Animal Trypanosomiasis: Challenges and Prospects for New Vaccination Strategies
Abstract
:1. Introduction
2. Different Trypanosome Species That Cause Animal Trypanosomiasis
2.1. Nagana
2.2. Surra
2.3. Dourine
3. Challenges in Vaccine Development for Animal Diseases Caused by Trypanosomes
3.1. Host Immune Response and Immunoregulatory Pathways During Trypanosoma Infections
3.2. Parasite Antigenic Variation
3.3. Immunosuppression of Host and Parasite Persistence
3.4. Limitation of Effective Laboratory Models
4. Advances in Research Towards the Development of Vaccines Against Animal Trypanosomes
4.1. Vaccines Based on Recombinant Proteins
4.2. The mRNA Vaccines
4.3. Vector-Based Vaccines
4.4. CRISPR-Attenuated Vaccines
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taye, M.; Belihu, K.; Bekana, M.; Sheferaw, D. Assessment of Impacts of Tsetse and Trypanosomosis Control Measures on Cattle Herd Composition and Performance in Southern Region, Ethiopia. Trop. Anim. Health Prod. 2012, 44, 1759–1763. [Google Scholar] [CrossRef]
- Cecchi, G.; Paone, M.; Argilés Herrero, R.; Vreysen, M.J.B.; Mattioli, R.C. Developing a Continental Atlas of the Distribution and Trypanosomal Infection of Tsetse Flies (Glossina Species). Parasit. Vectors 2015, 8, 284. [Google Scholar] [CrossRef]
- Baker, J.R. La Taxonomie Sous-Spécifique de Trypanosoma Brucei. Parasite 1995, 2, 3–12. [Google Scholar] [CrossRef]
- Bruce, D. Preliminary Report on the Tsetse Fly Disease or Nagana in Zululand; Bennett & Davis: Durban, South Africa, 1905; Volume 44, pp. 1–28. [Google Scholar]
- Silvester, E.; McWilliam, K.R.; Matthews, K.R. The Cytological Events and Molecular Control of Life Cycle Development of Trypanosoma Brucei in the Mammalian Bloodstream. Pathogens 2017, 6, 29. [Google Scholar] [CrossRef]
- Holmes, P. Tsetse-Transmitted Trypanosomes—Their Biology, Disease Impact and Control. J. Invertebr. Pathol. 2013, 112 (Suppl. 1), S11–S14. [Google Scholar] [CrossRef]
- Barrett, M.P.; Croft, S.L. Management of Trypanosomiasis and Leishmaniasis. Br. Med. Bull. 2012, 104, 175–196. [Google Scholar] [CrossRef]
- Giordani, F.; Morrison, L.J.; Rowan, T.G.; DE Koning, H.P.; Barrett, M.P. The Animal Trypanosomiases and Their Chemotherapy: A Review. Parasitology 2016, 143, 1862–1889. [Google Scholar] [CrossRef]
- Steverding, D. The History of African Trypanosomiasis. Parasit. Vectors 2008, 1, 3. [Google Scholar] [CrossRef]
- Simarro, P.P.; Franco, J.; Diarra, A.; Postigo, J.A.R.; Jannin, J. Update on Field Use of the Available Drugs for the Chemotherapy of Human African Trypanosomiasis. Parasitology 2012, 139, 842–846. [Google Scholar] [CrossRef]
- Boisson-Walsh, A. Chad Eliminates Gambiense Sleeping Sickness. Lancet Infect. Dis. 2024, 24, e551. [Google Scholar] [CrossRef]
- Crump, R.E.; Aliee, M.; Sutherland, S.A.; Huang, C.-I.; Crowley, E.H.; Spencer, S.E.F.; Keeling, M.J.; Shampa, C.; Mwamba Miaka, E.; Rock, K.S. Modelling Timelines to Elimination of Sleeping Sickness in the Democratic Republic of Congo, Accounting for Possible Cryptic Human and Animal Transmission. Parasit. Vectors 2024, 17, 332. [Google Scholar] [CrossRef]
