Low 25-Hydroxyvitamin D Post-Kidney Transplant Is Associated with Increased Risk of BK Polyomavirus-Associated Nephropathy
Abstract
1. Introduction
2. Materials and Methods
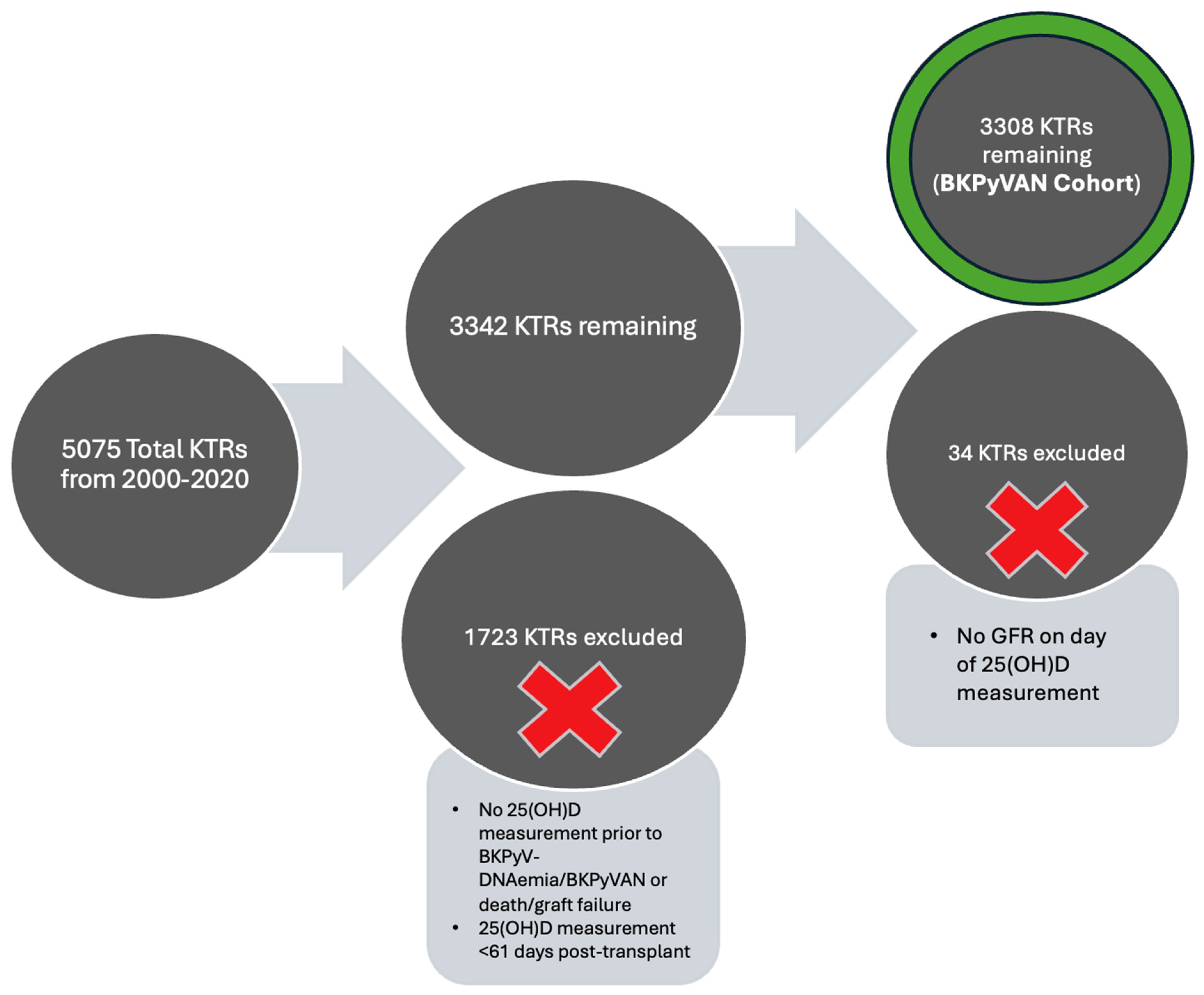
2.1. Study Population and Design
2.2. Follow-Up
2.3. Vitamin D and BK Virus Quantification
2.4. BK Monitoring and Treatment Protocols
2.5. Other Characteristics
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
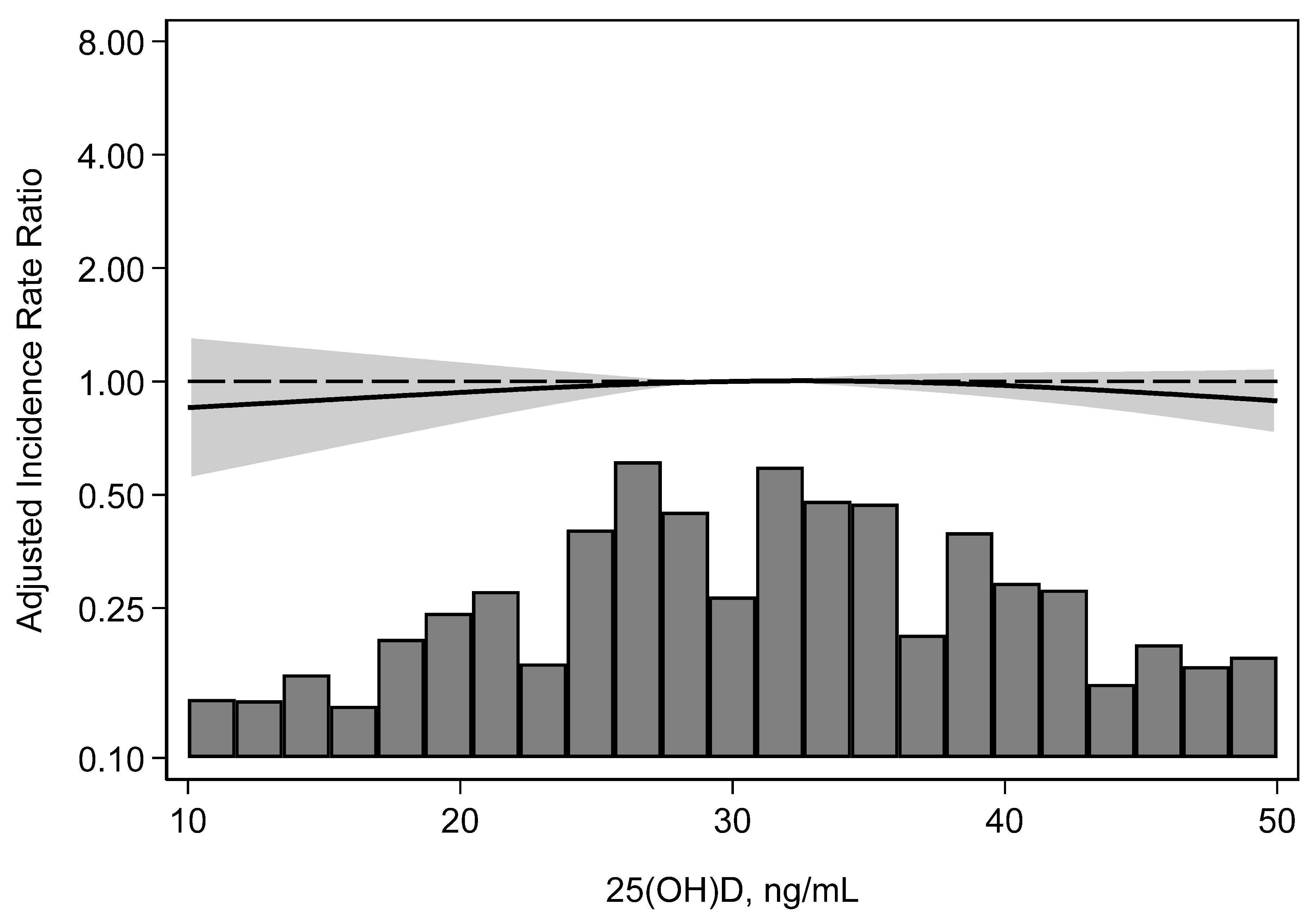
3.2. Incidence of BK Viremia
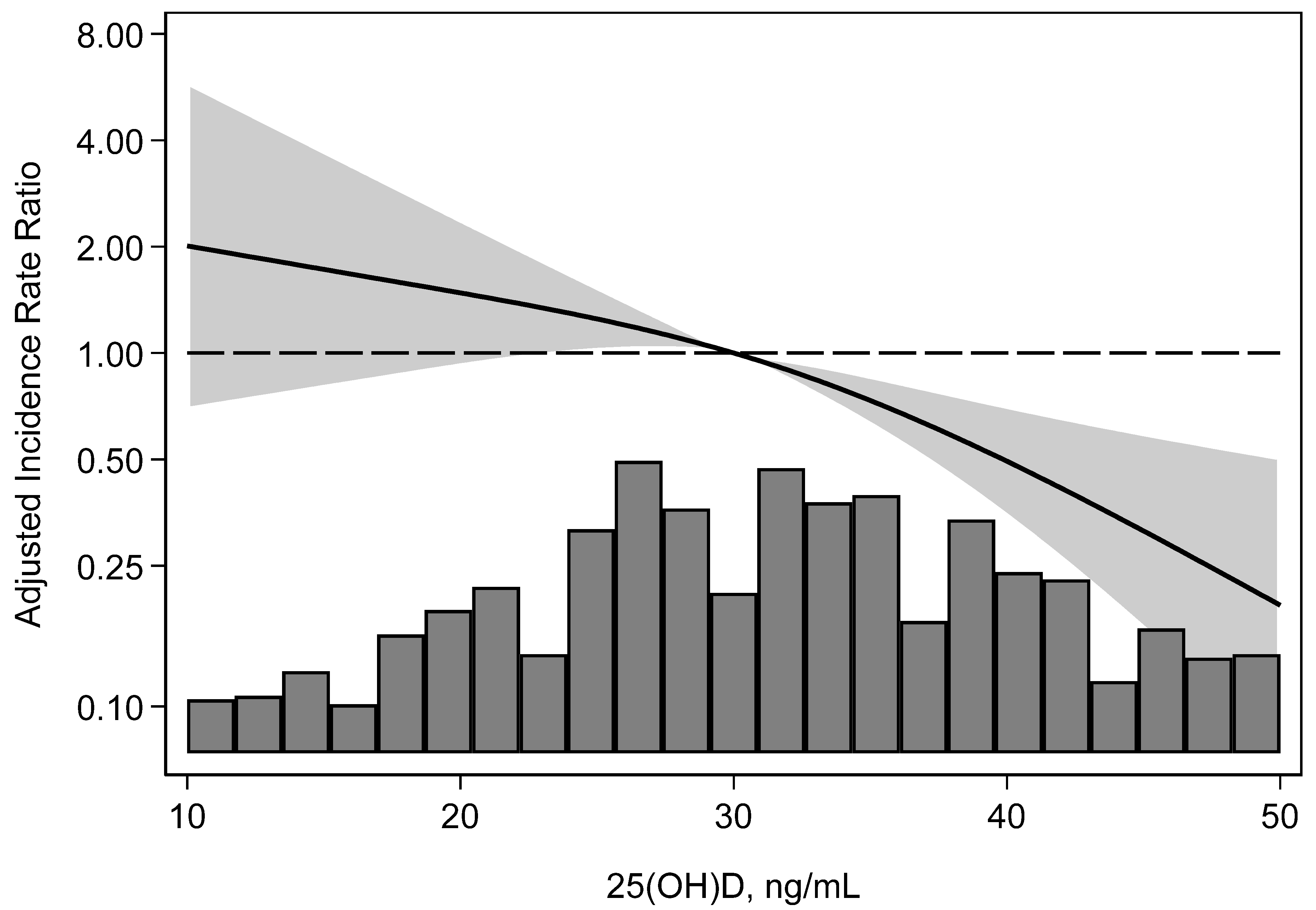
3.3. Incidence of BK Nephropathy
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1,25(OH)2-D | 1,25-dihydroxyvitamin D |
| 25(OH)D | 25-hydroxyvitamin D |
| aHR | adjusted hazard ratio |
| BKPyV-DNAemia | BK viremia |
| BKPyVAN | BK polyomavirus-associated nephropathy |
| BMI | body mass index |
| CMV | cytomegalovirus |
| CNI | calcineurin inhibitor |
| eGFR | estimated glomerular filtration rate |
| ESKD | end-stage kidney disease |
| IVIG | intravenous immunoglobulin |
| KTRs | kidney transplant recipients |
| PCR | polymerase chain reaction |
| PVAN | polyomavirus-associated nephropathy |
| RH | relative hazard |
| SD | standard deviation |
| WisARD | Wisconsin Allograft Recipient Database |
References
- Kharel, A.; Djamali, A.; Jorgenson, M.R.; Alzoubi, B.; Swanson, K.J.; Garg, N.; Aziz, F.; Mohamed, M.A.; Mandelbrot, D.A.; Parajuli, S. Risk factors for progression from low level BK dnaemia to unfavorable outcomes after BK management via immunosuppressive reduction. Transpl. Infect. Dis. 2021, 23, e13561. [Google Scholar] [CrossRef] [PubMed]
- Mylonakis, E.; Goes, N.; Rubin, R.H.; Cosimi, A.B.; Colvin, R.B.; Fishman, J.A. BK virus in solid organ transplant recipients: An emerging syndrome. Transplantation 2001, 72, 1587–1592. [Google Scholar] [CrossRef] [PubMed]
