The Clinical Anatomy of SARS-CoV-2 Variants of Concern in Central Greece During October 2020–July 2022
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Laboratory Analysis
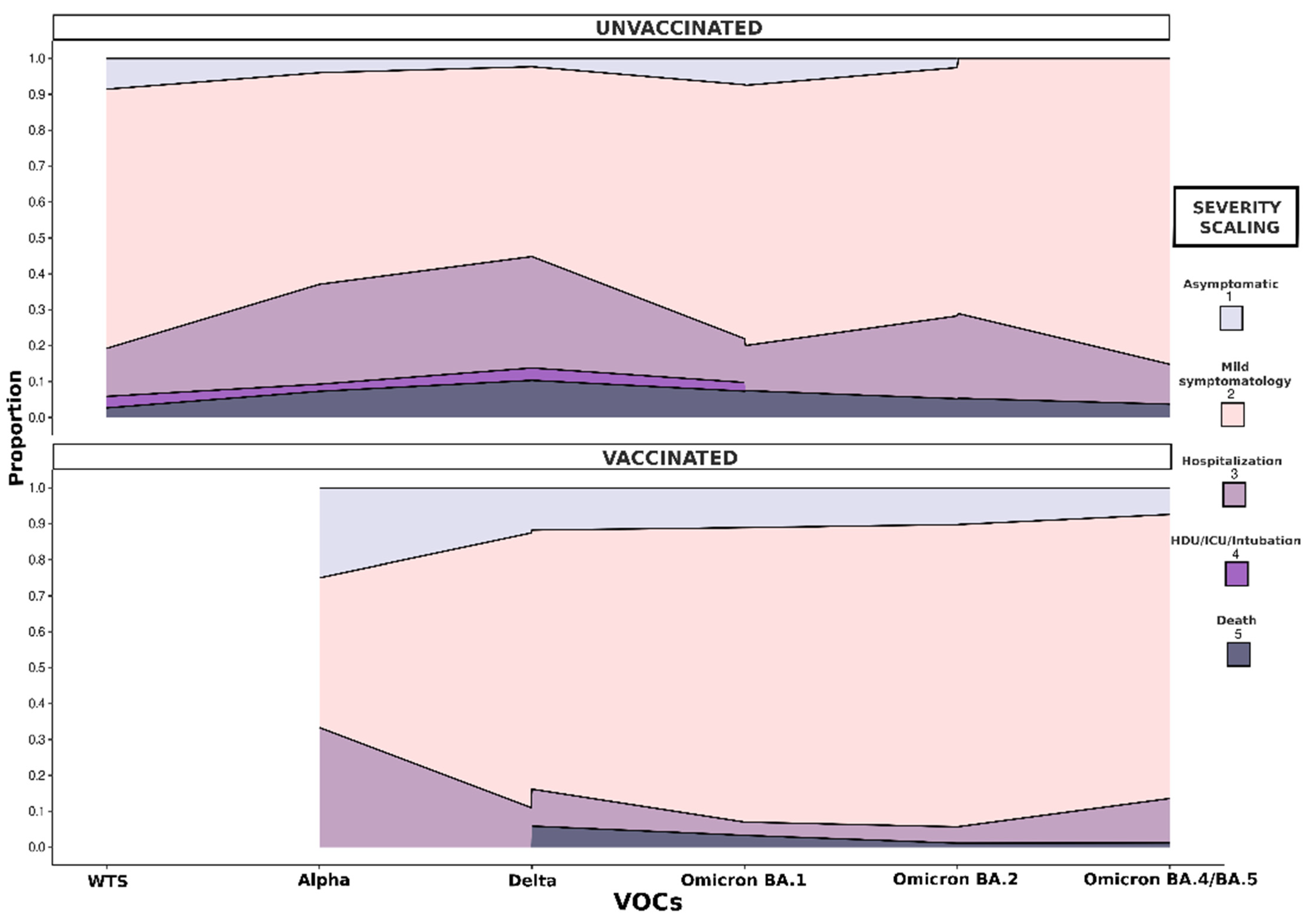
2.3. Classification of COVID-19 Severity and Vaccination Status
2.4. Statistical Analysis
2.5. Ethical Statement
3. Results