- Barrett, M.P.; Vincent, I.M.; Burchmore, R.J.S.; Kazibwe, A.J.N.; Matovu, E. Drug Resistance in Human African Trypanosomiasis. Future Microbiol. 2011, 6, 1037–1047. [Google Scholar] [CrossRef]
- Francesconi, V.; Rizzo, M.; Schenone, S.; Carbone, A.; Tonelli, M. State-of-the-Art Review on the Antiparasitic Activity of Benzimidazolebased Derivatives: Facing Malaria, Leishmaniasis, and Trypanosomiasis. Curr. Med. Chem. 2024, 31, 1955–1982. [Google Scholar] [CrossRef]
- Zoltner, M.; Horn, D.; Field, M.C. Pass the Boron: Benzoxaboroles as Antiparasite Drugs. Trends Parasitol. 2024, 40, 820–828. [Google Scholar] [CrossRef]
- Mesu, V.K.B.K.; Kalonji, W.M.; Bardonneau, C.; Mordt, O.V.; Blesson, S.; Simon, F.; Delhomme, S.; Bernhard, S.; Kuziena, W.; Lubaki, J.-P.F.; et al. Oral Fexinidazole for Late-Stage African Trypanosoma Brucei Gambiense Trypanosomiasis: A Pivotal Multicentre, Randomised, Non-Inferiority Trial. Lancet 2018, 391, 144–154. [Google Scholar] [CrossRef]
- Dickie, E.A.; Giordani, F.; Gould, M.K.; Mäser, P.; Burri, C.; Mottram, J.C.; Rao, S.P.S.; Barrett, M.P. New Drugs for Human African Trypanosomiasis: A Twenty First Century Success Story. Trop. Med. Infect. Dis. 2020, 5, 29. [Google Scholar] [CrossRef]
- Bastos, T.S.A.; Faria, A.M.; de Assis Cavalcante, A.S.; de Carvalho Madrid, D.M.; Zapa, D.M.B.; Nicaretta, J.E.; Cruvinel, L.B.; Heller, L.M.; Couto, L.F.M.; Soares, V.E.; et al. Comparison of Therapeutic Efficacy of Different Drugs Against Trypanosoma vivax on Experimentally Infected Cattle. Prev. Vet. Med. 2020, 181, 105040. [Google Scholar] [CrossRef]
- Thumbi, S.M.; de Clare Bronsvoort, B.M.; Poole, E.J.; Kiara, H.; Toye, P.G.; Mbole-Kariuki, M.N.; Conradie, I.; Jennings, A.; Handel, I.G.; Coetzer, J.A.W.; et al. Parasite Co-Infections and Their Impact on Survival of Indigenous Cattle. PLoS ONE 2014, 9, e76324. [Google Scholar] [CrossRef]
- Tola, N.; Wagari, A.; Lemu, G.H.; Kedir, M.; Gebremeskel, H.F.; Kebede, I.A. Trypanosome Infection in Cattle and Associated Vectors in Etang District of Gambella, Ethiopia. J. Parasitol. Res. 2024, 2024, 5548718. [Google Scholar] [CrossRef]
- Swetha, K.; Reddy, B.S.; Shobhamani, B.; Sivajothi, S. Clinico-Haematobiochemical and Cardiac Alterations in Trypanosoma Evansi Infected Buffaloes of Andhra Pradesh, India. Vet. Res. Commun. 2024, 48, 2171–2184. [Google Scholar] [CrossRef]
- N’Djetchi, M.K.; Camara, O.; Koffi, M.; Camara, M.; Kaba, D.; Kaboré, J.; Tall, A.; Rotureau, B.; Glover, L.; Traoré, M.B.; et al. Specificity of Serological Screening Tests and Reference Laboratory Tests to Diagnose Gambiense Human African Trypanosomiasis: A Prospective Clinical Performance Study. Infect. Dis. Poverty 2024, 13, 53. [Google Scholar] [CrossRef]
- de Melo-Junior, R.D.; Bastos, T.S.A.; Couto, L.F.M.; de Assis Cavalcante, A.S.; Zapa, D.M.B.; de Morais, I.M.L.; Heller, L.M.; Salvador, V.F.; Leal, L.L.L.L.; de Oliveira Franco, A.; et al. Trypanosoma vivax in and Outside Cattle Blood: Parasitological, Molecular, and Serological Detection, Reservoir Tissues, Histopathological Lesions, and Vertical Transmission Evaluation. Res. Vet. Sci. 2024, 174, 105290. [Google Scholar] [CrossRef]
