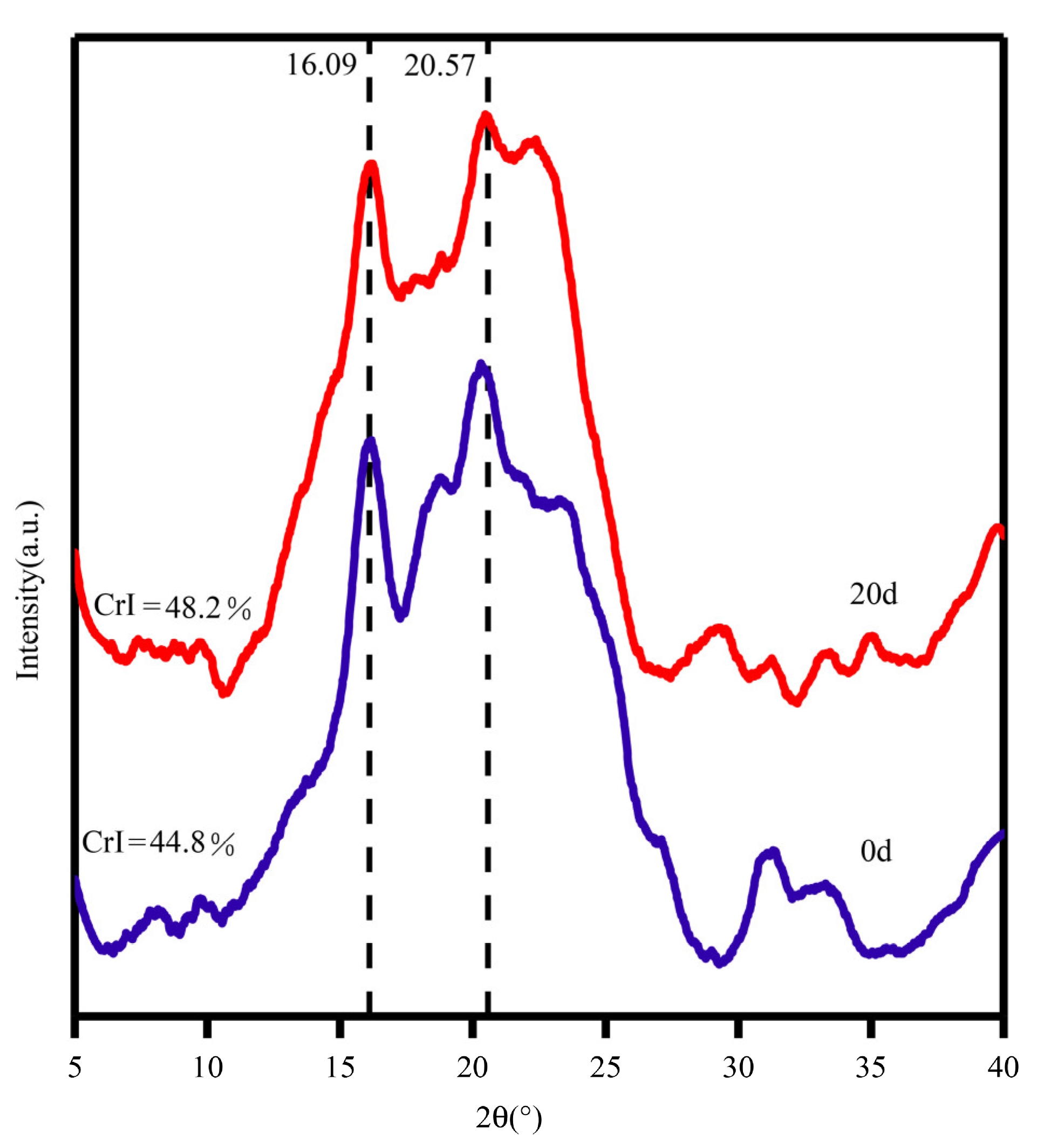
3.4.3. XRD Analysis
3.4.4. Color Analysis
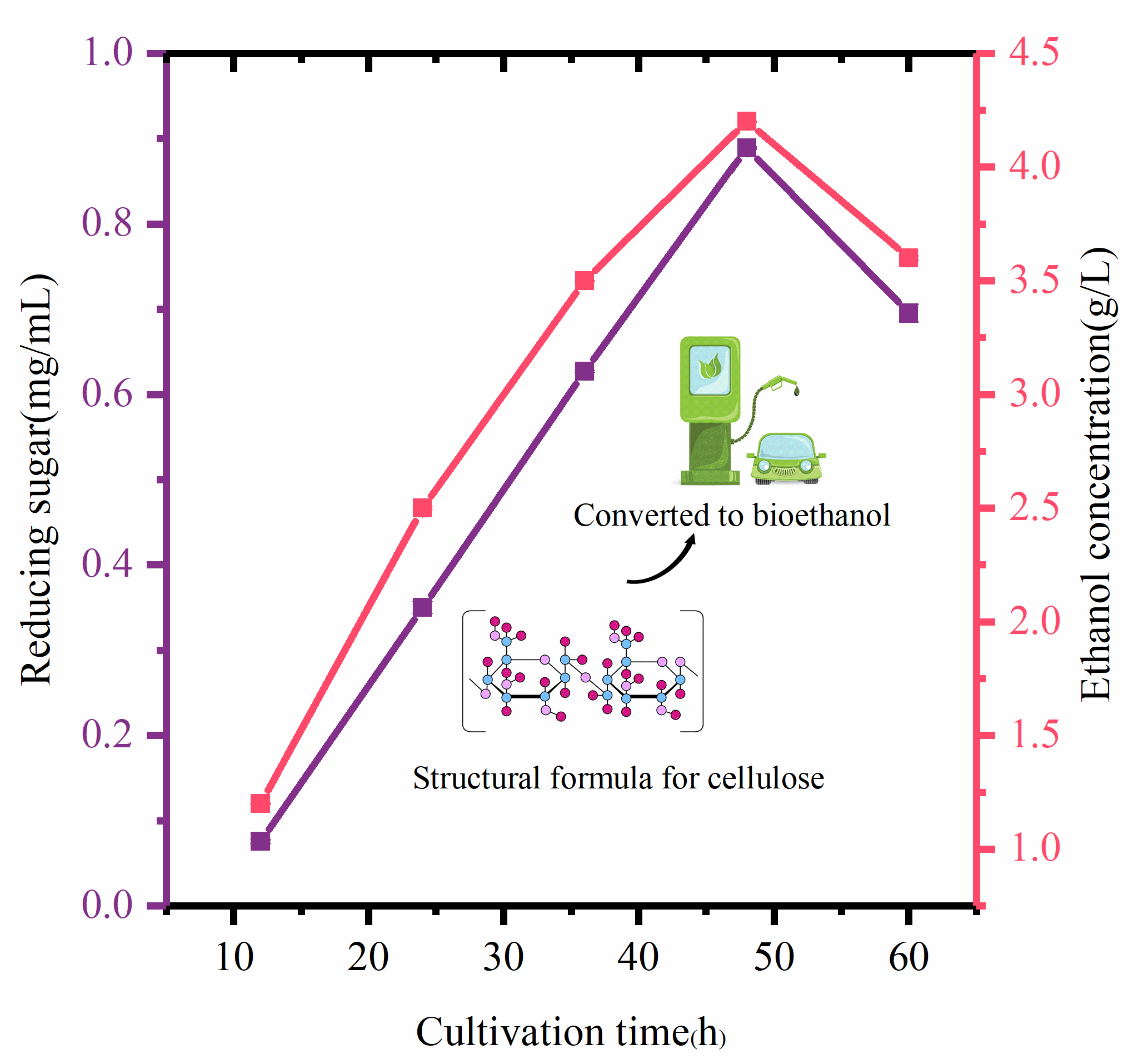
3.4.5. Bioethanol Production
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Prasad, R.K.; Chatterjee, S.; Mazumder, P.B.; Gupta, S.K.; Sharma, S.; Vairale, M.G.; Datta, S.; Dwivedi, S.K.; Gupta, D.K. Bioethanol production from waste lignocelluloses: A review on microbial degradation potential. Chemosphere 2019, 231, 588–606. [Google Scholar] [CrossRef]
- Samir Ali, S.; Al-Tohamy, R.; Sun, J.; Wu, J.; Huizi, L. Screening and construction of a novel microbial consortium SSA-6 enriched from the gut symbionts of wood-feeding termite, Coptotermes formosanus and its biomass-based biorefineries. Fuel 2019, 236, 1128–1145. [Google Scholar] [CrossRef]
- Santhi, V.S.; Gupta, A.; Saranya, S.; Jebakumar, S.R.D. A novel marine bacterium Isoptericola sp. JS-C42 with the ability to saccharifying the plant biomasses for the aid in cellulosic ethanol production. Biotechnol. Rep. 2014, 1, 8–14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Byadgi, S.A.; Kalburgi, P.B. Production of Bioethanol from Waste Newspaper. Procedia Environ. Sci. 2016, 35, 555–562. [Google Scholar] [CrossRef]
- Danso, B.; Ali, S.S.; Xie, R.; Sun, J. Valorisation of wheat straw and bioethanol production by a novel xylanase- and cellulase-producing Streptomyces strain isolated from the wood-feeding termite, Microcerotermes species. Fuel 2022, 310, 122333. [Google Scholar] [CrossRef]
- Khot, M.; Gupta, R.; Barve, K.; Zinjarde, S.; Govindwar, S.; Kumar, A.R. Fungal production of single cell oil using untreated copra cake and evaluation of its fuel properties for biodiesel. J. Microbiol. Biotechn. 2015, 25, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Auchová, K.; Fehér, C.; Ravn, J.L.; Bedő, S.; Biely, P.; Geijer, C. Cellulose- and xylan-degrading yeasts: Enzymes, applications and biotechnological potential. Biotechnol. Adv. 2022, 59, 107981. [Google Scholar]
- Chukwuma, O.B.; Rafatullah, M.; Tajarudin, H.A.; Ismail, N. A Review on Bacterial Contribution to Lignocellulose Breakdown into Useful Bio-Products. Int. J. Environ. Res. Public Health 2021, 18, 6001. [Google Scholar] [CrossRef]
- Liu, L.; Jiao, J.; Fang, B.; Lv, A.; Ming, Y.; Li, M.; Salam, N.; Li, W. Isolation of Clostridium from Yunnan-Tibet hot springs and description of Clostridium thermarum sp. Nov. With lignocellulosic ethanol production. Syst. Appl. Microbiol. 2020, 43, 126104. [Google Scholar] [CrossRef]