- Augustine, J. Kidney transplant: New opportunities and challenges. Clevel. Clin. J. Med. 2018, 85, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Stolt, A.; Sasnauskas, K.; Koskela, P.; Lehtinen, M.; Dillner, J. Seroepidemiology of the human polyomaviruses. J. Gen. Virol. 2003, 84 Pt 6, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Knowles, W.A.; Pipkin, P.; Andrews, N.; Vyse, A.; Minor, P.; Brown, D.W.; Miller, E. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J. Med. Virol. 2003, 71, 115–123. [Google Scholar] [CrossRef]
- Prelog, M.; Egli, A.; Zlamy, M.; Hirsch, H.H. JC and BK polyomavirus-specific immunoglobulin G responses in patients thymectomized in early childhood. J. Clin. Virol. 2013, 58, 553–558. [Google Scholar] [CrossRef]
- Simon-Santamaria, J.; Rinaldo, C.H.; Kardas, P.; Li, R.; Malovic, I.; Elvevold, K.; McCourt, P.; Smedsrød, B.; Hirsch, H.H.; Sørensen, K.K. Efficient uptake of blood-borne BK and JC polyomavirus-like particles in endothelial cells of liver sinusoids and renal vasa recta. PLoS ONE 2014, 9, e111762. [Google Scholar] [CrossRef] [PubMed]
- Chesters, P.M.; Heritage, J.; McCance, D.J. Persistence of DNA sequences of BK virus and JC virus in normal human tissues and in diseased tissues. J. Infect. Dis. 1983, 147, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Wilhelm, M.; Wilk, S.; Hirsch, H.H. BK polyomavirus-specific antibody and T-cell responses in kidney transplantation: Update. Curr. Opin. Infect. Dis. 2019, 32, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Barraclough, K.A.; Isbel, N.M.; Staatz, C.E.; Johnson, D.W. BK Virus in Kidney Transplant Recipients: The Influence of Immunosuppression. J. Transplant. 2011, 2011, 750836. [Google Scholar] [CrossRef] [PubMed]
- Sawinski, D.; Goral, S. BK virus infection: An update on diagnosis and treatment. Nephrol. Dial. Transplant. 2015, 30, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Kotton, C.N.; Kamar, N.; Wojciechowski, D.; Eder, M.; Hopfer, H.; Randhawa, P.; Sester, M.; Comoli, P.; Silva, H.T.; Knoll, G.; et al. The Second International Consensus Guidelines on the Management of BK Polyomavirus in Kidney Transplantation. Transplantation 2024, 108, 1834–1866. [Google Scholar] [CrossRef] [PubMed]