- de Almeida Castilho Neto, K.J.G.; da Cruz Favaro Garcia, A.B.; Fidelis Junior, O.L.; Nagata, W.B.; André, M.R.; Teixeira, M.M.G.; Machado, R.Z.; Cadioli, F.A. Follow-up of Dairy Cattle Naturally Infected by Trypanosoma vivax After Treatment with Isometamidium Chloride. Rev. Bras. Parasitol. Vet. 2021, 30, e020220. [Google Scholar] [CrossRef]
- Desquesnes, M.; Sazmand, A.; Gonzatti, M.; Boulangé, A.; Bossard, G.; Thévenon, S.; Gimonneau, G.; Truc, P.; Herder, S.; Ravel, S.; et al. Diagnosis of Animal Trypanosomoses: Proper Use of Current Tools and Future Prospects. Parasit. Vectors 2022, 15, 235. [Google Scholar] [CrossRef]
- Kamyingkird, K.; Chalermwong, P.; Inpankaew, T.; Ngasaman, R.; Tattiyapong, M.; Tiwananthagorn, S.; Chimnoi, W.; Choocherd, S.; Kengradomkij, C.; Klinkaew, N.; et al. Isolation and in Vitro Cultivation of Trypanosoma Evansi Thai Strains. Exp. Parasitol. 2022, 239, 108289. [Google Scholar] [CrossRef]
- Osório, A.L.A.R.; Madruga, C.R.; Desquesnes, M.; Soares, C.O.; Ribeiro, L.R.R.; da Costa, S.C.G. Trypanosoma (Duttonella) vivax: Its Biology, Epidemiology, Pathogenesis, and Introduction in the New World—A Review. Mem. Inst. Oswaldo Cruz 2008, 103, 1–13. [Google Scholar] [CrossRef]
- Wellde, B.T.; Chumo, D.A.; Adoyo, M.; Kovatch, R.M.; Mwongela, G.N.; Opiyo, E.A. Haemorrhagic Syndrome in Cattle Associated with Trypanosoma vivax Infection. Trop. Anim. Health Prod. 1983, 15, 95–102. [Google Scholar] [CrossRef]
- Bastos, T.S.A.; Faria, A.M.; Couto, L.F.M.; Nicaretta, J.E.; de Assis Cavalcante, A.S.; Zapa, D.M.B.; Ferreira, L.L.; Heller, L.M.; de Carvalho Madrid, D.M.; Cruvinel, L.B.; et al. Epidemiological and Molecular Identification of Trypanosoma vivax Diagnosed in Cattle during Outbreaks in Central Brazil. Parasitology 2020, 147, 1313–1319. [Google Scholar] [CrossRef]
- de Melo Junior, R.D.; Azeredo Bastos, T.S.; Heller, L.M.; Couto, L.F.M.; Zapa, D.M.B.; Souza de Assis Cavalcante, A.; Cruvinel, L.B.; Nicaretta, J.E.; Iuasse, H.V.; Ferreira, L.L.; et al. How Many Cattle Can Be Infected by Trypanosoma vivax by Reusing the Same Needle and Syringe, and What Is the Viability Time of This Protozoan in Injectable Veterinary Products?—ERRATUM. Parasitology 2022, 149, 283. [Google Scholar] [CrossRef]
- de Gier, J.; Cecchi, G.; Paone, M.; Dede, P.; Zhao, W. The Continental Atlas of Tsetse and African Animal Trypanosomosis in Nigeria. Acta Trop. 2020, 204, 105328. [Google Scholar] [CrossRef]
- Silva Pereira, S.; Brás, D.; Porqueddu, T.; Nascimento, A.M.; De Niz, M. Investigation of Trypanosoma-Induced Vascular Damage Sheds Insights into Trypanosoma vivax Sequestration. Cell Surf. 2023, 10, 100113. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.G. Clinical Features, Diagnosis, and Treatment of Human African Trypanosomiasis (Sleeping Sickness). Lancet Neurol. 2013, 12, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Morrison, L.J.; Vezza, L.; Rowan, T.; Hope, J.C. Animal African Trypanosomiasis: Time to Increase Focus on Clinically Relevant Parasite and Host Species. Trends Parasitol. 2016, 32, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Desquesnes, M.; Dargantes, A.; Lai, D.-H.; Lun, Z.-R.; Holzmuller, P.; Jittapalapong, S. Trypanosoma Evansi and Surra: A Review and Perspectives on Transmission, Epidemiology and Control, Impact, and Zoonotic Aspects. BioMed Res. Int. 2013, 2013, 321237. [Google Scholar] [CrossRef] [PubMed]
