Campylobacter jejuni/coli Infection: Is It Still a Concern?
Abstract
1. Introduction
2. Microbiology
| Phylogenetic Group | Campylobacter Species | Reported Host |
|---|---|---|
| C. jejuni group | C. jejuni subsp. jejuni | poultry [14], cattle [15] |
| C. jejuni subsp. doylei | human [16] | |
| C. coli | human [17], poultry [17], cattle [18], dog [19], pig [20], sheep [18] | |
| C. hepaticus | poultry [21] | |
| C. helveticus | human, dog, cat [22] | |
| C. upsaliensis | human, dog, cat [23] | |
| C. cuniculorum | rabbit [24] | |
| C. avium | poultry [25] | |
| C. troglodytis | human, monkey [26,27] | |
| C. canadensis | birds [7] | |
| C. lari group | C. lari subsp. lari | shellfish [28] |
| C. lari subsp. concheus | ||
| C. insulaenigrae | human [29], pinnipeds [30] | |
| C. volucris | human [31], gull [32] | |
| C. peloridis | shellfish [28] | |
| C. subantarticus | birds [33] | |
| C. ornithocola | birds [34] | |
| C. concisus group | C. concisus | human [35], dog, cat [36] |
| C. showae | human, dog [37] | |
| C. rectus | human, dog [38] | |
| C. curvus | ||
| C. mucosalis | ||
| C. pinnipediorum | pinnipeds [39] | |
| C. ureolyticus group | C. ureolyticus | human [40], cattle [41] |
| C. hominis | human [42] | |
| C. geochelonis | tortoise [43] | |
| C. corcagienesis | monkey [44] | |
| C. gracilis | human [45], dog [38] | |
| C. sputorum | human [46], cattle [47], dog [38] | |
| C. fetus group | C. fetus subsp. fetus | human, cattle [48] |
| C. fetus subsp. venerealis | ||
| C. fetus subsp. testudinum | ||
| C. hyointestinalis | human, cattle, dog, hamster [49] | |
| C. iguaniorum | reptiles [50] | |
| C. lanienae | human [51], cattle, pig [52], chinchilla [53] |
3. Epidemiology
4. History
5. Transmission, Environmental Reservoirs, and Risk Factors
6. Virulence Factors and Pathogenesis
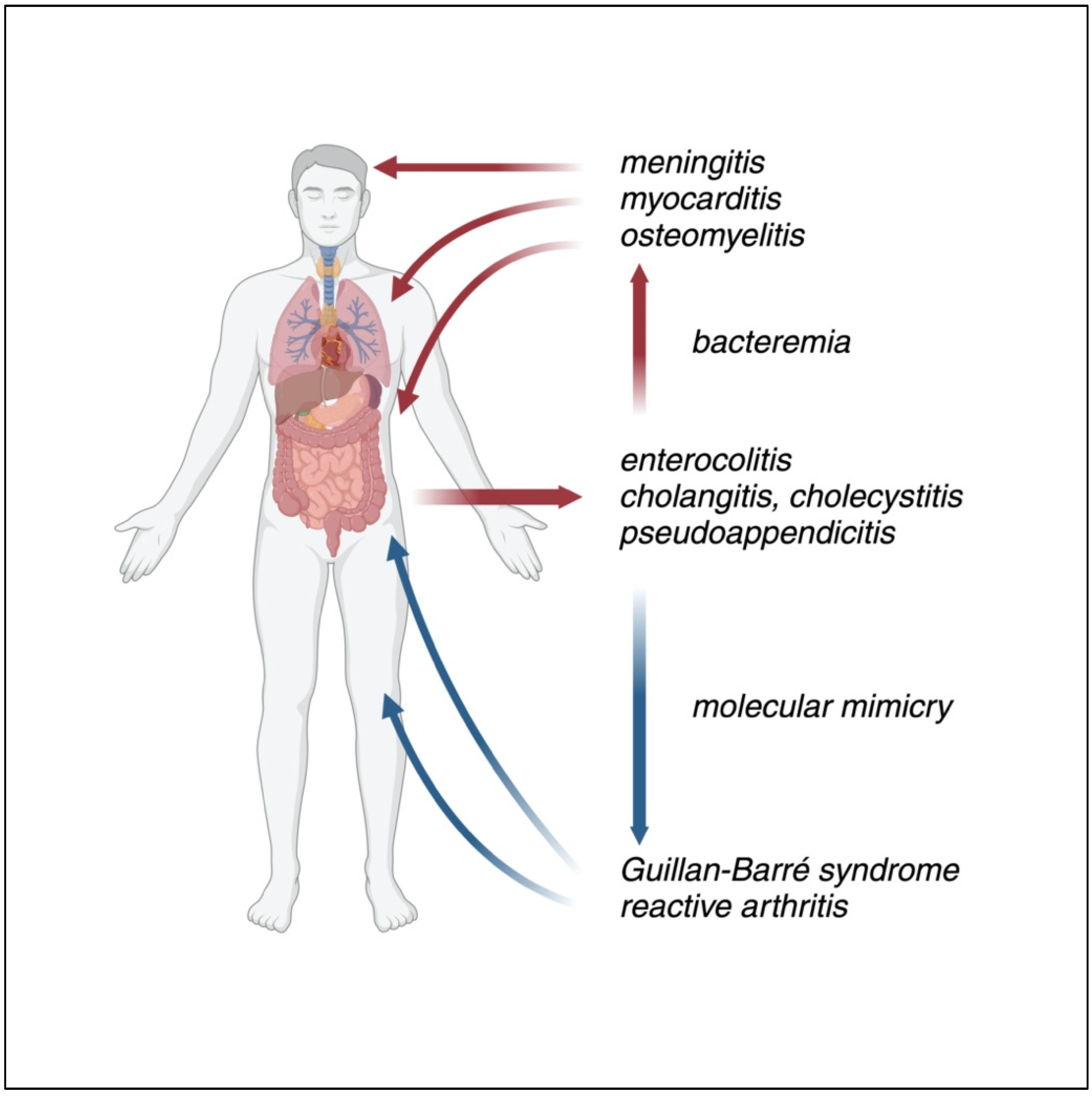
7. Clinical Manifestations
7.1. Gastrointestinal Manifestations
7.1.1. Gastroenteritis
7.1.2. Cholecystitis
7.1.3. Pseudoappendicits
7.2. Extraintestinal Manifestations
7.3. Late Complications
7.3.1. Guillan–Barré Syndrome, Miller Fisher Syndrome
7.3.2. Reactive Arthritis
8. Pediatric Population
9. Management: From Diagnosis to Treatment
10. Antimicrobial Resistance
10.1. Fluorquinolone Resistance
10.2. Macrolide Resistance
10.3. Beta-Lactam Resistance
10.4. Tetracycline Resistance
10.5. Aminoglycoside Resistance
10.6. Florfenicol Resistance
11. Conclusions and Future Prospects
Funding
Data Availability Statement
Conflicts of Interest
References
- Silva, J.; Leite, D.; Fernandes, M.; Mena, C.; Gibbs, P.A.; Teixeira, P. Campylobacter spp. as a Foodborne Pathogen: A Review. Front. Microbiol. 2011, 2, 200. [Google Scholar] [CrossRef]
- Corcionivoschi, N.; Gundogdu, O. Foodborne Pathogen Campylobacter. Microorganisms 2021, 9, 1241. [Google Scholar] [CrossRef] [PubMed]
- Latov, N. Campylobacter Jejuni Infection, Anti-Ganglioside Antibodies, and Neuropathy. Microorganisms 2022, 10, 2139. [Google Scholar] [CrossRef]
- Taha-Abdelaziz, K.; Singh, M.; Sharif, S.; Sharma, S.; Kulkarni, R.R.; Alizadeh, M.; Yitbarek, A.; Helmy, Y.A. Intervention Strategies to Control Campylobacter at Different Stages of the Food Chain. Microorganisms 2023, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- Best, E.L.; Fox, A.J.; Frost, J.A.; Bolton, F.J. Real-Time Single-Nucleotide Polymorphism Profiling Using Taqman Technology for Rapid Recognition of Campylobacter Jejuni Clonal Complexes. J. Med. Microbiol. 2005, 54, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Man, S.M. The Clinical Importance of Emerging Campylobacter Species. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 669–685. [Google Scholar] [CrossRef]
- Costa, D.; Iraola, G. Pathogenomics of Emerging Campylobacter Species. Clin. Microbiol. Rev. 2019, 32, 23. [Google Scholar] [CrossRef] [PubMed]
- Parte, A.C.; Sardà Carbasse, J.; Meier-Kolthoff, J.P.; Reimer, L.C.; Göker, M. List of Prokaryotic Names with Standing in Nomenclature (LPSN) Moves to the DSMZ. Int. J. Syst. Evol. Microbiol. 2020, 70, 5607–5612. [Google Scholar] [CrossRef] [PubMed]
- Igwaran, A.; Okoh, A.I. Human Campylobacteriosis: A Public Health Concern of Global Importance. Heliyon 2019, 5, e02814. [Google Scholar] [CrossRef]
- Carter, P.E.; McTavish, S.M.; Brooks, H.J.; Campbell, D.; Collins-Emerson, J.M.; Midwinter, A.C.; French, N.P. Novel Clonal Complexes with an Unknown Animal Reservoir Dominate Campylobacter Jejuni Isolates from River Water in New Zealand. Appl. Environ. Microbiol. 2009, 75, 6038–6046. [Google Scholar] [CrossRef] [PubMed]
- Dingle, K.E.; Colles, F.; Wareing, D.R.; Ure, R.; Fox, A.J. Multilocus Sequence Typing System for Campylobacter Jejuni. J. Clin. Microbiol. 2001, 39, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Dessouky, Y.E.; Elsayed, S.W.; Abdelsalam, N.A.; Saif, N.A.; Álvarez-Ordóñez, A.; Elhadidy, M. Genomic Insights into Zoonotic Transmission and Antimicrobial Resistance in Campylobacter Jejuni from Farm to Fork: A One Health Perspective. Gut Pathog. 2022, 14, 44. [Google Scholar] [CrossRef]
- Neimann, J.; Engberg, J.; Mølbak, K.; Wegener, H.C. A Case–Control Study of Risk Factors for Sporadic Campylobacter Infections in Denmark. Epidemiol. Infect. 2003, 130, 353–366. [Google Scholar] [CrossRef]
- Bronowski, C.; James, C.E.; Winstanley, C. Role of Environmental Survival in Transmission of Campylobacter Jejuni. FEMS Microbiol. Lett. 2014, 356, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Ellis-Iversen, J.; Pritchard, G.C.; Wooldridge, M.; Nielen, M. Risk Factors for Campylobacter Jejuni and Campylobacter Coli in Young Cattle on English and Welsh Farms. Prev. Vet. Med. 2009, 88, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Steele, T.W.; Owen, R.J. Campylobacter Jejuni Subsp. Doylei Subsp. nov., a Subspecies of Nitrate-Negative Campylobacters Isolated from Human Clinical Specimens. Int. J. Syst. Evol. Microbiol. 1988, 38, 316–318. [Google Scholar] [CrossRef]
- Cribb, D.M.; Biggs, P.J.; McLure, A.T.; Wallace, R.L.; French, N.P.; Glass, K.; Kirk, M.D. Genomic Diversity of Campylobacter Jejuni and Campylobacter Coli Isolates Recovered from Human and Poultry in Australia and New Zealand, 2017 to 2019. Microb. Genomics 2024, 10, 001319. [Google Scholar] [CrossRef] [PubMed]
- Ocejo, M.; Oporto, B.; Hurtado, A. Occurrence of Campylobacter Jejuni and Campylobacter Coli in Cattle and Sheep in Northern Spain and Changes in Antimicrobial Resistance in Two Studies 10-Years Apart. Pathogens 2019, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- Mughini Gras, L.; Smid, J.H.; Wagenaar, J.A.; Koene, M.G.J.; Havelaar, A.H.; Friesema, I.H.M.; French, N.P.; Flemming, C.; Galson, J.D.; Graziani, C.; et al. Increased Risk for Campylobacter Jejuni and C. Coli Infection of Pet Origin in Dog Owners and Evidence for Genetic Association between Strains Causing Infection in Humans and Their Pets. Epidemiol. Infect. 2013, 141, 2526–2535. [Google Scholar] [CrossRef] [PubMed]