- Kant, S.; Dasgupta, A.; Bagnasco, S.; Brennan, D.C. BK Virus Nephropathy in Kidney Transplantation: A State-of-the-Art Review. Viruses 2022, 14, 1616. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, H.H.; Randhawa, P.S. BK polyomavirus in solid organ transplantation-Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13528. [Google Scholar] [CrossRef] [PubMed]
- Hertz-Tang, A.L.; Astor, B.C.; Mandelbrot, D.A.; Mohamed, M.A.; Djamali, A.; Parajuli, S. BK viremia is not associated with adverse outcomes in the absence of BK nephropathy. Clin. Transplant. 2018, 32, e13283. [Google Scholar] [CrossRef]
- Lang, P.O.; Samaras, N.; Samaras, D.; Aspinall, R. How important is vitamin D in preventing infections? Osteoporos. Int. 2013, 24, 1537–1553. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Infections and Autoimmunity-The Immune System and Vitamin D: A Systematic Review. Nutrients 2023, 15, 3842. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.O.; Aspinall, R. Vitamin D Status and the Host Resistance to Infections: What It Is Currently (Not) Understood. Clin. Ther. 2017, 39, 930–945. [Google Scholar] [CrossRef] [PubMed]
- Pecora, F.; Persico, F.; Argentiero, A.; Neglia, C.; Esposito, S. The Role of Micronutrients in Support of the Immune Response against Viral Infections. Nutrients 2020, 12, 3198. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Christakos, S. Mechanisms Underlying the Regulation of Innate and Adaptive Immunity by Vitamin D. Nutrients 2015, 7, 8251–8260. [Google Scholar] [CrossRef]
- Ghaseminejad-Raeini, A.; Ghaderi, A.; Sharafi, A.; Nematollahi-Sani, B.; Moossavi, M.; Derakhshani, A.; Sarab, G.A. Immunomodulatory actions of vitamin D in various immune-related disorders: A comprehensive review. Front. Immunol. 2023, 14, 950465. [Google Scholar] [CrossRef] [PubMed]
- McGregor, R.; Li, G.; Penny, H.; Lombardi, G.; Afzali, B.; Goldsmith, D.J. Vitamin D in renal transplantation—From biological mechanisms to clinical benefits. Am. J. Transplant. 2014, 14, 1259–1270. [Google Scholar] [CrossRef]