- Zhu, L.; Feng, L.; Luo, H.; Dong, R.; Wang, M.; Yao, G.; Chen, J. Characterization of polyvinyl alcohol-nanocellulose composite film and its release effect on tetracycline hydrochloride. Ind. Crops Prod. 2022, 188, 115723. [Google Scholar] [CrossRef]
- Chambal, B.; Bergenståhl, B.; Dejmek, P. Edible proteins from coconut milk press cake; One step alkaline extraction and characterization by electrophoresis and mass spectrometry. Food Res. Int. 2012, 47, 146–151. [Google Scholar] [CrossRef]
- Nguyen, Q.A.; Yang, J.; Bae, H. Bioethanol production from individual and mixed agricultural biomass residues. Ind. Crops Prod. 2017, 95, 718–725. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Physicochemical and functional properties of protein concentrate from by-product of coconut processing. Food Chem. 2018, 241, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Leesing, R.; Somdee, T.; Siwina, S.; Ngernyen, Y.; Fiala, K. Production of 2G and 3G biodiesel, yeast oil, and sulfonated carbon catalyst from waste coconut meal: An integrated cascade biorefinery approach. Renew. Energy 2022, 199, 1093–1104. [Google Scholar] [CrossRef]
- Pham, V.H.T.; Kim, J.; Shim, J.; Chang, S.; Chung, W. Coconut Mesocarp-Based Lignocellulosic Waste as a Substrate for Cellulase Production from High Promising Multienzyme-Producing Bacillus amyloliquefaciens FW2 without Pretreatments. Microorganisms 2022, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Darwesh, O.M.; El-Maraghy, S.H.; Abdel-Rahman, H.M.; Zaghloul, R.A. Improvement of paper wastes conversion to bioethanol using novel cellulose degrading fungal isolate. Fuel 2020, 262, 116518. [Google Scholar] [CrossRef]
- Sperandio, G.B.; Ferreira Filho, E.X. Fungal co-cultures in the lignocellulosic biorefinery context: A review. Int. Biodeter. Biodegr. 2019, 142, 109–123. [Google Scholar] [CrossRef]
- Barbosa, K.L.; Malta, V.R.D.S.; Machado, S.S.; Leal Junior, G.A.; Da Silva, A.P.V.; Almeida, R.M.R.G.; Da Luz, J.M.R. Bacterial cellulase from the intestinal tract of the sugarcane borer. Int. J. Biol. Macromol. 2020, 161, 441–448. [Google Scholar] [CrossRef]
- Fu, Z.H.; Liu, J.; Zhong, L.B.; Huang, H.; Zhu, P.; Wang, C.X.; Bai, X.P. Screening of cellulose-degrading yeast and evaluation of its potential for degradation of coconut oil cake. Front. Microbiol. 2022, 13, 996930. [Google Scholar] [CrossRef]
- Dar, M.A.; Syed, R.; Pawar, K.D.; Dhole, N.P.; Xie, R.; Pandit, R.S.; Sun, J. Evaluation and characterization of the cellulolytic bacterium, Bacillus pumilus SL8 isolated from the gut of oriental leafworm Spodoptera litura: An assessment of its potential value for lignocellulose bioconversion. Environ. Technol. Innov. 2022, 27, 102459. [Google Scholar] [CrossRef]