- Denis, M.; Nagard, B.; Rose, V.; Bourgoin, K.; Cutimbo, M.; Kerouanton, A. No Clear Differences between Organic or Conventional Pig Farms in the Genetic Diversity or Virulence of Campylobacter Coli Isolates. Front. Microbiol. 2017, 8, 1016. [Google Scholar] [CrossRef]
- Petrovska, L.; Tang, Y.; van Rensburg, M.J.; Cawthraw, S.; Nunez, J.; Sheppard, S.K.; Ellis, R.J.; Whatmore, A.M.; Crawshaw, T.R.; Irvine, R.M. Genome Reduction for Niche Association in Campylobacter Hepaticus, A Cause of Spotty Liver Disease in Poultry. Front. Cell. Infect. Microbiol. 2017, 7, 354. [Google Scholar] [CrossRef]
- Bojanić, K.; Midwinter, A.C.; Acke, E.; Marshall, J.C.; Cornelius, A.J.; Biggs, P.J. Draft Genome Sequences of Eight Strains of Campylobacter Helveticus Isolated from Cats and a Dog in New Zealand. Microbiol. Resour. Announc. 2020, 9, e01244-19. [Google Scholar] [CrossRef]
- Bourke, B.; Chan, V.L.; Sherman, P. Campylobacter Upsaliensis: Waiting in the Wings. Clin. Microbiol. Rev. 1998, 11, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, R.G.; Debruyne, L.; Rossi, M.; Revez, J.; Vandamme, P. Campylobacter Cuniculorum sp. nov., from Rabbits. Int. J. Syst. Evol. Microbiol. 2009, 59, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Debruyne, L.; Zanoni, R.G.; Manfreda, G.; Revez, J.; Vandamme, P. Campylobacter Avium sp. nov., a Hippurate-Positive Species Isolated from Poultry. Int. J. Syst. Evol. Microbiol. 2009, 59, 2364–2369. [Google Scholar] [CrossRef] [PubMed]
- Kaur, T.; Singh, J.; Huffman, M.A.; Petrželková, K.J.; Taylor, N.S.; Xu, S.; Dewhirst, F.E.; Paster, B.J.; Debruyne, L.; Vandamme, P.; et al. Campylobacter Troglodytis sp. nov., Isolated from Feces of Human-Habituated Wild Chimpanzees (Pan Troglodytes Schweinfurthii) in Tanzania. Appl. Environ. Microbiol. 2011, 77, 2366–2373. [Google Scholar] [CrossRef]
- Platts-Mills, J.A.; Liu, J.; Gratz, J.; Mduma, E.; Amour, C.; Swai, N.; Taniuchi, M.; Begum, S.; Yori, P.P.; Tilley, D.H.; et al. Detection of Campylobacter in Stool and Determination of Significance by Culture, Enzyme Immunoassay, and PCR in Developing Countries. J. Clin. Microbiol. 2014, 52, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Debruyne, L.; On, S.L.W.; De Brandt, E.; Vandamme, P. Novel Campylobacter Lari-like Bacteria from Humans and Molluscs: Description of Campylobacter Peloridis sp. nov., Campylobacter Lari Subsp. Concheus Subsp. nov. and Campylobacter Lari Subsp. Lari Subsp. nov. Int. J. Syst. Evol. Microbiol. 2009, 59, 1126–1132. [Google Scholar] [CrossRef]
- Kyotani, M.; Kenzaka, T.; Akita, H.; Arakawa, S. Campylobacter Insulaenigrae Bacteremia with Meningitis: A Case Report. BMC Infect. Dis. 2021, 21, 633. [Google Scholar] [CrossRef]
- González, M.; Paz Villanueva, M.; Debruyne, L.; Vandamme, P.; Fernández, H. Campylobacter Insulaenigrae: First Isolation Report from South American Sea Lion (Otaria Flavescens, (Shaw, 1800). Braz. J. Microbiol. 2011, 42, 261–265. [Google Scholar] [CrossRef]
- Kweon, O.J.; Lim, Y.K.; Yoo, B.; Kim, H.R.; Kim, T.-H.; Lee, M.-K. First Case Report of Campylobacter Volucris Bacteremia in an Immunocompromised Patient. J. Clin. Microbiol. 2015, 53, 1976–1978. [Google Scholar] [CrossRef] [PubMed]
- Debruyne, L.; Broman, T.; Bergström, S.; Olsen, B.; On, S.L.W.; Vandamme, P. Campylobacter Volucris sp. nov., Isolated from Black-Headed Gulls (Larus Ridibundus). Int. J. Syst. Evol. Microbiol. 2010, 60, 1870–1875. [Google Scholar] [CrossRef]
- Debruyne, L.; Broman, T.; Bergström, S.; Olsen, B.; On, S.L.W.; Vandamme, P. Campylobacter Subantarcticus sp. nov., Isolated from Birds in the Sub-Antarctic Region. Int. J. Syst. Evol. Microbiol. 2010, 60, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, A.; Muñoz, I.; Iraola, G.; Díaz-Viraqué, F.; Collado, L. Campylobacter Ornithocola sp. nov., a Novel Member of the Campylobacter Lari Group Isolated from Wild Bird Faecal Samples. Int. J. Syst. Evol. Microbiol. 2017, 67, 1643–1649. [Google Scholar] [CrossRef]
- Akutko, K.; Matusiewicz, K. Campylobacter Concisus as the Etiologic Agent of Gastrointestinal Diseases. Adv. Clin. Exp. Med. 2017, 26, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Love, D.N.; Jones, R.F.; Bailey, M.; Calverley, A. Comparison of Strains of Gram-Negative, Anaerobic, Agar-Corroding Rods Isolated from Soft Tissue Infections in Cats and Dogs with Type Strains of Bacteroides Gracilis, Wolinella Recta, Wolinella Succinogenes, and Campylobacter Concisus. J. Clin. Microbiol. 1984, 20, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.; Gemmell, M.R.; Franzosa, E.A.; Berry, S.; Mukhopadhya, I.; Hansen, R.; Michaud, M.; Nielsen, H.; Miller, W.G.; Nielsen, H.; et al. Comparative Genomics and Genome Biology of Campylobacter Showae. Emerg. Microbes Infect. 2019, 8, 827–840. [Google Scholar] [CrossRef]
- Chaban, B.; Ngeleka, M.; Hill, J.E. Detection and Quantification of 14 Campylobacter Species in Pet Dogs Reveals an Increase in Species Richness in Feces of Diarrheic Animals. BMC Microbiol. 2010, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.J.; Miller, W.G.; Leger, J.S.; Chapman, M.H.; Timmerman, A.J.; Duim, B.; Foster, G.; Wagenaar, J.A. Campylobacter Pinnipediorum sp. nov., Isolated from Pinnipeds, Comprising Campylobacter Pinnipediorum Subsp. Pinnipediorum Subsp. nov. and Campylobacter Pinnipediorum Subsp. Caledonicus Subsp. Nov. Int. J. Syst. Evol. Microbiol. 2017, 67, 1961–1968. [Google Scholar] [CrossRef]
- Kaakoush, N.; Castaño-Rodríguez, N.; Mitchell, H.; Man, S.M. Global Epidemiology of Campylobacter Infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef] [PubMed]
- Koziel, M.; Lucey, B.; Bullman, S.; Corcoran, G.D.; Sleator, R.D. Molecular-Based Detection of the Gastrointestinal Pathogen Campylobacter Ureolyticus in Unpasteurized Milk Samples from Two Cattle Farms in Ireland. Gut Pathog. 2012, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Lawson, A.J.; On, S.L.; Logan, J.M.; Stanley, J. Campylobacter Hominis sp. nov., from the Human Gastrointestinal Tract. Int. J. Syst. Evol. Microbiol. 2001, 51, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, A.; Niero, G.; Calleros, L.; Pérez, R.; Naya, H.; Iraola, G. Campylobacter Geochelonis sp. nov. Isolated from the Western Hermann’s Tortoise (Testudo Hermanni Hermanni). Int. J. Syst. Evol. Microbiol. 2016, 66, 3468–3476. [Google Scholar] [CrossRef]
- Koziel, M.; Lucid, A.; Bullman, S.; Corcoran, G.D.; Lucey, B.; Sleator, R.D. Draft Genome Sequence of Campylobacter Corcagiensis Strain CIT045T, a Representative of a Novel Campylobacter Species Isolated from Lion-Tailed Macaques (Macaca Silenus). Genome Announc. 2014, 2, 2. [Google Scholar] [CrossRef]
- Siqueira, J.; Rôças, I. Campylobacter Gracilis and Campylobacter Rectus in Primary Endodontic Infections. Int. Endod. J. 2003, 36, 174–180. [Google Scholar] [CrossRef]