- Ungogo, M.A.; de Koning, H.P. Drug Resistance in Animal Trypanosomiases: Epidemiology, Mechanisms and Control Strategies. Int. J. Parasitol. Drugs Drug Resist. 2024, 25, 100533. [Google Scholar] [CrossRef]
- Luciani, M.; Armillotta, G.; Di Febo, T.; Krasteva, I.; Ulisse, S.; Di Pancrazio, C.; Laguardia, C.; Perletta, F.; Serroni, A.; Maggetti, M.; et al. Analysis of Trypanosoma Equiperdum Recombinant Proteins for the Serological Diagnosis of Dourine. Vet. Sci. 2024, 11, 127. [Google Scholar] [CrossRef]
- Sidney, R.; Andrew, M.; James, C.; Richard, N. Dourine—An Emerging Venereal Threat to European Horses. AHT/BEVA/DEFrA Equine Q. Dis. Surveill. Rep. 2011, 6, 15–18. [Google Scholar]
- Onyilagha, C.; Uzonna, J.E. Host Immune Responses and Immune Evasion Strategies in African Trypanosomiasis. Front. Immunol. 2019, 10, 2738. [Google Scholar] [CrossRef]
- Alfituri, O.A.; Quintana, J.F.; MacLeod, A.; Garside, P.; Benson, R.A.; Brewer, J.M.; Mabbott, N.A.; Morrison, L.J.; Capewell, P. To the Skin and Beyond: The Immune Response to African Trypanosomes as They Enter and Exit the Vertebrate Host. Front. Immunol. 2020, 11, 1250. [Google Scholar] [CrossRef]
- Wheeler, R.J. The Trypanolytic Factor–Mechanism, Impacts and Applications. Trends Parasitol. 2010, 26, 457–464. [Google Scholar] [CrossRef]
- Kiner, E.; Willie, E.; Vijaykumar, B.; Chowdhary, K.; Schmutz, H.; Chandler, J.; Schnell, A.; Thakore, P.I.; LeGros, G.; Mostafavi, S.; et al. Gut CD4(+) T Cell Phenotypes Are a Continuum Molded by Microbes, Not by T(H) Archetypes. Nat. Immunol. 2021, 22, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.-H.D.; Canto, F.B.; Gomes, A.; Brandao, L.M.; Lima, J.R.; Melo, G.A.; Granato, A.; Neves, E.G.A.; Dutra, W.O.; Oliveira, A.-C.; et al. Cytotoxic CD4(+) T Cells Driven by T-Cell Intrinsic IL-18R/MyD88 Signaling Predominantly Infiltrate Trypanosoma Cruzi-Infected Hearts. eLife 2022, 11, e74636. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Holetz, F.B.; Alves, L.R.; Ávila, A.R. Modulation of Virulence Factors during Trypanosoma Cruzi Differentiation. Pathogens 2022, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, G.; Grippi, F.; Di Bella, S.; Blanda, V.; Gucciardi, F.; Torina, A.; Guercio, A.; Cannella, V. A Review on the Immunological Response against Trypanosoma Cruzi. Pathogens 2023, 12, 282. [Google Scholar] [CrossRef]
- Kumar, S.; Tarleton, R.L. The Relative Contribution of Antibody Production and CD8+ T Cell Function to Immune Control of Trypanosoma Cruzi. Parasite Immunol. 1998, 20, 207–216. [Google Scholar] [CrossRef]
- Choi, B.; Vu, H.T.; Vu, H.T.; Radwanska, M.; Magez, S. Advances in the Immunology of the Host–Parasite Interactions in African Trypanosomosis, Including Single-Cell Transcriptomics. Pathogens 2024, 13, 188. [Google Scholar] [CrossRef]
- Chandra, M.; Đaković, S.; Foti, K.; Zeelen, J.P.; van Straaten, M.; Aresta-Branco, F.; Tihon, E.; Lübbehusen, N.; Ruppert, T.; Glover, L.; et al. Structural Similarities between the Metacyclic and Bloodstream Form Variant Surface Glycoproteins of the African Trypanosome. PLoS Negl. Trop. Dis. 2023, 17, e0011093. [Google Scholar] [CrossRef]