- Raja, M.G.; Tamilvendan, M.; Janani, R.; Kalaichelvan, P.T.; Klaus, H. Improvement of saccharification and delignification efficiency of trichoderma reesei Rut-C30 by genetic bioengineering. Microorganisms 2020, 8, 159. [Google Scholar]
- Neves, T.M.; Frantz, T.S.; Do Schenque, E.C.C.; Gelesky, M.A.; Mortola, V.B. An investigation into an alternative photocatalyst based on CeO2/Al2O3 in dye degradation. Environ. Technol. Innov. 2017, 8, 349–359. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Z.; Shi, S.; Lin, M. Lignocellulose degradation patterns, structural changes, and enzyme secretion by Inonotus obliquus on straw biomass under submerged fermentation. Bioresource Technol. 2017, 241, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Guo, J.; Nawaz, A.; Ul Haq, I.; Zhou, X.; Xu, Y. Comprehensive investigation of multiples factors in sulfuric acid pretreatment on the enzymatic hydrolysis of waste straw cellulose. Bioresource Technol. 2021, 340, 125740. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Li, Y.; Yu, H.; Wang, Y.; Piao, C. Insoluble dietary fibre from okara (soybean residue) modified by yeast Kluyveromyces marxianus. LWT 2020, 134, 110252. [Google Scholar] [CrossRef]
- Gsicka, A.; Borkowska, M.; Biaas, W.; Kaczmarek, P.; Celinska, E. Production of Raw Starch-Digesting Amylolytic Preparation in Yarrowia lipolytica and Its Application in Biotechnological Synthesis of Lactic Acid and Ethanol. Microorganisms 2020, 8, 717. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xie, Y.; Gao, X.; Shan, M.; Sun, C.; Niu, Y.D.; Shan, A. Screening of cellulose degradation bacteria from Min pigs and optimization of its cellulase production. Electron. J. Biotechn. 2020, 48, 29–35. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, A.; Sivakumar, N.; Verma, J.P. Deconstruction of lignocellulosic biomass for bioethanol production: Recent advances and future prospects. Fuel 2022, 327, 125109. [Google Scholar] [CrossRef]
- Dar, M.A.; Pawar, K.D.; Jadhav, J.P.; Pandit, R.S. Isolation of cellulolytic bacteria from the gastro-intestinal tract of Achatina fulica (Gastropoda: Pulmonata) and their evaluation for cellulose biodegradation. Int. Biodeter. Biodegr. 2015, 98, 73–80. [Google Scholar] [CrossRef]
- Jiaxing, X.; Zhen, G.; Bin, W.; Bingfang, H. Lactose-inducted production of a complete lignocellulolytic enzyme system by a novel bacterium Bacillus sp. BS-5 and its application for saccharification of alkali-pretreated corn cob. Cellulose 2017, 24, 2059–2070. [Google Scholar]