- Lindblom, G.-B.; Sjögren, E.; Hansson-Westerberg, J.; Kaijser, B. Campylobacter Upsaliensis, C. Sputorum Sputorum and C. Concisus as Common Causes of Diarrhoea in Swedish Children. Scand. J. Infect. Dis. 1995, 27, 187–188. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.G.; Williams, T.G.; Wood, D.F.; Chapman, M.H. Campylobacter Sputorum Subsp. Bovis Subsp. nov., Isolated from Cattle, and an Emended Description of Campylobacter Sputorum. Int. J. Syst. Evol. Microbiol. 2024, 74, 006571. [Google Scholar] [CrossRef]
- Pena-Fernández, N.; van der Graaf-van Bloois, L.; Duim, B.; Zomer, A.; Wagenaar, J.A.; Ocejo, M.; Lavín, J.L.; Collantes-Fernández, E.; Hurtado, A.; Aduriz, G. Campylobacter Fetus Plasmid Diversity: Comparative Analysis of Fully Sequenced Plasmids and Proposed Classification Scheme. Genome Biol. Evol. 2024, 16, evae203. [Google Scholar] [CrossRef]
- Costa, D.; Lévesque, S.; Kumar, N.; Fresia, P.; Ferrés, I.; Lawley, T.D.; Iraola, G. Pangenome Analysis Reveals Genetic Isolation in Campylobacter Hyointestinalis Subspecies Adapted to Different Mammalian Hosts. Sci. Rep. 2021, 11, 3431. [Google Scholar] [CrossRef]
- Gilbert, M.J.; Kik, M.; Miller, W.G.; Duim, B.; Wagenaar, J.A. Campylobacter Iguaniorum sp. nov., Isolated from Reptiles. Int. J. Syst. Evol. Microbiol. 2015, 65, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Logan, J.M.; Burnens, A.; Linton, D.; Lawson, A.J.; Stanley, J. Campylobacter Lanienae sp. nov., a New Species Isolated from Workers in an Abattoir. Int. J. Syst. Evol. Microbiol. 2000, 50, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.G.; Lopes, B.S.; Ramjee, M.; Jay-Russell, M.T.; Chapman, M.H.; Williams, T.G.; Wood, D.F.; Gruntar, I.; Papić, B.; Forbes, K.J. Campylobacter Devanensis sp. nov., Campylobacter Porcelli sp. nov., and Campylobacter Vicugnae sp. nov., Three Novel Campylobacter Lanienae-like Species Recovered from Swine, Small Ruminants, and Camelids. Int. J. Syst. Evol. Microbiol. 2024, 74, 006405. [Google Scholar] [CrossRef] [PubMed]
- Turowski, E.E.; Shen, Z.; Ducore, R.M.; Parry, N.M.A.; Kirega, A.; Dewhirst, F.E.; Fox, J.G. Isolation of a Ampylobacter Lanienae-like Bacterium from Laboratory Chinchillas (Hinchilla Laniger). Zoonoses Public Health 2014, 61, 571–580. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). The European Union One Health 2022 Zoonoses Report. EFSA J. 2023, 21, e8442. [Google Scholar] [CrossRef]
- NNDSS Annual Report Working Group. Australia’s Notifiable Disease Status, 2016: Annual Report of the National Notifiable Diseases Surveillance System. Commun. Dis. Intell. (2018) 2021, 45, 28. [Google Scholar]
- Kirk, M.D.; Pires, S.M.; Black, R.E.; Caipo, M.; Crump, J.A.; Devleesschauwer, B.; Döpfer, D.; Fazil, A.; Fischer-Walker, C.L.; Hald, T.; et al. World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med. 2015, 12, e1001921. [Google Scholar] [CrossRef]
- Liu, J.; Platts-Mills, J.A.; Juma, J.; Kabir, F.; Nkeze, J.; Okoi, C.; Operario, D.J.; Uddin, J.; Ahmed, S.; Alonso, P.L.; et al. Use of Quantitative Molecular Diagnostic Methods to Identify Causes of Diarrhoea in Children: A Reanalysis of the GEMS Case-Control Study. Lancet 2016, 388, 1291–1301. [Google Scholar] [CrossRef]
- Amour, C.; Gratz, J.; Mduma, E.; Svensen, E.; Rogawski, E.T.; McGrath, M.; Seidman, J.C.; McCormick, B.J.J.; Shrestha, S.; Samie, A.; et al. Epidemiology and Impact of Campylobacter Infection in Children in 8 Low-Resource Settings: Results From the MAL-ED Study. Clin. Infect. Dis. 2016, 63, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Patrick, M.E.; Henao, O.L.; Robinson, T.; Geissler, A.L.; Cronquist, A.; Hanna, S.; Hurd, S.; Medalla, F.; Pruckler, J.; Mahon, B.E. Features of Illnesses Caused by Five Species of Campylobacter, Foodborne Diseases Active Surveillance Network (FoodNet)—2010–2015. Epidemiol. Infect. 2018, 146, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, S.; Hanning, I.; Biswas, D.; Ricke, S.C. Evaluation of Whole-Genome Sequencing as a Genotyping Tool for Campylobacter Jejuni in Comparison with Pulsed-Field Gel Electrophoresis and FlaA Typing1 1Presented as Part of the Next-Generation Sequencing Tools: Applications for Poultry Production and Fo. Poult. Sci. 2013, 92, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Casey, E.; Fitzgerald, E.; Lucey, B. Towards Understanding Clinical Campylobacter Infection and Its Transmission: Time for a Different Approach? Br. J. Biomed. Sci. 2017, 74, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Samie, A.; Obi, C.L.; Barrett, L.J.; Powell, S.M.; Guerrant, R.L. Prevalence of Campylobacter Species, Helicobacter Pylori and Arcobacter Species in Stool Samples from the Venda Region, Limpopo, South Africa: Studies Using Molecular Diagnostic Methods. J. Infect. 2007, 54, 558–566. [Google Scholar] [CrossRef] [PubMed]
- McFadyean, J.; Stockman, S. Report of the Deparmental Committee Appointed by the Board of Agriculture and Fisheries to Enquire into Epizootic Abortion. In Abortion in Sheep; His Majesty’s Stationery Office: London, UK, 1913. [Google Scholar]
- Smith, T.; Taylor, M.S. Some Morphological and Biological Characters of the Spirilla (Vibrio fetus, n. sp.) Associated with Disease of the Fetal Membranes in Cattle. J. Exp. Med. 1919, 30, 299–311. [Google Scholar] [CrossRef]
- Jones, F.S.; Little, R.B. Vibrionic Enteritis in Calves. J. Exp. Med. 1931, 53, 845–851. [Google Scholar] [CrossRef]
- Moore, J.E.; Gilpin, D.; Crothers, E.; Canney, A.; Kaneko, A.; Matsuda, M. Occurrence of Campylobacter spp. and Cryptosporidium spp. in Seagulls (Larus spp.). Vector-Borne Zoonotic Dis. 2002, 2, 111–114. [Google Scholar] [CrossRef]
- King, E.O. Human Infections with Vibrio Fetus and a Closely Related Vibrio. J. Infect. Dis. 1957, 101, 119–128. [Google Scholar] [CrossRef]
- Vinzent, R. A Poorly Known Condition of Pregnancy; Placental Vibrio Fetal Infection. Press. Med 1949, 57, 1230–1232. [Google Scholar] [PubMed]
- Dekeyser, P.; Gossuin-Detrain, M.; Butzler, J.P.; Sternon, J. Acute Enteritis Due to Related Vibrio: First Positive Stool Cultures. J. Infect. Dis. 1972, 125, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Butzler, J.P.; Dekeyser, P.; Detrain, M.; Dehaen, F. Related Vibrio in Stools. J. Pediatr. 1973, 82, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Butzler, J.P.; Dekeyser, P.; Lafontaine, T. Susceptibility of Related Vibrios and Vibrio Fetus to Twelve Antibiotics. Antimicrob. Agents Chemother. 1974, 5, 86–89. [Google Scholar] [CrossRef]
- Friedman, C.R.; Hoekstra, R.M.; Samuel, M.; Marcus, R.; Bender, J.; Shiferaw, B.; Reddy, S.; Ahuja, S.D.; Helfrick, D.L.; Hardnett, F.; et al. Risk Factors for Sporadic Campylobacter Infection in the United States: A Case-Control Study in FoodNet Sites. Clin. Infect. Dis. 2004, 38, S285–S296. [Google Scholar] [CrossRef] [PubMed]
- Taniuchi, M.; Sobuz, S.U.; Begum, S.; Platts-Mills, J.A.; Liu, J.; Yang, Z.; Wang, X.-Q.; Petri, W.A.J.; Haque, R.; Houpt, E.R. Etiology of Diarrhea in Bangladeshi Infants in the First Year of Life Analyzed Using Molecular Methods. J. Infect. Dis. 2013, 208, 1794–1802. [Google Scholar] [CrossRef] [PubMed]