- Sadlier, D.M.; Magee, C.C. Prevalence of 25(OH) vitamin D (calcidiol) deficiency at time of renal transplantation: A prospective study. Clin. Transplant. 2007, 21, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Ducloux, D.; Courivaud, C.; Bamoulid, J.; Kazory, A.; Dumoulin, G.; Chalopin, J.M. Pretransplant serum vitamin D levels and risk of cancer after renal transplantation. Transplantation 2008, 85, 1755–1759. [Google Scholar] [CrossRef] [PubMed]
- Astor, B.C.; Djamali, A.; Mandelbrot, D.A.; Parajuli, S.; Melamed, M.L. The Association of 25-Hydroxyvitamin D Levels with Late Cytomegalovirus Infection in Kidney Transplant Recipients: The Wisconsin Allograft Recipient Database. Transplantation 2019, 103, 1683–1688. [Google Scholar] [CrossRef]
- Rech, M.A.; Fleming, J.N.; Moore, C.L. 25-hydroxyvitamin D deficiency and opportunistic viral infections after kidney transplant. Exp. Clin. Transplant. 2014, 12, 95–100. [Google Scholar]
- Fernández-Ruiz, M.; Corbella, L.; Morales-Cartagena, A.; González, E.; Polanco, N.; Ruiz-Merlo, T.; Parra, P.; Silva, J.T.; López-Medrano, F.; San Juan, R.; et al. Vitamin D deficiency and infection risk in kidney transplant recipients: A single-center cohort study. Transpl. Infect. Dis. 2018, 20, e12988. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, H.V.; Sacha, L.M.; Ingemi, A.I.; Shullo, M.A.; Johnson, H.J.; Sood, P.; Tevar, A.D.; Humar, A.; Venkataramanan, R. Low vitamin D exposure is associated with higher risk of infection in renal transplant recipients. Clin. Transplant. 2017, 31, e12955. [Google Scholar] [CrossRef]
- Messa, P.; Regalia, A.; Alfieri, C.M. Nutritional Vitamin D in Renal Transplant Patients: Speculations and Reality. Nutrients 2017, 9, 550. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Jorgenson, M.R.; Descourouez, J.L.; Lyu, B.; Astor, B.C.; Saddler, C.M.; Mandelbrot, D.A.; Parajuli, S. Management of BK viremia is associated with a lower risk of subsequent cytomegalovirus infection in kidney transplant recipients. Clin. Transplant. 2020, 34, e13798. [Google Scholar] [CrossRef]