- Duhan, P.; Bansal, P.; Rani, S. Isolation, identification and characterization of endophytic bacteria from medicinal plant Tinospora cordifolia. S. Afr. J. Bot. 2020, 134, 43–49. [Google Scholar] [CrossRef]
- Zhu, G.; Xing, F.; Tao, J.; Chen, S.; Li, K.; Cao, L.; Yan, N.; Zhang, Y.; Rittmann, B.E. Synergy of strains that accelerate biodegradation of pyridine and quinoline. J. Environ. Manag. 2021, 285, 112119. [Google Scholar] [CrossRef] [PubMed]
- Kazeem, M.O.; Shah, U.K.M.; Baharuddin, A.S.; AbdulRahman, N.A. Prospecting agro-waste cocktail: Supplementation for cellulase production by a newly isolated thermophilic b-licheniformis 2D55. Appl. Biochem. Biotechnol. Part A Enzym. Eng. Biotechnol. 2017, 182, 1318–1340. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.A.; Dhole, N.P.; Xie, R.; Pawar, K.D.; Ullah, K.; Rahi, P.; Pandit, R.S.; Sun, J. Valorization Potential of a Novel Bacterial Strain, Bacillus altitudinis RSP75, towards Lignocellulose Bioconversion: An Assessment of Symbiotic Bacteria from the Stored Grain Pest, Tribolium castaneum. Microorganisms 2021, 9, 1952. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.J.; Choi, J.I.; Kim, S.J.; Park, C.S. Characterization of Cellulase from Bacillus sp. JB1 from Forest Soil. J. Chitin Chitosan 2017, 22, 235–240. [Google Scholar] [CrossRef]
- Sang, J.K.; Park, C.S. Characterization of Cellulase from Bacillus subtilis NSC Isolated from Soil. J. Chitin Chitosan 2018, 23, 228–233. [Google Scholar]
- Yan, S.; Sun, X.; Zhang, W.; Zhu, L. Isolation, identification and cellulase-producing condition optimization of Bacillus subtilis Q3 strain. AIP Conf. Proc. 2019, 2110, 020004. [Google Scholar]
- Sharma, M.; Bajaj, B.K. Cellulase Production from Bacillus subtilis MS 54 and its Potential for Saccharification of Biphasic-Acid-Pretreated Rice Straw. J. Biobased Mater. Bioenergy 2014, 8, 449–456. [Google Scholar] [CrossRef]
- Ariffin, H.; Abdullah, N.; Kalsom, M.S.U.; Shirai, Y.; Hassan, M.A. Production and characterization of cellulase by Bacillus pumilus EB3. Int. J. Eng. Technol. 2006, 3, 47–53. [Google Scholar]
- Makky, E.A. Avicelase Production by a Thermophilic Geobacillus stearothermophilus Isolated from Soil using Sugarcane Bagasse. Int. J. Bioeng. Life Sci. 2009, 3, 469–473. [Google Scholar]