- Coker, A.; Isokpehi, R.; Thomas, B.; Amisu, K.; Obi, L. Human Campylobacteriosis in Developing Countries. Emerg. Infect. Dis. 2002, 8, 237–243. [Google Scholar] [CrossRef]
- Kapperud, G.; Espeland, G.; Wahl, E.; Walde, A.; Herikstad, H.; Gustavsen, S.; Tveit, I.; Natås, O.; Bevanger, L.; Digranes, A. Factors Associated with Increased and Decreased Risk of Campylobacter Infection: A Prospective Case-Control Study in Norway. Am. J. Epidemiol. 2003, 158, 234–242. [Google Scholar] [CrossRef]
- Kownhar, H.; Muthu Shankar, E.; Rajan, R.; Vengatesan, A.; Rao, U.A. Prevalence of Campylobacter Jejuni and Enteric Bacterial Pathogens among Hospitalized HIV Infected versus Non-HIV Infected Patients with Diarrhoea in Southern India. Scand. J. Infect. Dis. 2007, 39, 862–866. [Google Scholar] [CrossRef]
- Ellis-Iversen, J.; Ridley, A.; Morris, V.; Sowa, A.; Harris, J.; Atterbury, R.; Sparks, N.; Allen, V. Persistent Environmental Reservoirs on Farms as Risk Factors for Campylobacter in Commercial Poultry. Epidemiol. Infect. 2012, 140, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O.; Sodhi, N.; Chenu, J.W.; Cox, J.M.; Riordan, S.M.; Mitchell, H.M. The Interplay between Campylobacter and Helicobacter Species and Other Gastrointestinal Microbiota of Commercial Broiler Chickens. Gut Pathog. 2014, 6, 18. [Google Scholar] [CrossRef]
- Kittl, S.; Korczak, B.; Niederer, L.; Baumgartner, A.; Buettner, S.; Overesch, G.; Kuhnert, P. Comparison of Genotypes and Antibiotic Resistances of Campylobacter Jejuni and Campylobacter Coli on Chicken Retail Meat and at Slaughter. Appl. Environ. Microbiol. 2013, 79, 3875–3878. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, C.; Zychowska, M.A.; Stephan, R. Prevalence and Characteristics of Shiga Toxin-Producing Escherichia Coli, Salmonella spp. and Campylobacter spp. Isolated from Slaughtered Sheep in Switzerland. Int. J. Food Microbiol. 2004, 92, 45–53. [Google Scholar] [CrossRef]
- Cohen, E.J.; Nakane, D.; Kabata, Y.; Hendrixson, D.R.; Nishizaka, T.; Beeby, M. Campylobacter Jejuni Motility Integrates Specialized Cell Shape, Flagellar Filament, and Motor, to Coordinate Action of Its Opposed Flagella. PLoS Pathog. 2020, 16, e1008620. [Google Scholar] [CrossRef]
- Galán, J.E.; Wolf-Watz, H. Protein Delivery into Eukaryotic Cells by Type III Secretion Machines. Nature 2006, 444, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Svensson, S.L.; Pryjma, M.; Gaynor, E.C. Flagella-Mediated Adhesion and Extracellular DNA Release Contribute to Biofilm Formation and Stress Tolerance of Campylobacter Jejuni. PLoS ONE 2014, 9, e106063. [Google Scholar] [CrossRef] [PubMed]
- Buelow, D.R.; Christensen, J.E.; Neal-McKinney, J.M.; Konkel, M.E. Campylobacter Jejuni Survival within Human Epithelial Cells Is Enhanced by the Secreted Protein CiaI. Mol. Microbiol. 2011, 80, 1296–1312. [Google Scholar] [CrossRef]
- Neal-McKinney, J.; Konkel, M. The Campylobacter Jejuni CiaC Virulence Protein Is Secreted from the Flagellum and Delivered to the Cytosol of Host Cells. Front. Cell. Infect. Microbiol. 2012, 2, 31. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Amill, V.; Kim, B.J.; Seshu, J.; Konkel, M.E. Secretion of the Virulence-Associated Campylobacter Invasion Antigens from Campylobacter Jejuni Requires a Stimulatory Signal. J. Infect. Dis. 2001, 183, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, D.R.; Eucker, T.P.; Bell, J.A.; Dybas, L.; Mansfield, L.S.; Konkel, M.E. The Campylobacter JejuniCiaD Effector Protein Activates MAP Kinase Signaling Pathways and Is Required for the Development of Disease. Cell Commun. Signal. 2013, 11, 79. [Google Scholar] [CrossRef]
- Barrero-Tobon, A.M.; Hendrixson, D.R. Identification and Analysis of Flagellar Coexpressed Determinants (Feds) of Campylobacter Jejuni Involved in Colonization. Mol. Microbiol. 2012, 84, 352–369. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.O.; Galán, J.E. Campylobacter Jejuni Survives within Epithelial Cells by Avoiding Delivery to Lysosomes. PLOS Pathog. 2008, 4, e14. [Google Scholar] [CrossRef] [PubMed]
- Noto, J.M.; Peek, R.M. The Helicobacter Pylori Cag Pathogenicity Island BT—Helicobacter Species: Methods and Protocols. In; Houghton, J., Ed.; Humana Press: Totowa, NJ, USA, 2012; pp. 41–50. ISBN 978-1-62703-005-2. [Google Scholar]
- Grohmann, E.; Christie, P.J.; Waksman, G.; Backert, S. Type IV Secretion in Gram-Negative and Gram-Positive Bacteria. Mol. Microbiol. 2018, 107, 455–471. [Google Scholar] [CrossRef]
- Bacon, D.; Alm, R.; Burr, D.; Hu, L.; Kopecko, D.; Ewing, C.; Trust, T.; Guerry, P. Involvement of a Plasmid in Virulence of Campylobacter Jejuni 81-176. Infect. Immun. 2000, 68, 4384–4390. [Google Scholar] [CrossRef] [PubMed]
- Gabbert, A.D.; Mydosh, J.L.; Talukdar, P.K.; Gloss, L.M.; McDermott, J.E.; Cooper, K.K.; Clair, G.C.; Konkel, M.E. The Missing Pieces: The Role of Secretion Systems in Campylobacter Jejuni Virulence. Biomolecules 2023, 13, 135. [Google Scholar] [CrossRef] [PubMed]
- Lertpiriyapong, K.; Gamazon, E.R.; Feng, Y.; Park, D.S.; Pang, J.; Botka, G.; Graffam, M.E.; Ge, Z.; Fox, J.G. Campylobacter Jejuni Type VI Secretion System: Roles in Adaptation to Deoxycholic Acid, Host Cell Adherence, Invasion, and In Vivo Colonization. PLoS ONE 2012, 7, e42842. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.; Liaw, J.; Omole, Z.; Xia, D.; van Vliet, A.H.M.; Corcionivoschi, N.; Hachani, A.; Gundogdu, O. Bioinformatic Analysis of the Campylobacter Jejuni Type VI Secretion System and Effector Prediction. Front. Microbiol. 2021, 12, 694824. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.; Porte, L.; Weitzel, T.; Varela, C.; Muñoz-Rehbein, C.; Ugalde, J.A.; Grim, C.; González-Escalona, N.; Blondel, C.J.; Bravo, V. Whole-Genome Sequencing Reveals Changes in Genomic Diversity and Distinctive Repertoires of T3SS and T6SS Effector Candidates in Chilean Clinical Campylobacter Strains. Front. Cell. Infect. Microbiol. 2023, 13, 1208825. [Google Scholar] [CrossRef]
- Boehm, M.; Simson, D.; Escher, U.; Schmidt, A.-M.; Bereswill, S.; Tegtmeyer, N.; Backert, S.; Heimesaat, M.M. Function of Serine Protease HtrA in the Lifecycle of the Foodborne Pathogen Campylobacter Jejuni. Eur. J. Microbiol. Immunol. EuJMI 2018, 8, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Karlyshev, A.; Brendan, W. Detection and Initial Characterization of Novel Capsular Polysaccharide among Diverse Campylobacter JejuniStrains Using Alcian Blue Dye. J. Clin. Microbiol. 2001, 39, 279–284. [Google Scholar] [CrossRef]
- Monteiro, M.A.; Chen, Y.-H.; Ma, Z.; Ewing, C.P.; Mohamad Nor, N.; Omari, E.; Song, E.; Gabryelski, P.; Guerry, P.; Poly, F. Relationships of Capsular Polysaccharides Belonging to Campylobacter Jejuni HS1 Serotype Complex. PLoS ONE 2021, 16, e0247305. [Google Scholar] [CrossRef]
- Keo, T.; Collins, J.; Kunwar, P.; Blaser, M.J.; Iovine, N.M. Campylobacter Capsule and Lipooligosaccharide Confer Resistance to Serum and Cationic Antimicrobials. Virulence 2011, 2, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Zilbauer, M.; Dorrell, N.; Parjeet, B.; Harris, A.; Brendan, W.; Nigel, K.; Bajaj-Elliott, M. Intestinal Innate Immunity to Campylobacter Jejuni Results in Induction of Bactericidal Human Beta-Defensins 2 and 3. Infect. Immun. 2005, 73, 7281–7289. [Google Scholar] [CrossRef]