3.1. Characteristics and Clinical Image of Participants with Laboratory-Confirmed SARS-CoV-2 Infection
3.1.1. Demographic Characteristics of Children (<18 Years Old) with Laboratory-Confirmed SARS-CoV-2 Infection
3.1.2. Characteristics of Symptoms Due to COVID-19 in Children (<18 Years Old)
3.1.3. Demographic Characteristics of Adults (≥18 Years Old) with Laboratory-Confirmed SARS-CoV-2 Infection
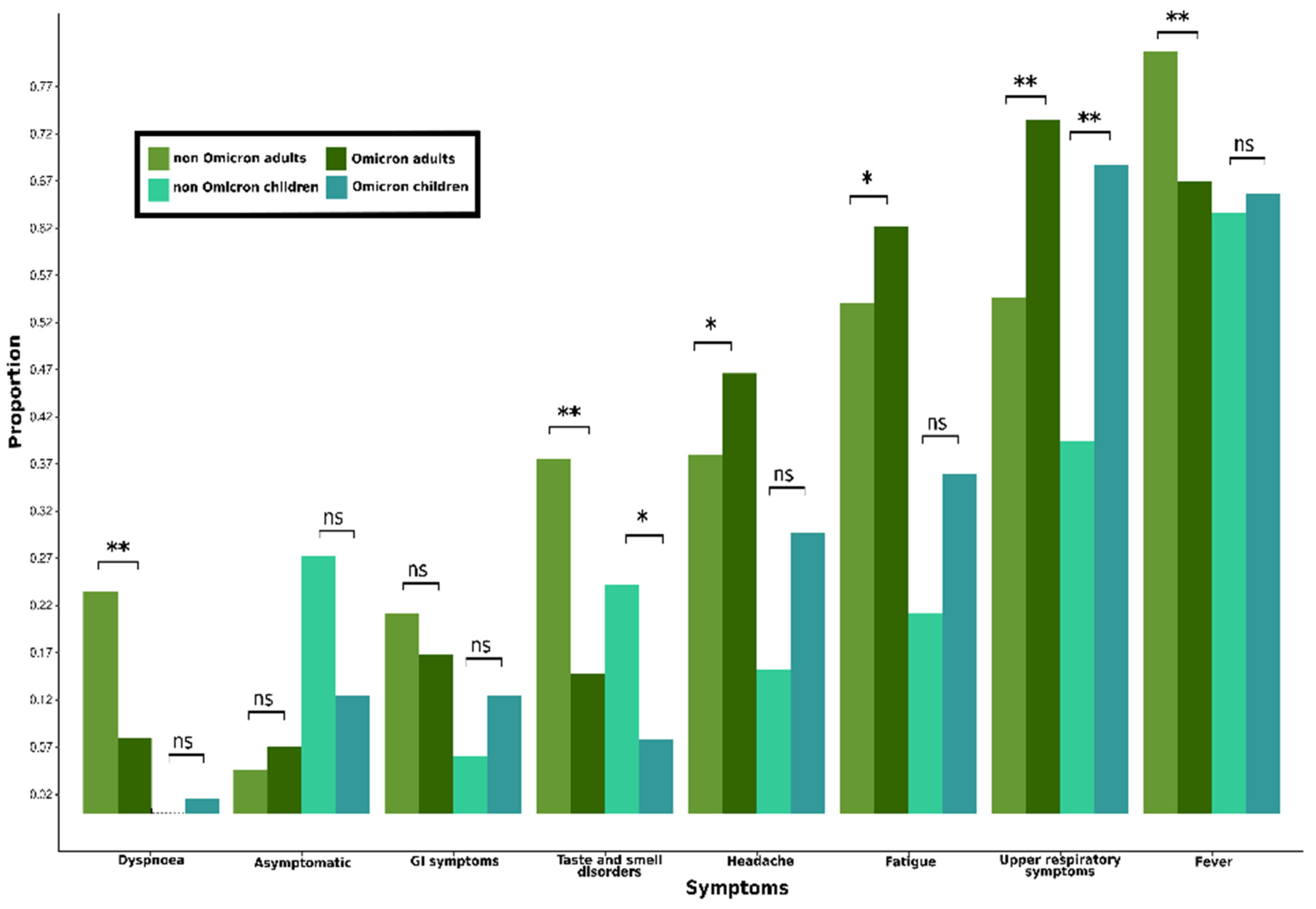
3.1.4. Characteristics of Symptoms Due to COVID-19 in Adults (≥18 Years Old)
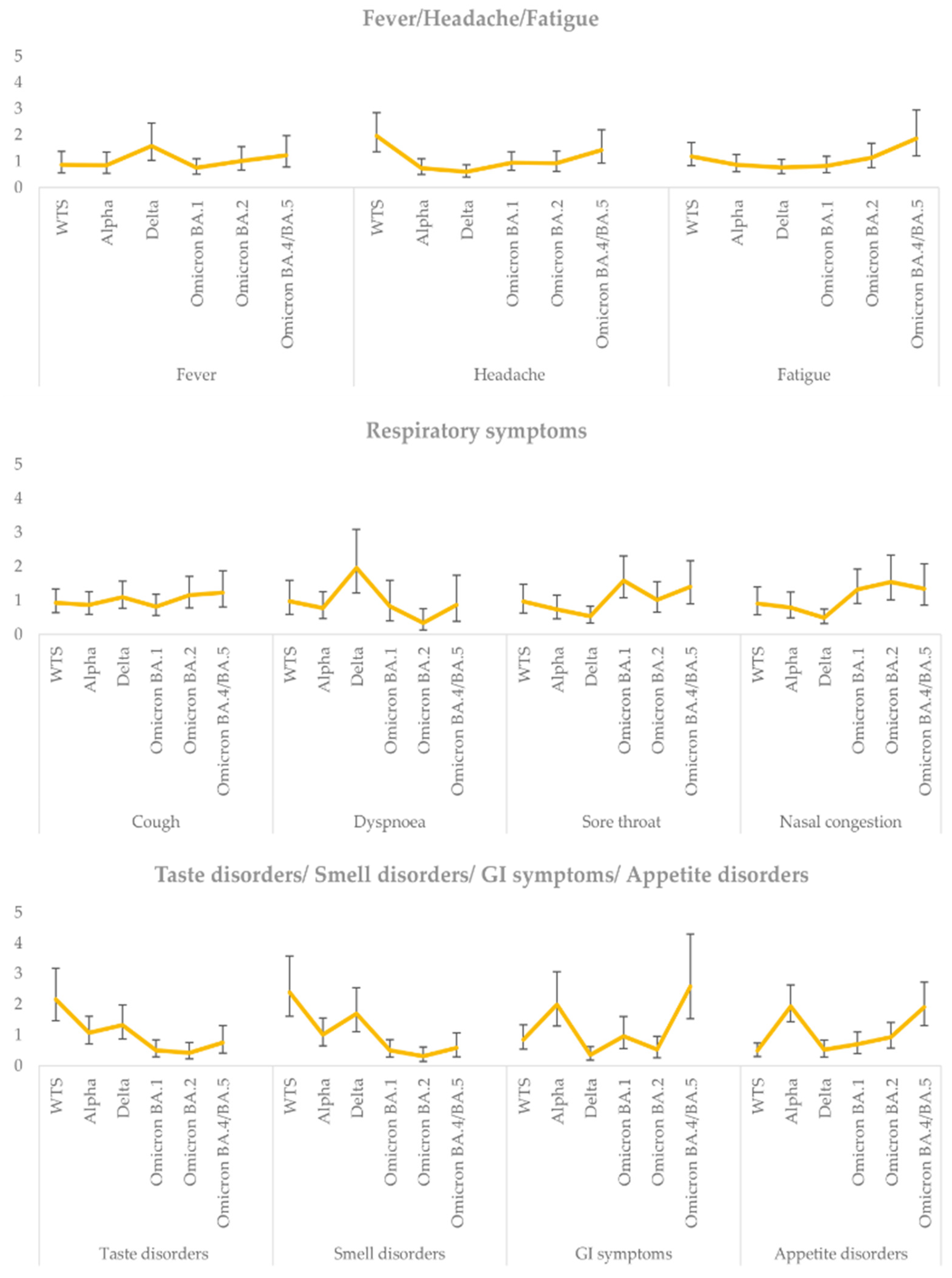
3.2. Total Findings Regarding Clinical Manifestations in the Entire Study Population
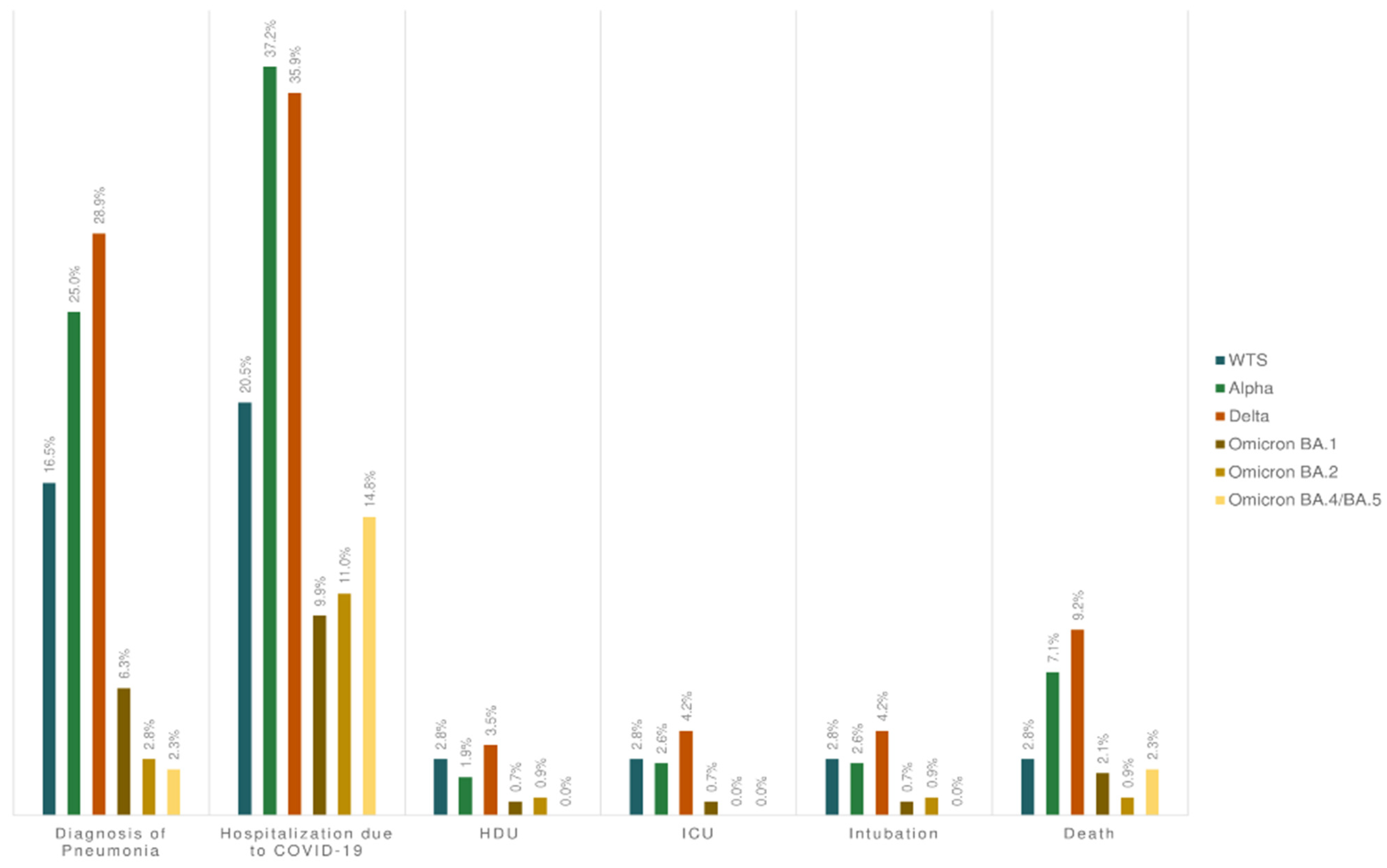
3.3. Severity of COVID-19 in Adults (≥18 Years Old)
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aleem, A.; Samad, A.B.A.; Vaqar, S. Emerging Variants of SARS-CoV-2 and Novel Therapeutics Against Corona-virus (COVID-19). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://pubmed.ncbi.nlm.nih.gov/34033342/ (accessed on 18 April 2024).