- Aresta-Branco, F.; Erben, E.; Papavasiliou, F.N.; Stebbins, C.E. Mechanistic Similarities between Antigenic Variation and Antibody Diversification during Trypanosoma Brucei Infection. Trends Parasitol. 2019, 35, 302–315. [Google Scholar] [CrossRef]
- Manna, P.T.; Boehm, C.; Leung, K.F.; Natesan, S.K.; Field, M.C. Life and Times: Synthesis, Trafficking, and Evolution of VSG. Trends Parasitol. 2014, 30, 251–258. [Google Scholar] [CrossRef]
- Berriman, M.; Ghedin, E.; Hertz-Fowler, C.; Blandin, G.; Renauld, H.; Bartholomeu, D.C.; Lennard, N.J.; Caler, E.; Hamlin, N.E.; Haas, B.; et al. The Genome of the African Trypanosome Trypanosoma Brucei. Science 2005, 309, 416–422. [Google Scholar] [CrossRef]
- Tahir Aleem, M.; Munir, F.; Shakoor, A.; Ud Din Sindhu, Z.; Gao, F. Advancement in the Development of DNA Vaccines against Trypanosoma Brucei and Future Perspective. Int. Immunopharmacol. 2024, 140, 112847. [Google Scholar] [CrossRef] [PubMed]
- Stijlemans, B.; Radwanska, M.; De Trez, C.; Magez, S. African Trypanosomes Undermine Humoral Responses and Vaccine Development: Link with Inflammatory Responses? Front. Immunol. 2017, 8, 582. [Google Scholar] [CrossRef] [PubMed]
- Hudson, K.M.; Byner, C.; Freeman, J.; Terry, R.J. Immunodepression, High IgM Levels and Evasion of the Immune Response in Murine Trypanosomiasis. Nature 1976, 264, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Mertens, B.; Taylor, K.; Muriuki, C.; Rocchi, M. Cytokine MRNA Profiles in Trypanotolerant and Trypanosusceptible Cattle Infected with the Protozoan Parasite Trypanosoma Congolense: Protective Role for Interleukin-4? J. Interf. cytokine Res. Off. J. Int. Soc. Interf. Cytokine Res. 1999, 19, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Mekori, Y.A.; Metcalfe, D.D. Mast Cells in Innate Immunity. Immunol. Rev. 2000, 173, 131–140. [Google Scholar] [CrossRef]
- Ngaira, J.M.; Nantulya, V.M.; Musoke, A.J.; Hirumi, K. Phagocytosis of Antibody-Sensitized Trypanosoma Brucei in Vitro by Bovine Peripheral Blood Monocytes. Immunology 1983, 49, 393–400. [Google Scholar]
- Mulat, G.; Maru, M.; Tarekegn, Z.S.; Dejene, H. A Systematic Review and Meta-Analysis on Prevalence of Bovine Trypanosomosis in East Africa. Parasite Epidemiol. Control 2024, 26, e00371. [Google Scholar] [CrossRef]
- Quintana, J.F.; Sinton, M.C.; Chandrasegaran, P.; Kumar Dubey, L.; Ogunsola, J.; Al Samman, M.; Haley, M.; McConnell, G.; Kuispond Swar, N.-R.; Ngoyi, D.M.; et al. The Murine Meninges Acquire Lymphoid Tissue Properties and Harbour Autoreactive B Cells during Chronic Trypanosoma Brucei Infection. PLoS Biol. 2023, 21, e3002389. [Google Scholar] [CrossRef]
- Ekloh, W.; Sunter, J.D.; Gwira, T.M. African Trypanosome Infection Patterns in Cattle in a Farm Setting in Southern Ghana. Acta Trop. 2023, 237, 106721. [Google Scholar] [CrossRef]
- Hirumi, H.; Hirumi, K.; Moloo, S.K.; Shaw, M.K. In Vitro Cultivation of Blood Stream Trypomastigotes of Trypanosoma vivax without Feeder Cell Layers. J. Protozool. Res. 1991, 1, 1–12. [Google Scholar]