- Konkel, M.E.; Talukdar, P.K.; Negretti, N.M.; Klappenbach, C.M. Taking Control: Campylobacter Jejuni Binding to Fibronectin Sets the Stage for Cellular Adherence and Invasion. Front. Microbiol. 2020, 11, 564. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.M.; Lior, H. A New Heat-Labile Cytolethal Distending Toxin (CLDT) Produced by Campylobacter spp. Microb. Pathog. 1988, 4, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Lara-Tejero, M.; Galán, J.E. A Bacterial Toxin That Controls Cell Cycle Progression as a Deoxyribonuclease I-Like Protein. Science 2000, 290, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Guerra, L.; Cortes-Bratti, X.; Guidi, R.; Frisan, T. The Biology of the Cytolethal Distending Toxins. Toxins 2011, 3, 172–190. [Google Scholar] [CrossRef] [PubMed]
- Okuda, J.; Kurazono, H.; Takeda, Y. Distribution of the Cytolethal Distending Toxin A Gene (CdtA) among Species of Shigella and Vibrio, and Cloning and Sequencing of the Cdt Gene from Shigella Dysenteriae. Microb. Pathog. 1995, 18, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Cope, L.D.; Lumbley, S.; Latimer, J.L.; Klesney-Tait, J.; Stevens, M.K.; Johnson, L.S.; Purven, M.; Munson, R.S.; Lagergard, T.; Radolf, J.D.; et al. A Diffusible Cytotoxin of Haemophilus Ducreyi. Proc. Natl. Acad. Sci. USA 1997, 94, 4056–4061. [Google Scholar] [CrossRef]
- Hickey, T.; McVeigh, A.; Scott, D.; Michielutti, R.; Bixby, A.; Carroll, S.; Bourgeois, A.; Guerry, P. Campylobacter Jejuni Cytolethal Distending Toxin Mediates Release of Interleukin-8 from Intestinal Epithelial Cells. Infect. Immun. 2000, 68, 6535–6541. [Google Scholar] [CrossRef] [PubMed]
- Culotti, A.; Packman, A.I. Pseudomonas Aeruginosa Facilitates Campylobacter Jejuni Growth in Biofilms under Oxic Flow Conditions. FEMS Microbiol. Ecol. 2015, 91, fiv136. [Google Scholar] [CrossRef] [PubMed]
- Turonova, H.; Briandet, R.; Rodrigues, R.; Hernould, M.; Hayek, N.; Stintzi, A.; Pazlarova, J.; Tresse, O. Biofilm Spatial Organization by the Emerging Pathogen Campylobacter Jejuni: Comparison between NCTC 11168 and 81-176 Strains under Microaerobic and Oxygen-Enriched Conditions. Front. Microbiol. 2015, 6, 709. [Google Scholar] [CrossRef]
- Reuter, M.; Mallett, A.; Pearson, B.; van Vliet, A. Biofilm Formation by Campylobacter Jejuni Is Increased under Aerobic Conditions. Appl. Environ. Microbiol. 2010, 76, 2122–2128. [Google Scholar] [CrossRef]
- Same, R.G.; Tamma, P.D. Campylobacter Infections in Children. Pediatr. Rev. 2018, 39, 533–541. [Google Scholar] [CrossRef]
- Platts-Mills, J.A.; Kosek, M. Update on the Burden of Campylobacter in Developing Countries. Curr. Opin. Infect. Dis. 2014, 27, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Hara-Kudo, Y.; Takattori, K. Contamination Level and Ingestion Dose of Foodborne Pathogens Associated with Infections. Epidemiol. Infect. 2011, 139, 1505–1510. [Google Scholar] [CrossRef]
- Kapperud, G.; Lassen, J.; Ostroff, S.M.; Aasen, S. Clinical Features of Sporadic Campylobacter Infections in Norway. Scand. J. Infect. Dis. 1992, 24, 741–749. [Google Scholar] [CrossRef]
- Blaser, M.J. Epidemiologic and Clinical Features of Campylobacter Jejuni Infections. J. Infect. Dis. 1997, 176, S103–S105. [Google Scholar] [CrossRef] [PubMed]
- Udayakumar, D.; Sanaullah, M. Campylobacter Cholecystitis. Int. J. Med. Sci. 2009, 6, 374–375. [Google Scholar] [CrossRef] [PubMed]
- Acheson, D.; Allos, B.M. Campylobacter Jejuni Infections: Update on Emerging Issues and Trends. Clin. Infect. Dis. 2001, 32, 1201–1206. [Google Scholar] [CrossRef]
- Tauxe, R. V Salad and Pseudoappendicitis: Yersinia Pseudotuberculosis as a Foodborne Pathogen. J. Infect. Dis. 2004, 189, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Hartman, J.; Westerman, M.; Wagenaar, J.F.P. Two-Sided Femoral Campylobacter Jejuni Osteomyelitis in a Patient with Acquired Hypogammaglobulinemia: A Case Report. BMC Infect. Dis. 2020, 20, 199. [Google Scholar] [CrossRef] [PubMed]
- Kirk, K.F.; Boel, J.; Nielsen, H.L. Vertebral Osteomyelitis Caused by Campylobacter Jejuni in an Immunocompetent Patient. Gut Pathog. 2023, 15, 61. [Google Scholar] [CrossRef]
- Puljiz, I.; Topic, A. Campylobacter Jejuni Vertebral Osteomyelitis. Lancet Infect. Dis. 2017, 17, 1002. [Google Scholar] [CrossRef] [PubMed]
- Kusulja, M.; Santini, M.; Margetić, K.; Guzvinec, M.; Šoprek, S.; Butić, I.; Tambić Andrašević, A. Meningitis Caused by Campylobacter Jejuni: A Case Presentation and Literature Review. Acta Clin. Belg. 2021, 76, 318–323. [Google Scholar] [CrossRef]
- Tsoni, K.; Papadopoulou, E.; Michailidou, E.; Kavaliotis, I. Campylobacter Jejuni Meningitis in a Neonate: A Rare Case Report. J. Neonatal. Perinatal. Med. 2013, 6, 183–185. [Google Scholar] [CrossRef]
- Greenfield, G.M.; Mailey, J.; Lyons, K.; Trouton, T.G. Acute Myocarditis Secondary to Acute Campylobacter Jejuni Infection. Clin. Med. 2018, 18, 98–99. [Google Scholar] [CrossRef]
- Becker, S.; Ejlertsen, T.; Kristensen, B.; Nørgaard, M.; Nielsen, H. Is the Incidence of Perimyocarditis Increased Following Campylobacter Jejuni Infection? Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 927–929. [Google Scholar] [CrossRef] [PubMed]
- Shahrizaila, N.; Lehmann, H.C.; Kuwabara, S. Guillain-Barré Syndrome. Lancet 2021, 397, 1214–1228. [Google Scholar] [CrossRef] [PubMed]
- Islam, Z.; Nabila, F.H.; Asad, A.; Begum, R.; Jahan, I.; Hayat, S.; Endtz, H. Draft Genome Sequences of Three Strains of Campylobacter Jejuni Isolated from Patients with Guillain-Barré Syndrome in Bangladesh. Microbiol. Resour. Announc. 2021, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, K.M.; Tattersfield, A.E. Guillain-Barre Syndrome Associated with Campylobacter Infection. Br. Med. J. (Clin. Res. Ed) 1982, 285, 173–174. [Google Scholar] [CrossRef]
- Leonhard, S.E.; van der Eijk, A.A.; Andersen, H.; Antonini, G.; Arends, S.; Attarian, S.; Barroso, F.A.; Bateman, K.J.; Batstra, M.R.; Benedetti, L.; et al. An International Perspective on Preceding Infections in Guillain-Barré Syndrome. Neurology 2022, 99, e1299–e1313. [Google Scholar] [CrossRef] [PubMed]
- Poropatich, K.O.; Fischer-Walker, C.L.; Black, R.E. Quantifying the Association between Campylobacter Infection and Guillain-Barré Syndrome: A Systematic Review. J. Heal. Popul. Nutr. 2010, 28, 545–552. [Google Scholar] [CrossRef]
- Allos, B.M. Association between Campylobacter Infection and Guillain-Barré Syndrome. J. Infect. Dis. 1997, 176, S125–S128. [Google Scholar] [CrossRef] [PubMed]