- Nurika, I.; Shabrina, E.N.; Azizah, N.; Suhartini, S.; Bugg, T.D.H.; Barker, G.C. Application of ligninolytic bacteria to the enhancement of lignocellulose breakdown and methane production from oil palm empty fruit bunches (OPEFB). Bioresour. Technol. Rep. 2022, 17, 100951. [Google Scholar] [CrossRef]
- Sriwong, C.; Sukyai, P. Simulated elephant colon for cellulose extraction from sugarcane bagasse: An effective pretreatment to reduce chemical use. Sci. Total Environ. 2022, 835, 155281. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Y. Physicochemical and functional properties of coconut (Cocos nucifera L.) cake dietary fibres: Effects of cellulase hydrolysis, acid treatment and particle size distribution. Food Chem. 2018, 257, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Feng, J.; Zhong, T.; Zheng, Z.; Chen, T. Effects of volatile chemical components of wood species on mould growth susceptibility and termite attack resistance of wood plastic composites. Int. Biodeter. Biodegr. 2015, 100, 106–115. [Google Scholar] [CrossRef]
- Dar, M.A.; Shaikh, A.A.; Pawar, K.D.; Pandit, R.S. Exploring the gut of Helicoverpa armigera for cellulose degrading bacteria and evaluation of a potential strain for lignocellulosic biomass deconstruction. Process Biochem. 2018, 73, 142–153. [Google Scholar] [CrossRef]
- Nie, D.; Yao, L.; Xu, X.; Zhang, Z.; Li, Y. Promoting corn stover degradation via sequential processing of steam explosion and cellulase/lactic acid bacteria-assisted ensilage. Bioresour. Technol. 2021, 337, 125392. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Tian, H.; Li, Y.; Wang, X.; Shi, P. Effects of carboxymethylation, hydroxypropylation and dual enzyme hydrolysis combination with heating on physicochemical and functional properties and antioxidant activity of coconut cake dietary fibre. Food Chem. 2021, 336, 127688. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Sun, Y.; Xu, G.; Wang, W.; Piao, R.; Cui, Z.; Zhao, H. Degradation of lignocelluloses in straw using AC-1, a thermophilic composite microbial system. PeerJ 2021, 9, e12364. [Google Scholar] [CrossRef]
- Singh, J.K.; Chaurasia, B.; Dubey, A.; Faneite, N.A.M.; Gupta, A.; Kothari, R.; Upadhyaya, C.P.; Kumar, A.; Hashem, A.; Alqarawi, A.A.; et al. Biological Characterization and Instrumental Analytical Comparison of Two Biorefining Pretreatments for Water Hyacinth Eichhornia crassipes Biomass Hydrolysis. Sustainability 2020, 13, 245. [Google Scholar] [CrossRef]