- Parajuli, S.; Astor, B.C.; Kaufman, D.; Muth, B.; Mohamed, M.; Garg, N.; Djamali, A.; Mandelbrot, D.A. Which is more nephrotoxic for kidney transplants: BK nephropathy or rejection? Clin. Transplant. 2018, 32, e13216. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Haas, M.; Solez, K.; Racusen, L.; Glotz, D.; Seron, D.; Nankivell, B.J.; Colvin, R.B.; Afrouzian, M.; Akalin, E.; et al. The Banff 2015 kidney meeting report: Current challenges in rejection classification and prospects for adopting molecular pathology. Am. J. Transplant. 2017, 17, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Moscarelli, L.; Antognoli, G.; Buti, E.; Dervishi, E.; Fani, F.; Caroti, L.; Tsalouchos, A.; Romoli, E.; Ghiandai, G.; Minetti, E. 1,25 Dihydroxyvitamin D circulating levels, calcitriol administration, and incidence of acute rejection, CMV infection, and polyoma virus infection in renal transplant recipients. Clin. Transplant. 2016, 30, 1347–1359. [Google Scholar] [CrossRef] [PubMed]
- Musavi Mehdiabadi, F.; Ahmadi, F.; Lesan Pezeshki, M.; Razeghi, E. The Relationship Between Serum Level of 25-hydroxy Vitamin D and Cytomegalovirus Infection in Kidney Transplant Recipients. Iran. J. Kidney Dis. 2019, 13, 225–231. [Google Scholar] [PubMed]
- Fernández-Ruiz, M.; Rodríguez-Goncer, I.; Ruiz-Merlo, T.; Parra, P.; López-Medrano, F.; Andrés, A.; Aguado, J.M. Low 25-hydroxyvitamin D Levels and the Risk of Late CMV Infection After Kidney Transplantation: Role for CMV-specific Mediated Immunity. Transplantation 2019, 103, e216–e217. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D for health and in chronic kidney disease. Semin. Dial. 2005, 18, 266–275. [Google Scholar] [CrossRef] [PubMed]
| Overall (N = 3308) | Serum 25(OH)D | |||||
|---|---|---|---|---|---|---|
| ≥30 ng/mL (N = 1993) | 21–29 ng/mL (N = 916) | ≤20 ng/mL (N = 399) | p | |||