- Meidaninikjeh, S.; Sabouni, N.; Taheri, M.; Borjkhani, M.; Bengar, S.; Majidi Zolbanin, N.; Khalili, A.; Jafari, R. SARS-CoV-2 and Guillain–Barré Syndrome: Lessons from Viral Infections. Viral Immunol. 2022, 35, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Jahan, I.; Hayat, S.; Khalid, M.M.; Ahammad, R.U.; Asad, A.; Islam, B.; Mohammad, Q.D.; Jacobs, B.C.; Islam, Z. Association of Mannose-Binding Lectin 2 Gene Polymorphisms with Guillain-Barré Syndrome. Sci. Rep. 2022, 12, 5791. [Google Scholar] [CrossRef]
- Heikema, A.P.; Strepis, N.; Horst-Kreft, D.; Huynh, S.; Zomer, A.; Kelly, D.J.; Cooper, K.K.; Parker, C.T. Biomolecule Sulphation and Novel Methylations Related to Guillain-Barré Syndrome-Associated Campylobacter Jejuni Serotype HS:19. Microb. Genomics 2021, 7, 000660. [Google Scholar] [CrossRef] [PubMed]
- Willison, H.J.; Yuki, N. Peripheral Neuropathies and Anti—glycolipid Antibodies. Brain 2002, 125, 2591–2625. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Koga, M.; Yokoyama, K.; Yuki, N. Epidemiology of Campylobacter Jejuni Isolated from Patients with Guillain-Barré and Fisher Syndromes in Japan. J. Clin. Microbiol. 2005, 43, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Koga, M.; Gilbert, M.; Li, J.; Koike, S.; Takahashi, M.; Furukawa, K.; Hirata, K.; Yuki, N. Antecedent Infections in Fisher Syndrome. Neurology 2005, 64, 1605–1611. [Google Scholar] [CrossRef]
- Generali, E.; Bose, T.; Selmi, C.; Voncken, J.W.; Damoiseaux, J.G.M.C. Nature versus Nurture in the Spectrum of Rheumatic Diseases: Classification of Spondyloarthritis as Autoimmune or Autoinflammatory. Autoimmun. Rev. 2018, 17, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Townes, J.M.; Deodhar, A.A.; Laine, E.S.; Smith, K.; Krug, H.E.; Barkhuizen, A.; Thompson, M.E.; Cieslak, P.R.; Sobel, J. Reactive Arthritis Following Culture-Confirmed Infections with Bacterial Enteric Pathogens in Minnesota and Oregon: A Population-Based Study. Ann. Rheum. Dis. 2008, 67, 1689–1696. [Google Scholar] [CrossRef]
- Pope, J.E.; Krizova, A.; Garg, A.X.; Thiessen-Philbrook, H.; Ouimet, J.M. Campylobacter Reactive Arthritis: A Systematic Review. Semin. Arthritis Rheum. 2007, 37, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Schielke, A.; Rosner, B.M.; Stark, K. Epidemiology of Campylobacteriosis in Germany—Insights from 10 Years of Surveillance. BMC Infect. Dis. 2014, 14, 30. [Google Scholar] [CrossRef]
- Smith, A.; Reacher, M.; Smerdon, W.; Adak, G.K.; Nichols, G.; Chalmers, R.M. Outbreaks of Waterborne Infectious Intestinal Disease in England and Wales, 1992–2003. Epidemiol. Infect. 2006, 134, 1141–1149. [Google Scholar] [CrossRef]
- Szczepanska, B.; Andrzejewska, M.; Spica, D.; Klawe, J.J. Prevalence and Antimicrobial Resistance of Campylobacter Jejuni and Campylobacter Coli Isolated from Children and Environmental Sources in Urban and Suburban Areas. BMC Microbiol. 2017, 17, 80. [Google Scholar] [CrossRef] [PubMed]
- Colles, F.M.; Dingle, K.; Cody, A.J.; Maiden, M.C.J. Comparison of Campylobacter Populations in Wild Geese with Those in Starlings and Free-Range Poultry on the Same Farm. Appl. Environ. Microbiol. 2008, 74, 3583–3590. [Google Scholar] [CrossRef] [PubMed]
- Colles, F.M.; McCarthy, N.D.; Layton, R.; Maiden, M.C.J. The Prevalence of Campylobacter amongst a Free-Range Broiler Breeder Flock Was Primarily Affected by Flock Age. PLoS ONE 2011, 6, e22825. [Google Scholar] [CrossRef]
- Kebede, A.; Aragie, S.; Shimelis, T. The Common Enteric Bacterial Pathogens and Their Antimicrobial Susceptibility Pattern among HIV-Infected Individuals Attending the Antiretroviral Therapy Clinic of Hawassa University Hospital, Southern Ethiopia. Antimicrob. Resist. Infect. Control 2017, 6, 128. [Google Scholar] [CrossRef]
- Fullerton, K.E.; Ingram, L.A.; Jones, T.F.; Anderson, B.J.; McCarthy, P.V.; Hurd, S.; Shiferaw, B.; Vugia, D.; Haubert, N.; Hayes, T.; et al. Sporadic Campylobacter Infection in Infants: A Population-Based Surveillance Case-Control Study. Pediatr. Infect. Dis. J. 2007, 26, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Pyra, M.; Conover, C.; Howland, J.; Soyemi, K. Determinants of Campylobacteriosis Notifications in New Zealand. Epidemiol. Infect. 2012, 140, 2087–2088. [Google Scholar] [CrossRef]
- Diriba, K.; Awulachew, E.; Anja, A. Prevalence and Associated Factor of Campylobacter Species among Less than 5-Year-Old Children in Ethiopia: A Systematic Review and Meta-Analysis. Eur. J. Med. Res. 2021, 26, 2. [Google Scholar] [CrossRef]
- Lengerh, A.; Moges, F.; Unakal, C.; Anagaw, B. Prevalence, Associated Risk Factors and Antimicrobial Susceptibility Pattern of Campylobacter Species among under Five Diarrheic Children at Gondar University Hospital, Northwest Ethiopia. BMC Pediatr. 2013, 13, 82. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; McKune, S.L.; Singh, N.; Yousuf Hassen, J.; Gebreyes, W.; Manary, M.J.; Bardosh, K.; Yang, Y.; Diaz, N.; Mohammed, A.; et al. Campylobacter Colonization, Environmental Enteric Dysfunction, Stunting, and Associated Risk Factors Among Young Children in Rural Ethiopia: A Cross-Sectional Study From the Campylobacter Genomics and Environmental Enteric Dysfunction (CAGED) Project. Front. Public Heal. 2021, 8, 615793. [Google Scholar] [CrossRef]
- Rogawski, E.T.; Liu, J.; Platts-Mills, J.A.; Kabir, F.; Lertsethtakarn, P.; Siguas, M.; Khan, S.S.; Praharaj, I.; Murei, A.; Nshama, R.; et al. Use of Quantitative Molecular Diagnostic Methods to Investigate the Effect of Enteropathogen Infections on Linear Growth in Children in Low-Resource Settings: Longitudinal Analysis of Results from the MAL-ED Cohort Study. Lancet Glob. Heal. 2018, 6, e1319–e1328. [Google Scholar] [CrossRef] [PubMed]
- Karmali, M.; Fleming, P. Campylobacter Enteritis. Can. Med. Assoc. J. 1979, 121, 279. [Google Scholar]
- DeWitt, T.G.; Humphrey, K.F.; Doern, G. V White Blood Cell Counts in Patients with Campylobacter-Induced Diarrhea and in Controls. J. Infect. Dis. 1985, 152, 427–428. [Google Scholar] [CrossRef]
- Schattner, A. Campylobacter Jejuni and Cytopenias. Am. J. Med. 2013, 126, 1020–1021. [Google Scholar] [CrossRef]
- Tabata, H.; Horinishi, Y.; Sano, C. A Case of Pseudoappendicitis Caused by Campylobacter Enteritis Diagnosed by Gram Staining and Direct Microscopic Investigation of Stool Specimen. Cureus 2023, 15, e33980. [Google Scholar] [CrossRef] [PubMed]
- Bonilauri, P.; Bardasi, L.; Leonelli, R.; Ramini, M.; Luppi, A.; Giacometti, F.; Merialdi, G. Detection of Food Hazards in Foods: Comparison of Real Time Polymerase Chain Reaction and Cultural Methods. Ital J Food Saf. 2016, 5, 5641. [Google Scholar] [CrossRef]
- Ternhag, A.; Asikainen, T.; Giesecke, J.; Ekdahl, K. A Meta-Analysis on the Effects of Antibiotic Treatment on Duration of Symptoms Caused by Infection with Campylobacter Species. Clin. Infect. Dis. 2007, 44, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Travelers’ Diarrhea. In CDC Yellow-Book 2024; Oxford University Press: New York, NY, USA, 2024. [Google Scholar]
- World Gastroenterology Organisation Acute Diarrhea in Adults and Children: A Global Perspective. Available online: https://www.worldgastroenterology.org/guidelines/acute-diarrhea/acute-diarrhea-english (accessed on 3 December 2024).