- D’Archivio, S.; Medina, M.; Cosson, A.; Chamond, N.; Rotureau, B.; Minoprio, P.; Goyard, S. Genetic Engineering of Trypanosoma (Dutonella) vivax and in Vitro Differentiation under Axenic Conditions. PLoS Negl. Trop. Dis. 2011, 5, e1461. [Google Scholar] [CrossRef] [PubMed]
- Autheman, D.; Crosnier, C.; Clare, S.; Goulding, D.A.; Brandt, C.; Harcourt, K.; Tolley, C.; Galaway, F.; Khushu, M.; Ong, H.; et al. An Invariant Trypanosoma vivax Vaccine Antigen Induces Protective Immunity. Nature 2021, 595, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Coustou, V.; Guegan, F.; Plazolles, N.; Baltz, T. Complete in Vitro Life Cycle of Trypanosoma Congolense: Development of Genetic Tools. PLoS Negl. Trop. Dis. 2010, 4, e618. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, A.; Tanaka, Y.; Watanabe, K.; Sato, M.; Kawazu, S.-I.; Kita, K.; Inoue, N.; van Rensburg, H.D.J.; N’Da, D.D.; Suganuma, K. Prophylactic Activity of Orally Administered Dry-Heat-Sterilized Acremonium Egyptiacum against Trypanosoma Congolense-Induced Animal African Trypanosomosis. Acta Trop. 2024, 254, 107185. [Google Scholar] [CrossRef] [PubMed]
- Obishakin, E.; de Trez, C.; Magez, S. Chronic Trypanosoma Congolense Infections in Mice Cause a Sustained Disruption of the B-Cell Homeostasis in the Bone Marrow and Spleen. Parasite Immunol. 2014, 36, 187–198. [Google Scholar] [CrossRef]
- Tanaka, Y.; Suganuma, K.; Watanabe, K.; Kobayashi, Y. Pathology of Female Mice Experimentally Infected with an in Vitro Cultured Strain of Trypanosoma Equiperdum. J. Vet. Med. Sci. 2021, 83, 1212–1218. [Google Scholar] [CrossRef]
- Magez, S.; Caljon, G. Mouse Models for Pathogenic African Trypanosomes: Unravelling the Immunology of Host-Parasite-Vector Interactions. Parasite Immunol. 2011, 33, 423–429. [Google Scholar] [CrossRef]
- Jogi, H.R.; Smaraki, N.; Rajak, K.K.; Yadav, A.K.; Bhatt, M.; Einstien, C.; Revathi, A.; Thakur, R.; Kamothi, D.J.; Dedeepya, P.V.S.S.; et al. Revolutionizing Veterinary Health with Viral Vector-Based Vaccines. Indian J. Microbiol. 2024, 64, 867–878. [Google Scholar] [CrossRef]
- Sánchez-Valdéz, F.J.; Pérez Brandán, C.; Ferreira, A.; Basombrío, M.Á. Gene-Deleted Live-Attenuated Trypanosoma Cruzi Parasites as Vaccines to Protect against Chagas Disease. Expert Rev. Vaccines 2015, 14, 681–697. [Google Scholar] [CrossRef]
- Iavarone, C.; O’hagan, D.T.; Yu, D.; Delahaye, N.F.; Ulmer, J.B. Mechanism of Action of MRNA-Based Vaccines. Expert Rev. Vaccines 2017, 16, 871–881. [Google Scholar] [CrossRef]
- Mirtaleb, M.S.; Falak, R.; Heshmatnia, J.; Bakhshandeh, B.; Taheri, R.A.; Soleimanjahi, H.; Zolfaghari Emameh, R. An Insight Overview on COVID-19 MRNA Vaccines: Advantageous, Pharmacology, Mechanism of Action, and Prospective Considerations. Int. Immunopharmacol. 2023, 117, 109934. [Google Scholar] [CrossRef] [PubMed]
- Hargrave, K.E.; MacLeod, M.K.L.; Worrell, J.C. Antigen Presenting Cells: Professionals, Amateurs, and Spectators in the “long Game” of Lung Immunity. Int. J. Biochem. Cell Biol. 2022, 153, 106331. [Google Scholar] [CrossRef] [PubMed]
- Romero-Ramirez, A.; Casas-Sánchez, A.; Autheman, D.; Duffy, C.W.; Brandt, C.; Clare, S.; Harcourt, K.; André, M.R.; de Almeida Castilho Neto, K.J.G.; Teixeira, M.M.G.; et al. Vivaxin Genes Encode Highly Immunogenic, Non-Variant Antigens on the Trypanosoma vivax Cell-Surface. PLoS Negl. Trop. Dis. 2022, 16, e0010791. [Google Scholar] [CrossRef] [PubMed]