| Characteristics at time of transplant | ||||||
| Age, years (SD) | 52.6 (12.9) | 53.7 (12.4) | 52.0 (13.2) | 48.6 (13.5) | <0.001 | |
| Female, % | 39.8 | 38.4 | 42.0 | 41.1 | 0.11 | |
| Non-white, % | 20.9 | 18.0 | 24.2 | 28.1 | <0.001 | |
| Cause of ESKD, % | ||||||
| Diabetes mellitus | 25.0 | 21.0 | 29.9 | 33.6 | <0.001 | |
| Hypertension | 12.3 | 12.9 | 11.2 | 12.3 | ||
| PKD | 14.1 | 17.4 | 9.5 | 8.8 | ||
| Glomerulonephritis | 25.0 | 26.2 | 24.0 | 21.1 | ||
| Other | 23.6 | 22.6 | 25.3 | 24.3 | ||
| Body mass index, kg/m2 (SD) | 28.1 (5.3) | 27.9 (5.2) | 28.5 (5.3) | 28.2 (5.4) | 0.04 | |
| Living donor, % | 38.9 | 42.4 | 34.9 | 30.3 | <0.001 | |
| Prior transplant, % | 20.7 | 38.9 | 41.4 | 45.4 | <0.001 | |
| Delayed graft function, % | 18.3 | 36.0 | 40.6 | 44.5 | <0.001 | |
| Induction immunosuppression | ||||||
| Thymoglobulin | 31.6 | 30.0 | 33.1 | 36.6 | 0.003 | |
| Basiliximab | 55.6 | 57.1 | 55.8 | 47.9 | ||
| Alemtuzumab | 11.1 | 10.8 | 10.3 | 14.0 | ||
| Other | 1.7 | 2.1 | 0.9 | 1.5 | ||
| Preemptive transplantation | 23.5 | 29.3 | 16.4 | 10.8 | <0.001 | |
| HLA mismatch, % | ||||||
| 0–2 | 19.4 | 19.6 | 18.7 | 19.8 | 0.90 | |
| 3–4 | 43.8 | 43.0 | 44.4 | 41.9 | ||
| 5–6 | 36.8 | 36.5 | 36.9 | 38.4 | ||
| High-risk cytomegalovirus serostatus, % | 11.1 | 30.6 | 32.9 | 32.6 | 0.16 | |
| Tacrolimus-based immunosuppression | 92.0 | 93.7 | 90.8 | 86.2 | <0.001 | |
| History of acute rejection before the first serum 25(OH)D measurement, % | 10.3 | 9.2 | 8.8 | 19.5 | <0.001 | |
| eGFR at the time of the first serum 25(OH)D measurement (SD) | 71.4 (25.2) | 69.9 (24.1) | 73.7 (26.6) | 73.7 (27.0) | <0.001 | |
| Time of first serum 25(OH)D measurement, number of days post-transplant (SD) | 247 (141) | 247 (133) | 240 (147) | 263 (161) | 0.24 | |
| Serum 25(OH)D | ||||
|---|---|---|---|---|
| ≥30 ng/mL | 21–29 ng/mL | ≤20 ng/mL | P-Trend | |
| Numbers of events | 120 | 44 | 20 | - |
| Incidence rate/100 person-years | 2.88 | 2.22 | 2.37 | - |