- World Health Organization (WHO). Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed on 18 April 2024).
- Duong, D. Alpha, Beta, Delta, Gamma: What’s Important to Know about SARS-CoV-2 Variants of Concern? Can. Med. Assoc. J. 2021, 193, E1059–E1060. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Enhancing Response to Omicron SARS-CoV-2 Variant: Technical Brief and Priority Actions for Member States. Available online: https://www.who.int/publications/m/item/enhancing-readiness-for-omicron-(b.1.1.529)-technical-brief-and-priority-actions-for-member-states (accessed on 18 April 2024).
- McMillen, T.; Jani, K.; Robilotti, E.V.; Kamboj, M.; Babady, N.E. The Spike Gene Target Failure (SGTF) Genomic Signature Is Highly Accurate for the Identification of Alpha and Omicron SARS-CoV-2 Variants. Sci. Rep. 2022, 12, 18968. [Google Scholar] [CrossRef]
- Walker, A.S.; Vihta, K.D.; Gethings, O.; Pritchard, E.; Jones, J.; House, T.; Bell, I.; Bell, J.I.; Newton, J.N.; Farrar, J.; et al. Tracking the Emergence of SARS-CoV-2 Alpha Variant in the United Kingdom. N. Engl. J. Med. 2021, 385, 2582–2585. [Google Scholar] [CrossRef]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 10 May 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 3rd ed.; Springer: New York, NY, USA, 2016; Available online: https://ggplot2-book.org/ (accessed on 10 May 2024).
- Dethioux, L.; Dauby, N.; Montesinos, I.; Rebuffat, E.; Hainaut, M. SARS-CoV-2 Seroprevalence in Children and Their Family Members, July–October 2020, Brussels. Eur. J. Pediatr. 2022, 181, 1009–1016. [Google Scholar] [CrossRef]
- Sumner, M.W.; Xie, J.; Zemek, R.; Winston, K.; Freire, G.; Burstein, B.; Kam, A.; Emsley, J.; Gravel, J.; Porter, R.; et al. Comparison of Symptoms Associated with SARS-CoV-2 Variants among Children in Canada. JAMA Netw. Open 2023, 6, e232328. [Google Scholar] [CrossRef]
- Buitrago-Garcia, D.; Egli-Gany, D.; Counotte, M.J.; Hossmann, S.; Imeri, H.; Ipekci, A.M.; Salanti, G.; Low, N. Occurrence and Transmission Potential of Asymptomatic and Presymptomatic SARS-CoV-2 Infections: A Living Systematic Review and Meta-Analysis. PLoS Med. 2020, 17, e1003346. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Liu, J.; Liu, Q.; Kang, L.; Liu, R.; Jing, W.; Wu, Y.; Liu, M. Global Percentage of Asymptomatic SARS-CoV-2 Infections among the Tested Population and Individuals with Confirmed COVID-19 Diagnosis: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2021, 4, e2137257. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Ang, J.Y. COVID-19 Infection in Children: Diagnosis and Management. Curr. Infect. Dis. Rep. 2022, 24, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. COVID-19 in Children, Pregnancy and Neonates: A Review of Epidemiologic and Clinical Features. Pediatr. Infect. Dis. J. 2020, 39, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Flisiak, R.; Rzymski, P.; Zarębska-Michaluk, D.; Ciechanowski, P.; Dobrowolska, K.; Rogalska, M.; Jaroszewicz, J.; Szymanek-Pasternak, A.; Rorat, M.; Kozielewicz, D.; et al. Variability in the Clinical Course of COVID-19 in a Retrospective Analysis of a Large Real-World Database. Viruses 2023, 15, 149. [Google Scholar] [CrossRef] [PubMed]