- Magez, S.; Li, Z.; Nguyen, H.T.T.; Pinto Torres, J.E.; Van Wielendaele, P.; Radwanska, M.; Began, J.; Zoll, S.; Sterckx, Y.G.-J. The History of Anti-Trypanosome Vaccine Development Shows That Highly Immunogenic and Exposed Pathogen-Derived Antigens Are Not Necessarily Good Target Candidates: Enolase and ISG75 as Examples. Pathogens 2021, 10, 1050. [Google Scholar] [CrossRef] [PubMed]
- Maharana, B.R.; Sudhakar, N.R.; Jawalagatti, V.; Saravanan, B.C.; Blake, D.P.; Tewari, A.K. Evaluation of the Immunoprotective Potential of Recombinant Paraflagellar Rod Proteins of Trypanosoma Evansi in Mice. Vaccines 2020, 8, 84. [Google Scholar] [CrossRef]
- Tewari, A.K.; Kurup, S.P.; Baidya, S.; Barta, J.R.; Sharma, B. Protective Antibody and Cytokine Responses in Mice Following Immunization with Recombinant Beta-Tubulin and Subsequent Trypanosoma Evansi Challenge. Parasit. Vectors 2015, 8, 580. [Google Scholar] [CrossRef]
- Li, S.-Q.; Yang, W.-B.; Lun, Z.-R.; Ma, L.-J.; Xi, S.-M.; Chen, Q.-L.; Song, X.-W.; Kang, J.; Yang, L.-Z. Immunization with Recombinant Actin from Trypanosoma Evansi Induces Protective Immunity against T. evansi, T. equiperdum and T. b. brucei Infection. Parasitol. Res. 2009, 104, 429–435. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, W.; Qi, H. Recent Advancements in MRNA Vaccines: From Target Selection to Delivery Systems. Vaccines 2024, 12, 873. [Google Scholar] [CrossRef]
- Maldonado, E.; Morales-Pison, S.; Urbina, F.; Solari, A. Vaccine Design against Chagas Disease Focused on the Use of Nucleic Acids. Vaccines 2022, 10, 587. [Google Scholar] [CrossRef]
- Poveda, C.; Leão, A.C.; Mancino, C.; Taraballi, F.; Chen, Y.-L.; Adhikari, R.; Villar, M.J.; Kundu, R.; Nguyen, D.M.; Versteeg, L.; et al. Heterologous MRNA-Protein Vaccination with Tc24 Induces a Robust Cellular Immune Response against Trypanosoma Cruzi, Characterized by an Increased Level of Polyfunctional CD8(+) T-Cells. Curr. Res. Immunol. 2023, 4, 100066. [Google Scholar] [CrossRef]
- Travieso, T.; Li, J.; Mahesh, S.; Mello, J.D.F.R.E.; Blasi, M. The Use of Viral Vectors in Vaccine Development. npj Vaccines 2022, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Jenkin, D.; Ritchie, A.J.; Aboagye, J.; Fedosyuk, S.; Thorley, L.; Provstgaad-Morys, S.; Sanders, H.; Bellamy, D.; Makinson, R.; Xiang, Z.Q.; et al. Safety and Immunogenicity of a Simian-Adenovirus-Vectored Rabies Vaccine: An Open-Label, Non-Randomised, Dose-Escalation, First-in-Human, Single-Centre, Phase 1 Clinical Trial. Lancet Microbe 2022, 3, e663–e671. [Google Scholar] [CrossRef] [PubMed]
- Custers, J.; Kim, D.; Leyssen, M.; Gurwith, M.; Tomaka, F.; Robertson, J.; Heijnen, E.; Condit, R.; Shukarev, G.; Heerwegh, D.; et al. Vaccines Based on Replication Incompetent Ad26 Viral Vectors: Standardized Template with Key Considerations for a Risk/Benefit Assessment. Vaccine 2021, 39, 3081–3101. [Google Scholar] [CrossRef] [PubMed]