| Relative hazard (95% confidence interval) | 1.0 Reference | 0.70 (0.50, 0.99) p = 0.05 | 0.80 (0.50, 1.28) p = 0.35 | 0.10 |
| Adjusted * relative hazard (95% confidence interval) | 1.0 Reference | 0.72 (0.50, 1.02) p = 0.07 | 0.88 (0.55, 1.42) p = 0.61 | 0.22 |
| Serum 25(OH)D | ||||
|---|---|---|---|---|
| ≥30 ng/mL | 21–29 ng/mL | ≤20 ng/mL | P-Trend | |
| Numbers of events | 15 | 17 | 12 | - |
| Incidence rate/100 person-years | 0.30 | 0.75 | 1.28 | - |
| Relative hazard (95% confidence interval) | 1.0 Reference | 2.41 (1.20, 4.83) p = 0.01 | 4.15 (1.94, 8.86) p < 0.001 | <0.001 |
| Adjusted * relative hazard (95% confidence interval) | 1.0 Reference | 2.22 (1.11, 4.45) p = 0.02 | 3.92 (1.66, 9.23) p = 0.002 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raj, S.A.; Zhou, A.L.; Fedorova, E.; Yuan, Z.; Mandelbrot, D.A.; Astor, B.C.; Parajuli, S. Low 25-Hydroxyvitamin D Post-Kidney Transplant Is Associated with Increased Risk of BK Polyomavirus-Associated Nephropathy. Microorganisms 2024, 12, 2588. https://doi.org/10.3390/microorganisms12122588
Raj SA, Zhou AL, Fedorova E, Yuan Z, Mandelbrot DA, Astor BC, Parajuli S. Low 25-Hydroxyvitamin D Post-Kidney Transplant Is Associated with Increased Risk of BK Polyomavirus-Associated Nephropathy. Microorganisms. 2024; 12(12):2588. https://doi.org/10.3390/microorganisms12122588
Chicago/Turabian StyleRaj, Suseela A., Angela L. Zhou, Ekaterina Fedorova, Zhongyu Yuan, Didier A. Mandelbrot, Brad C. Astor, and Sandesh Parajuli. 2024. "Low 25-Hydroxyvitamin D Post-Kidney Transplant Is Associated with Increased Risk of BK Polyomavirus-Associated Nephropathy" Microorganisms 12, no. 12: 2588. https://doi.org/10.3390/microorganisms12122588
APA StyleRaj, S. A., Zhou, A. L., Fedorova, E., Yuan, Z., Mandelbrot, D. A., Astor, B. C., & Parajuli, S. (2024). Low 25-Hydroxyvitamin D Post-Kidney Transplant Is Associated with Increased Risk of BK Polyomavirus-Associated Nephropathy. Microorganisms, 12(12), 2588. https://doi.org/10.3390/microorganisms12122588