- Ma, M.; Mu, T. Modification of deoiled cumin dietary fiber with laccase and cellulase under high hydrostatic pressure. Carbohyd. Polym. 2016, 136, 87–94. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, J.; Chen, F.; Wang, X.; Zhu, Q.; Ao, Q. Surface characterization of corn stalk superfine powder studied by FTIR and XRD. Colloids Surf. B Biointerfaces 2013, 104, 207–212. [Google Scholar] [CrossRef]
- Menshawy, M.N.; Abdel-Hamid, A.M.; Mohamed, S.K.; El-Katatny, M.H. Isolation and molecular identification of cellulose/hemicellulose degrading bacteria from agricultural compost and determination of their hydrolytic potential. S. Afr. J. Bot. 2022, 149, 617–621. [Google Scholar] [CrossRef]


| Bacterial Strain | Isolated From | CMCase (U/mL) | Substrate | Pre-Treatment | Reference |
|---|---|---|---|---|---|
| B. tropicus | decayed dahlias | 167.3 | COC | NA | This study |
| Bacillus sp. PM06 | Agro-waste Cocktail | 0.150 | Rice husk | NA | [33] |
| Bacillus altitudinis RSP75 | The gut system of red flour beetle | 47.1 | Wheat husk | NA | [34] |
| Bacillus sp. JB1 | Forest soil | 5 | Carboxymethylcellulose (CMC) | NA | [35] |
| Bacillus subtilis CNS | Forest soil | 0.26 | CMC | NA | [36] |
| Bacillus Subtilis Q3 | Silage corn | 18.667 | CMC | NA | [37] |
| Bacillus subtilis MS 54 | Paper and pulp industry waste | 23.49 | Maize bran | Sulfuric acid | [38] |
| Bacillus pumilus EB3 | oil palm empty fruit bunch | 0.079 | CMC | NA | [39] |
| Geobacillus stearothermophilus | soil samples | 1.94 | sugarcane bagasse | NA | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Z.; Zhong, L.; Tian, Y.; Bai, X.; Liu, J. Identification of Cellulose-Degrading Bacteria and Assessment of Their Potential Value for the Production of Bioethanol from Coconut Oil Cake Waste. Microorganisms 2024, 12, 240. https://doi.org/10.3390/microorganisms12020240
Fu Z, Zhong L, Tian Y, Bai X, Liu J. Identification of Cellulose-Degrading Bacteria and Assessment of Their Potential Value for the Production of Bioethanol from Coconut Oil Cake Waste. Microorganisms. 2024; 12(2):240. https://doi.org/10.3390/microorganisms12020240
Chicago/Turabian StyleFu, Zihuan, Longbin Zhong, Yan Tian, Xinpeng Bai, and Jing Liu. 2024. "Identification of Cellulose-Degrading Bacteria and Assessment of Their Potential Value for the Production of Bioethanol from Coconut Oil Cake Waste" Microorganisms 12, no. 2: 240. https://doi.org/10.3390/microorganisms12020240
APA StyleFu, Z., Zhong, L., Tian, Y., Bai, X., & Liu, J. (2024). Identification of Cellulose-Degrading Bacteria and Assessment of Their Potential Value for the Production of Bioethanol from Coconut Oil Cake Waste. Microorganisms, 12(2), 240. https://doi.org/10.3390/microorganisms12020240