- Shane, A.; Mody, R.; Crump, J.; Tar, P.; Steiner, T.; Kotlof, K.; Langley, J.; Wanke, C.; Alcantara Warren, C.; Cheng, A.; et al. IDSA 2017—Clinical Practice Guidelines for the Diagnosis and Management of Infectious Diarrhea. Clin. Infect. Dis. 2017, 65, e45–e80. [Google Scholar] [CrossRef]
- Alfredson, D.A.; Korolik, V. Identification of Putative Zinc Hydrolase Genes of the Metallo-β-Lactamase Superfamily from Campylobacter Jejuni. FEMS Immunol. Med. Microbiol. 2007, 49, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention(CDC). Antibiotic Resistance Threats in the United States, 2019; Department of Health and Human Services: Atlanta, GA, USA, 2019. [Google Scholar]
- Tang, Y.; Fang, L.; Xu, C.; Zhang, Q. Antibiotic Resistance Trends and Mechanisms in the Foodborne Pathogen, Campylobacter. Anim. Heal. Res. Rev. 2017, 18, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Su, Y.; Ma, L.; Ma, L.; Li, P.; Du, X.; Gölz, G.; Wang, S.; Lu, X. Prevalence and Characterization of Campylobacter Jejuni Isolated from Retail Chicken in Tianjin, China. J. Food Prot. 2017, 80, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.P.; Pace, A.; Varriale, L.; Borrelli, L.; Gargiulo, A.; Pompameo, M.; Fioretti, A.; Dipineto, L. Prevalence and Antimicrobial Resistance of Enteropathogenic Bacteria in Yellow-Legged Gulls (Larus Michahellis) in Southern Italy. Animals 2021, 11, 275. [Google Scholar] [CrossRef]
- Di Donato, G.; Marotta, F.; Nuvoloni, R.; Zilli, K.; Neri, D.; Di Sabatino, D.; Calistri, P.; Di Giannatale, E. Prevalence, Population Diversity and Antimicrobial Resistance of Campylobacter Coli Isolated in Italian Swine at Slaughterhouse. Microorganisms 2020, 8, 222. [Google Scholar] [CrossRef] [PubMed]
- Santos-Ferreira, N.; Ferreira, V.; Teixeira, P. Occurrence and Multidrug Resistance of Campylobacter in Chicken Meat from Different Production Systems. Foods 2022, 11, 1827. [Google Scholar] [CrossRef]
- Dias, T.S.; Nascimento, R.J.; Machado, L.S.; Abreu, D.L.C.; do Nascimento, E.R.; Pereira, V.L.A.; de Aquino, M.H.C. Comparison of Antimicrobial Resistance in Thermophilic Campylobacter Strains Isolated from Conventional Production and Backyard Poultry Flocks. Br. Poult. Sci. 2021, 62, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.; Oh, Y.; Kim, M.; Jung, J.; Lee, Y. Antimicrobial Resistance Patterns and Corresponding Multilocus Sequence Types of the Campylobacter Jejuni Isolates from Human Diarrheal Samples. Microb. Drug Resist. 2012, 19, 110–116. [Google Scholar] [CrossRef]
- Thomrongsuwannakij, T.; Blackall, P.J.; Chansiripornchai, N. A Study on Campylobacter Jejuni and Campylobacter Coli through Commercial Broiler Production Chains in Thailand: Antimicrobial Resistance, the Characterization of DNA Gyrase Subunit A Mutation, and Genetic Diversity by Flagellin A Gene Restriction Fragmen. Avian Dis. 2017, 61, 186–197. [Google Scholar] [CrossRef]
- Wallace, R.L.; Bulach, D.; McLure, A.; Varrone, L.; Jennison, A.V.; Valcanis, M.; Smith, J.J.; Polkinghorne, B.G.; Glass, K.; Kirk, M.D. Antimicrobial Resistance of Campylobacter spp. Causing Human Infection in Australia: An International Comparison. Microb. Drug Resist. 2020, 27, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Guerry, P.; Pope, P.M.; Burr, D.H.; Leifer, J.; Joseph, S.W.; Bourgeois, A.L. Development and Characterization of RecA Mutants of Campylobacter Jejuni for Inclusion in Attenuated Vaccines. Infect. Immun. 1994, 62, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.E.; Timms, A.R.; Connerton, P.L.; Loc Carrillo, C.; Adzfa Radzum, K.; Connerton, I.F. Genome Dynamics of Campylobacter Jejuni in Response to Bacteriophage Predation. PLoS Pathog. 2007, 3, e119. [Google Scholar] [CrossRef] [PubMed]
- Steffan, S.M.; Shakeri, G.; Kehrenberg, C.; Peh, E.; Rohde, M.; Plötz, M.; Kittler, S. Campylobacter Bacteriophage Cocktail Design Based on an Advanced Selection Scheme. Antibiotics 2022, 11, 228. [Google Scholar] [CrossRef]
- Peh, E.; Szott, V.; Reichelt, B.; Friese, A.; Rösler, U.; Plötz, M.; Kittler, S. Bacteriophage Cocktail Application for Campylobacter Mitigation—From in Vitro to in Vivo. BMC Microbiol. 2023, 23, 209. [Google Scholar] [CrossRef] [PubMed]
- Payot, S.; Bolla, J.-M.; Corcoran, D.; Fanning, S.; Mégraud, F.; Zhang, Q. Mechanisms of Fluoroquinolone and Macrolide Resistance in Campylobacter spp. Microbes Infect. 2006, 8, 1967–1971. [Google Scholar] [CrossRef]
- Piddock, L.J.V.; Ricci, V.; Pumbwe, L.; Everett, M.J.; Griggs, D.J. Fluoroquinolone Resistance in Campylobacter Species from Man and Animals: Detection of Mutations in Topoisomerase Genes. J. Antimicrob. Chemother. 2003, 51, 19–26. [Google Scholar] [CrossRef]
- Bachoual, R.; Ouabdesselam, S.; Mory, F.; Lascols, C.; Soussy, C.-J.; Tankovic, J. Single or Double Mutational Alterations of GyrA Associated with Fluoroquinolone Resistance in Campylobacter jejuni and Campylobacter coli. Microb. Drug Resist. 2001, 7, 257–261. [Google Scholar] [CrossRef]
- Smith, J.; Fratamico, P.M. Fluoroquinolone Resistance in Campylobacter. J. Food Prot. 2010, 73, 1141–1152. [Google Scholar] [CrossRef]
- Beilei, G.; McDermott, P.F.; White, D.G.; Jianghong, M. Role of Efflux Pumps and Topoisomerase Mutations in Fluoroquinolone Resistance in Campylobacter jejuni and Campylobacter coli. Antimicrob. Agents Chemother. 2005, 49, 3347–3354. [Google Scholar] [CrossRef]
- Naidan, L.; Orhan, S.; Jun, L.; Michel, L.O.; Qijing, Z. In Vivo Selection of Campylobacter Isolates with High Levels of Fluoroquinolone Resistance Associated with GyrA Mutations and the Function of the CmeABC Efflux Pump. Antimicrob. Agents Chemother. 2003, 47, 390–394. [Google Scholar] [CrossRef]
- Cagliero, C.; Mouline, C.; Payot, S.; Cloeckaert, A. Involvement of the CmeABC Efflux Pump in the Macrolide Resistance of Campylobacter coli. J. Antimicrob. Chemother. 2005, 56, 948–950. [Google Scholar] [CrossRef]
- Pumbwe, L.; Randall, L.P.; Woodward, M.J.; Piddock, L.J.V. Evidence for Multiple-Antibiotic Resistance in Campylobacter Jejuni Not Mediated by CmeB or CmeF. Antimicrob. Agents Chemother. 2005, 49, 1289–1293. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Shen, Z.; Wang, Y.; Deng, F.; Liu, D. Emergence of a Potent Multidrug Efflux Pump Variant That Enhances Campylobacter Resistance to Multiple Antibiotics. MBio 2016, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Sahin, O.; Lin, J.; Zhang, Q. Role of the CmeABC Efflux Pump in the Emergence of Fluoroquinolone-Resistant Campylobacter under Selection Pressure. J. Antimicrob. Chemother. 2006, 58, 1154–1159. [Google Scholar] [CrossRef]
- Hoang, K.V.; Stern, N.J.; Lin, J. Development and Stability of Bacteriocin Resistance in Campylobacter spp. J. Appl. Microbiol. 2011, 111, 1544–1550. [Google Scholar] [CrossRef]
- Sahin, O.; Shen, Z.; Zhang, Q. Methods to Study Antimicrobial Resistance in Campylobacter Jejuni; Butcher, J., Stintzi, A., Eds.; Springer: New York, NY, USA, 2017; pp. 29–42. ISBN 978-1-4939-6536-6. [Google Scholar]
- Jeon, B.; Wang, Y.; Hao, H.; Barton, Y.-W.; Zhang, Q. Contribution of CmeG to Antibiotic and Oxidative Stress Resistance in Campylobacter jejuni. J. Antimicrob. Chemother. 2011, 66, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, M.; Yang, W.; Fang, Y.; Wang, G.; Hou, F. A Seventeen-Year Observation of the Antimicrobial Susceptibility of Clinical Campylobacter Jejuni and the Molecular Mechanisms of Erythromycin-Resistant Isolates in Beijing, China. Int. J. Infect. Dis. 2016, 42, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, Y.; Deng, F.; Liu, D.; Yao, H.; Zhang, Q.; Shen, J.; Liu, Z.; Gao, Y.; Wu, C.; et al. Species Shift and Multidrug Resistance of Campylobacter from Chicken and Swine, China, 2008–2014. J. Antimicrob. Chemother. 2016, 71, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ma, L.; Li, Y.; Jia, H.; Wei, J.; Shao, D.; Liu, K.; Shi, Y.; Qiu, Y.; Ma, Z. Antimicrobial Resistance of Campylobacter Species Isolated from Broilers in Live Bird Markets in Shanghai, China. Foodborne Pathog. Dis. 2016, 14, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.M.; Hotzel, H.; El-Adawy, H.; Tran, H.T.; Le, M.T.H.; Tomaso, H.; Neubauer, H.; Hafez, H.M. Genotyping and Antibiotic Resistance of Thermophilic Campylobacter Isolated from Chicken and Pig Meat in Vietnam. Gut Pathog. 2016, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Pezzotti, G.; Serafin, A.; Luzzi, I.; Mioni, R.; Milan, M.; Perin, R. Occurrence and Resistance to Antibiotics of Campylobacter Jejuni and Campylobacter Coli in Animals and Meat in Northeastern Italy. Int. J. Food Microbiol. 2003, 82, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Gallay, A.; Prouzet-Mauléon, V.; Kempf, I.; Lehours, P.; Labadi, L.; Camou, C.; Denis, M.; de Valk, H.; Desenclos, J.-C.; Mégraud, F. Campylobacter Antimicrobial Drug Resistance among Humans, Broiler Chickens, and Pigs, France. Emerg. Infect. Dis. J. 2007, 13, 259. [Google Scholar] [CrossRef]