- Jawalagatti, V.; Kirthika, P.; Singh, P.; Vinodhkumar, O.R.; Buddhi Chandrasekaran, S.; Chittlangia, R.K.; Tewari, A.K. Expression Kinetics of Cytokines and the Humoral Antibody Response Concerning Short-Term Protection Induced by Radiation-Attenuated Trypanosoma Evansi in Bovine Calves. Vaccine 2023, 41, 1668–1678. [Google Scholar] [CrossRef] [PubMed]
- de A Burle-Caldas, G.; Dos Santos, N.S.A.; de Castro, J.T.; Mugge, F.L.B.; Grazielle-Silva, V.; Oliveira, A.E.R.; Pereira, M.C.A.; Reis-Cunha, J.L.; Dos Santos, A.C.; Gomes, D.A.; et al. Disruption of Active Trans-Sialidase Genes Impairs Egress from Mammalian Host Cells and Generates Highly Attenuated Trypanosoma Cruzi Parasites. MBio 2022, 13, e0347821. [Google Scholar] [CrossRef]
- Diawara, H.; Healy, S.A.; Mwakingwe-Omari, A.; Issiaka, D.; Diallo, A.; Traore, S.; Soumbounou, I.H.; Gaoussou, S.; Zaidi, I.; Mahamar, A.; et al. Safety and Efficacy of PfSPZ Vaccine against Malaria in Healthy Adults and Women Anticipating Pregnancy in Mali: Two Randomised, Double-Blind, Placebo-Controlled, Phase 1 and 2 Trials. Lancet Infect. Dis. 2024. [Google Scholar] [CrossRef]
- Rooholamini, Z.; Dianat-Moghadam, H.; Esmaeilifallah, M.; Khanahmad, H. From Classical Approaches to New Developments in Genetic Engineering of Live Attenuated Vaccine against Cutaneous Leishmaniasis: Potential and Immunization. Front. Public Health 2024, 12, 1382996. [Google Scholar] [CrossRef]
- Abdi Ghavidel, A.; Aghamiri, S.; Raee, P.; Mohammadi-Yeganeh, S.; Noori, E.; Bandehpour, M.; Kazemi, B.; Jajarmi, V. Recent Advances in CRISPR/Cas9-Mediated Genome Editing in Leishmania Strains. Acta Parasitol. 2024, 69, 121–134. [Google Scholar] [CrossRef]
- Minet, C.; Chantal, I.; Berthier, D. Recent Advances in Genome Editing of Bloodstream Forms of Trypanosoma Congolense Using CRISPR-Cas9 Ribonucleoproteins: Proof of Concept. Exp. Parasitol. 2023, 252, 108589. [Google Scholar] [CrossRef]
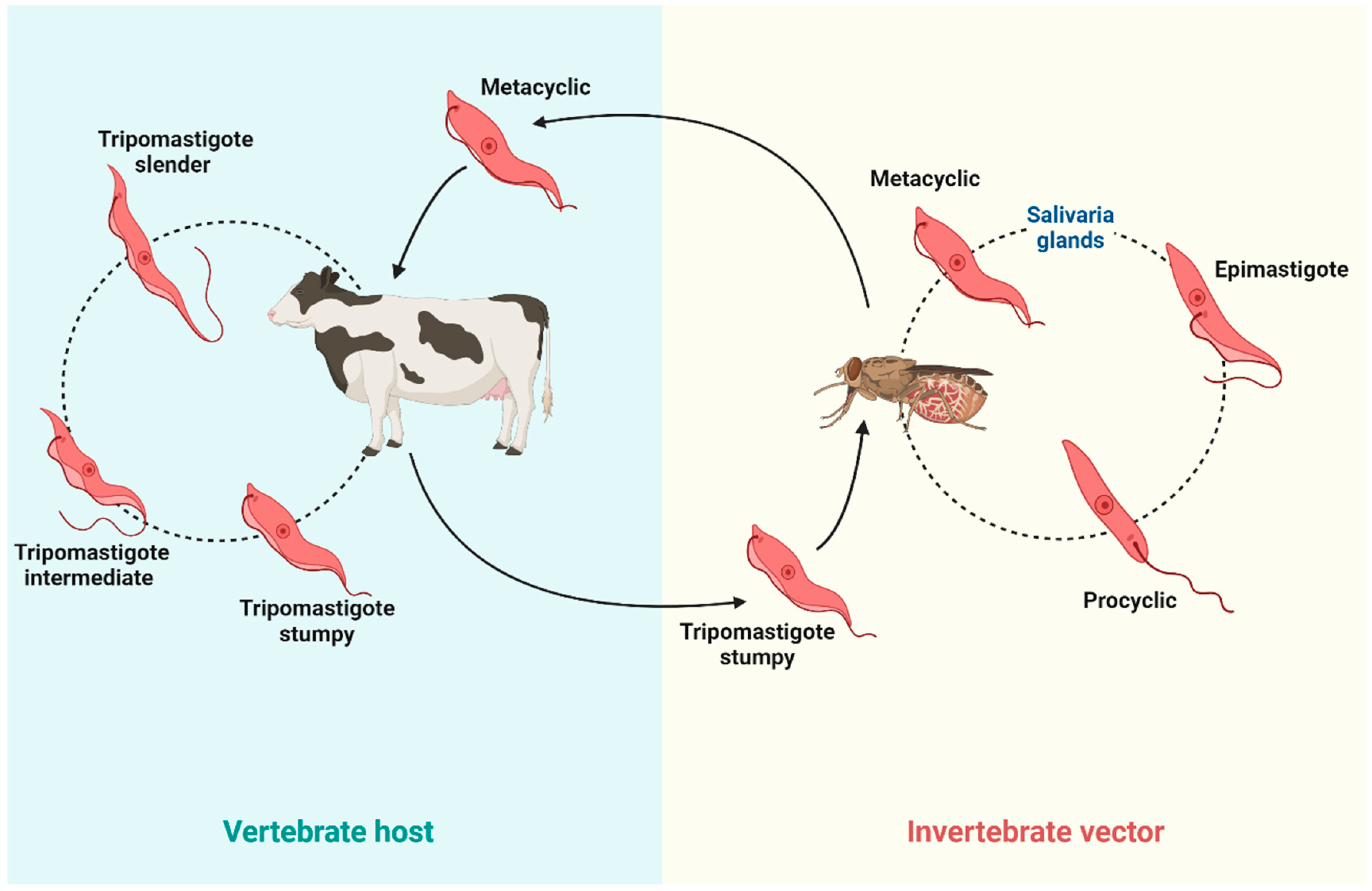
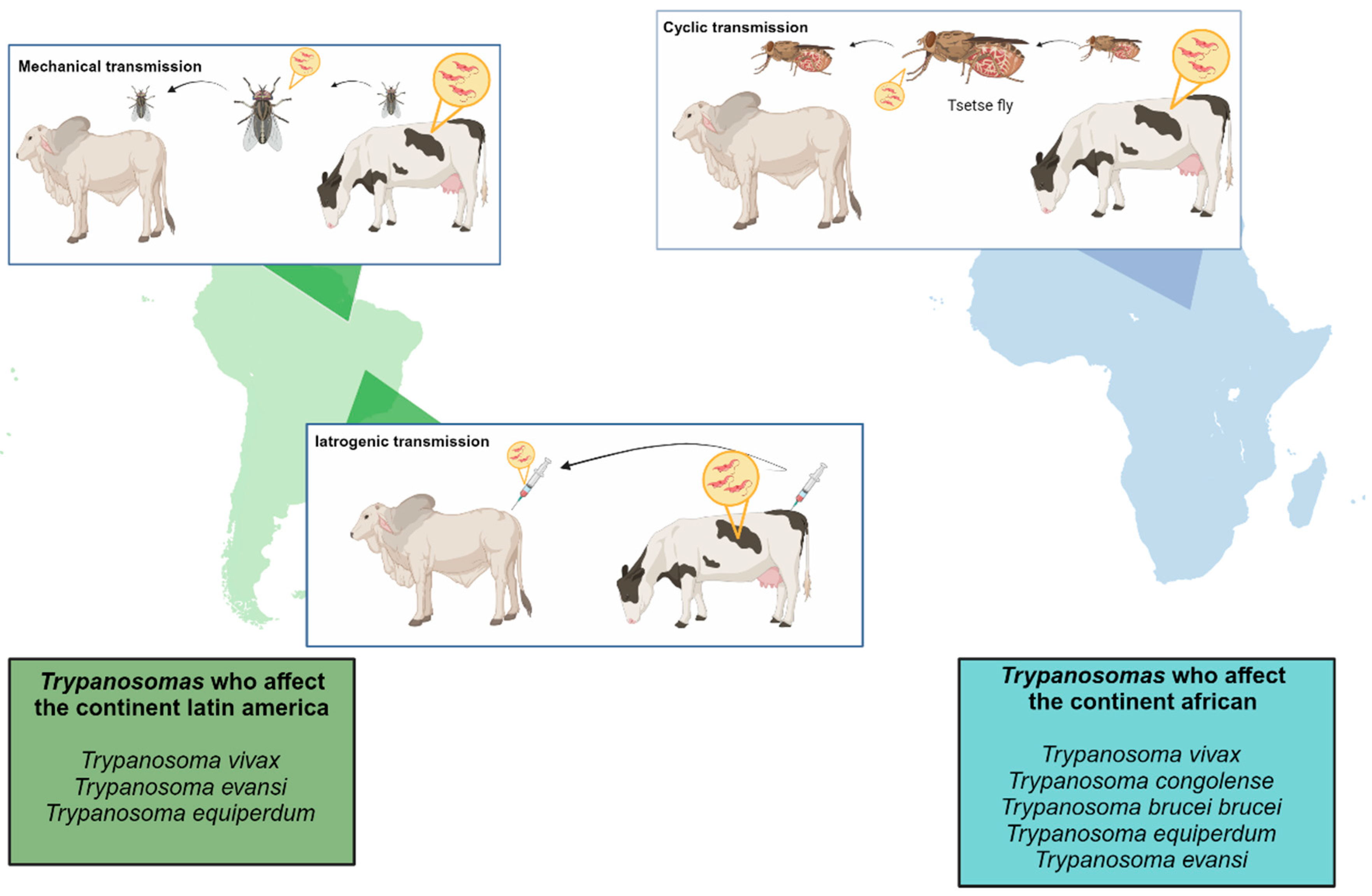

| Trypanosoma | Main host | Other hosts |
|---|---|---|
| Trypanosoma vivax | Bovine | Sheep Goat Equine Pig |
| Trypanosoma congolense | Bovine | Sheep Goat Equine Pig |
| Trypanosoma brucei brucei | Bovine | Sheep Goat Pig |
| Trypanosoma evansi | Equine | Bovine Camelids Goat Sheep Pig |
| Trypanosoma equiperdum | Equine | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, S.H.; Alves, F.P.; Teixeira, S.M.R. Animal Trypanosomiasis: Challenges and Prospects for New Vaccination Strategies. Microorganisms 2024, 12, 2575. https://doi.org/10.3390/microorganisms12122575
Pereira SH, Alves FP, Teixeira SMR. Animal Trypanosomiasis: Challenges and Prospects for New Vaccination Strategies. Microorganisms. 2024; 12(12):2575. https://doi.org/10.3390/microorganisms12122575
Chicago/Turabian StylePereira, Samille Henriques, Felipe Paladino Alves, and Santuza Maria Ribeiro Teixeira. 2024. "Animal Trypanosomiasis: Challenges and Prospects for New Vaccination Strategies" Microorganisms 12, no. 12: 2575. https://doi.org/10.3390/microorganisms12122575
APA StylePereira, S. H., Alves, F. P., & Teixeira, S. M. R. (2024). Animal Trypanosomiasis: Challenges and Prospects for New Vaccination Strategies. Microorganisms, 12(12), 2575. https://doi.org/10.3390/microorganisms12122575