- Woźniak-Biel, A.; Bugla-Płoskońska, G.; Kielsznia, A.; Korzekwa, K.; Tobiasz, A.; Korzeniowska-Kowal, A.; Wieliczko, A. High Prevalence of Resistance to Fluoroquinolones and Tetracycline Campylobacter spp. Isolated from Poultry in Poland. Microb. Drug Resist. 2017, 24, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, M.; Salata, C.; Martini, M.; Montesissa, C.; Piccirillo, A. Antimicrobial Resistance of Campylobacter Jejuni and Campylobacter Coli from Poultry in Italy. Microb. Drug Resist. 2013, 20, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Boto, D.; García-Peña, F.J.; Abad-Moreno, J.C.; Echeita, M.A. Antimicrobial Susceptibilities of Campylobacter Jejuni and Campylobacter Coli Strains Isolated from Two Early Stages of Poultry Production. Microb. Drug Resist. 2013, 19, 323–330. [Google Scholar] [CrossRef]
- Nachamkin, I.; Ung, H.; Li, M. Increasing Fluoroquinolone Resistance in Campylobacter Jejuni, Pennsylvania, USA,1982–20011. Emerg. Infect. Dis. J. 2002, 8, 1501. [Google Scholar] [CrossRef]
- Sierra-Arguello, Y.M.; Perdoncini, G.; Morgan, R.B.; Salle, C.T.P.; Moraes, H.L.S.; Gomes, M.J.P.; do Nascimento, V.P. Fluoroquinolone and Macrolide Resistance in Campylobacter Jejuni Isolated from Broiler Slaughterhouses in Southern Brazil. Avian Pathol. 2016, 45, 66–72. [Google Scholar] [CrossRef]
- Tenson, T.; Lovmar, M.; Ehrenberg, M. The Mechanism of Action of Macrolides, Lincosamides and Streptogramin B Reveals the Nascent Peptide Exit Path in the Ribosome. J. Mol. Biol. 2003, 330, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, D.; Quinn, T.; Cotter, L.; Fanning, S. An Investigation of the Molecular Mechanisms Contributing to High-Level Erythromycin Resistance in Campylobacter. Int. J. Antimicrob. Agents 2006, 27, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Vacher, S.; Menard, A.; Bernard, E.; Santos, A.; Megraud, F. Detection of Mutations Associated with Macrolide Resistance in Thermophilic Campylobacter spp. by Real-Time PCR. Microb. Drug Resist. 2005, 11, 40–47. [Google Scholar] [CrossRef]
- Gibreel, A.; Kos, V.N.; Keelan, M.; Trieber, C.A.; Levesque, S.; Michaud, S.; Taylor, D. Macrolide Resistance in Campylobacter Jejuni and Campylobacter Coli: Molecular Mechanism and Stability of the Resistance Phenotype. Antimicrob. Agents Chemother. 2005, 49, 2753–2759. [Google Scholar] [CrossRef] [PubMed]
- Ohno, H.; Wachino, J.; Saito, R.; Jin, W.; Yamada, K.; Kimura, K.; Arakawa, Y. A Highly Macrolide-Resistant Campylobacter Jejuni Strain with Rare A2074T Mutations in 23S RRNA Genes. Antimicrob. Agents Chemother. 2016, 60, 2580–2581. [Google Scholar] [CrossRef]
- Cagliero, C.; Mouline, C.; Cloeckaert, A.; Payot, S. Synergy between Efflux Pump CmeABC and Modifications in Ribosomal Proteins L4 and L22 in Conferring Macrolide Resistance in Campylobacter Jejuni and Campylobacter coli. Antimicrob. Agents Chemother. 2006, 50, 3893–3896. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Wang, Y.; Zhang, Q.; Zhang, M.; Deng, F.; Shen, Z.; Wu, C.; Wang, S.; Zhang, J.; Shen, J. Report of Ribosomal RNA Methylase Gene Erm(B) in Multidrug-Resistant Campylobacter coli. J. Antimicrob. Chemother. 2014, 69, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Florez-Cuadrado, D.; Ugarte-Ruiz, M.; Quesada, A.; Palomo, G.; Domínguez, L.; Porrero, M.C. Description of an Erm(B)-Carrying Campylobacter Coli Isolate in Europe. J. Antimicrob. Chemother. 2016, 71, 841–843. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Shen, Z.; Jeon, B.; Dai, L.; Zhang, Q. Synergistic Effects of Anti-CmeA and Anti-CmeB Peptide Nucleic Acids on Sensitizing Campylobacter Jejuni to Antibiotics. Antimicrob. Agents Chemother. 2013, 57, 4575–4577. [Google Scholar] [CrossRef]
- Komba, E.V.G.; Mdegela, R.H.; Msoffe, P.L.M.; Nielsen, L.N.; Ingmer, H. Prevalence, Antimicrobial Resistance and Risk Factors for Thermophilic Campylobacter Infections in Symptomatic and Asymptomatic Humans in Tanzania. Zoonoses Public Health 2015, 62, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Brown, S.; Gillespie, B.; Lin, J. A Single Nucleotide in the Promoter Region Modulates the Expression of the β-Lactamase OXA-61 in Campylobacter jejuni. J. Antimicrob. Chemother. 2014, 69, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Akiba, M.; Lin, J.; Barton, Y.-W.; Zhang, Q. Interaction of CmeABC and CmeDEF in Conferring Antimicrobial Resistance and Maintaining Cell Viability in Campylobacter jejuni. J. Antimicrob. Chemother. 2006, 57, 52–60. [Google Scholar] [CrossRef]
- Connell, S.R.; Trieber, C.A.; Dinos, G.P.; Einfeldt, E.; Taylor, D.E.; Nierhaus, K.H. Mechanism of Tet (O)-mediated Tetracycline Resistance. EMBO J. 2003, 22, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Iovine, N.M. Resistance Mechanisms in Campylobacter jejuni. Virulence 2013, 4, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.D.; Sanchez, S.; Zimmer, M.; Idris, U.; Berrang, M.E.; McDermott, P.F. Class 1 Integron-Associated Tobramycin-Gentamicin Resistance in Campylobacter Jejuni Isolated from the Broiler Chicken House Environment. Antimicrob. Agents Chemother. 2002, 46, 3660–3664. [Google Scholar] [CrossRef]
- Nirdnoy, W.; Mason, C.J.; Guerry, P. Mosaic Structure of a Multiple-Drug-Resistant, Conjugative Plasmid from Campylobacter jejuni. Antimicrob. Agents Chemother. 2005, 49, 2454–2459. [Google Scholar] [CrossRef] [PubMed]
- Hansson, I.; Sandberg, M.; Habib, I.; Lowman, R.; Engvall, E.O. Knowledge Gaps in Control of Campylobacter for Prevention of Campylobacteriosis. Transbound. Emerg. Dis. 2018, 65, 30–48. [Google Scholar] [CrossRef]
- Sibanda, N.; McKenna, A.; Richmond, A.; Ricke, S.C.; Callaway, T.; Stratakos, A.C.; Gundogdu, O.; Corcionivoschi, N. A Review of the Effect of Management Practices on Campylobacter Prevalence in Poultry Farms. Front. Microbiol. 2018, 9, 2002. [Google Scholar] [CrossRef] [PubMed]
- Arsi, K.; Donoghue, A.M.; Woo-Ming, A.; Blore, P.J.; Donoghue, D.J. Intracloacal Inoculation, an Effective Screening Method for Determining the Efficacy of Probiotic Bacterial Isolates against Campylobacter Colonization in Broiler Chickens. J. Food Prot. 2015, 78, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Jang, M.J.; Kim, S.Y.; Yang, Y.; Pavlidis, H.O.; Ricke, S.C. Potential for Prebiotics as Feed Additives to Limit Foodborne Campylobacter Establishment in the Poultry Gastrointestinal Tract. Front. Microbiol. 2019, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Sahin, O.; Grover, M.; Zhang, Q. New and Alternative Strategies for the Prevention, Control, and Treatment of Antibiotic-Resistant Campylobacter. Transl. Res. 2020, 223, 76–88. [Google Scholar] [CrossRef]
- Rice, B.E.; Rollins, D.M.; Mallinson, E.T.; Carr, L.; Joseph, S.W. Campylobacter Jejuni in Broiler Chickens: Colonization and Humoral Immunity Following Oral Vaccination and Experimental Infection. Vaccine 1997, 15, 1922–1932. [Google Scholar] [CrossRef]
- Radomska, K.A.; Ordoñez, S.R.; Wösten, M.M.S.M.; Wagenaar, J.A.; van Putten, J.P.M. Feedback Control of Campylobacter Jejuni Flagellin Levels through Reciprocal Binding of FliW to Flagellin and the Global Regulator CsrA. Mol. Microbiol. 2016, 102, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Taha-Abdelaziz, K.; Hodgins, D.C.; Alkie, T.N.; Quinteiro-Filho, W.; Yitbarek, A.; Astill, J.; Sharif, S. Oral Administration of PLGA-Encapsulated CpG ODN and Campylobacter Jejuni Lysate Reduces Cecal Colonization by Campylobacter Jejuni in Chickens. Vaccine 2018, 36, 388–394. [Google Scholar] [CrossRef]
- Kobierecka, P.A.; Wyszyńska, A.K.; Gubernator, J.; Kuczkowski, M.; Wiśniewski, O.; Maruszewska, M.; Wojtania, A.; Derlatka, K.E.; Adamska, I.; Godlewska, R.; et al. Chicken Anti-Campylobacter Vaccine—Comparison of Various Carriers and Routes of Immunization. Front. Microbiol. 2016, 7, 740. [Google Scholar] [CrossRef] [PubMed]


| Source | Clinical Manifestations | Treatment | First-Line Antibiotic | Second-Line Antibiotics |
|---|---|---|---|---|
| CDC Yellow Book [160] (2024) | mild | not recommended | none | none |
| moderate | possible | azithromycin | fluoroquinolones, rifaximin (>12 years) | |
| severe | recommended | |||
| World Gastroenterology Organisation [161] (2012) | dysentery | consider | azithromycin | fluoroquinolones, trimethoprim-sulfamethoxazole (children) |
| persistent dysentery | recommended | |||
| patients at high risk of dehydration | recommended | |||
| Infectious Diseases Society of America [162] (2017) | immunocompromised patient | consider | azithromycin | fluoroquinolones (should be avoided in Asian countries), trimethoprim-sulfamethoxazole |
| severe | recommended | |||
| infants < 3 months | recommended | |||
| sepsis-like | recommended |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veronese, P.; Dodi, I. Campylobacter jejuni/coli Infection: Is It Still a Concern? Microorganisms 2024, 12, 2669. https://doi.org/10.3390/microorganisms12122669
Veronese P, Dodi I. Campylobacter jejuni/coli Infection: Is It Still a Concern? Microorganisms. 2024; 12(12):2669. https://doi.org/10.3390/microorganisms12122669
Chicago/Turabian StyleVeronese, Piero, and Icilio Dodi. 2024. "Campylobacter jejuni/coli Infection: Is It Still a Concern?" Microorganisms 12, no. 12: 2669. https://doi.org/10.3390/microorganisms12122669
APA StyleVeronese, P., & Dodi, I. (2024). Campylobacter jejuni/coli Infection: Is It Still a Concern? Microorganisms, 12(12), 2669. https://doi.org/10.3390/microorganisms12122669