- DeWitt, M.E.; Tjaden, A.H.; Herrington, D.; Schieffelin, J.; Gibbs, M.; Weintraub, W.S.; Sanders, J.W.; Edelstein, S.L. COVID-19 Symptoms by Variant Period in the North Carolina COVID-19 Community Research Partnership, North Carolina, USA. Emerg. Infect. Dis. 2023, 29, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Brewster, R.C.L.; Parsons, C.; Laird-Gion, J.; Hilker, S.; Irwin, M.; Sommerschield, A.; A Michaelis, K.; Lam, M.; Parsons, A.; Mansbach, J.M. COVID-19-Associated Croup in Children. Pediatrics 2022, 149, e2022056492. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Valdes, A.M.; Polidori, L.; Antonelli, M.; Penamakuri, S.; Nogal, A.; Louca, P.; May, A.; Figueiredo, J.C.; Hu, C.; et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: A prospective observational study from the ZOE COVID Study. Lancet 2022, 399, 1618–1624. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.P.Y.; Ho, J.C.W.; Cheung, M.C.; Ng, K.-C.; Ching, R.H.H.; Lai, K.-L.; Kam, T.T.; Gu, H.; Sit, K.-Y.; Hsin, M.K.Y.; et al. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature 2022, 603, 715–720. [Google Scholar] [CrossRef]
- Meng, B.; Abdullahi, A.; Ferreira, I.A.T.M.; Goonawardane, N.; Saito, A.; Kimura, I.; Yamasoba, D.; Gerber, P.P.; Fatihi, S.; Rathore, S.; et al. Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity. Nature 2022, 603, 706–714. [Google Scholar] [CrossRef]
- Hui, K.P.Y.; Ng, K.C.; Ho, J.C.W.; Yeung, H.-W.; Ching, R.H.; Gu, H.; Chung, J.C.; Chow, V.L.; Sit, K.-Y.; Hsin, M.K.; et al. Replication of SARS-CoV-2 Omicron BA.2 variant in ex vivo cultures of the human upper and lower respiratory tract. EBioMedicine 2022, 83, 104232. [Google Scholar] [CrossRef] [PubMed]
- Coelho, D.H.; Reiter, E.R.; French, E.; Costanzo, R.M. Decreasing Incidence of Chemosensory Changes by COVID-19 Variant. Otolaryngol. Head Neck Surg. 2023, 168, 704–706. [Google Scholar] [CrossRef] [PubMed]
- Westerhof, I.; De Hoog, M.; Ieven, M.; Lammens, C.; van Beek, J.; Rozhnova, G.; Eggink, D.; Euser, S.; Wildenbeest, J.; Duijts, L.; et al. The impact of variant and vaccination on SARS-CoV-2 symptomatology: Three prospective household cohorts. Int. J. Infect. Dis. 2023, 128, 140–147. [Google Scholar] [CrossRef]
- Iijima, H.; Kubota, M.; Ogimi, C. Clinical characteristics of pediatric patients with COVID-19 between Omicron era vs. pre-Omicron era. J. Infect. Chemother. 2022, 28, 1501–1505. [Google Scholar] [CrossRef]
- Tian, Y.; Rong, L.; Nian, W.; He, Y. Review article: Gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment. Pharmacol. Ther. 2020, 51, 843–851. [Google Scholar] [CrossRef]
- Shi, Y.; Mei, Z.; Wang, H. Characteristics and implications of Omicron variant associated digestive system infections—Correspondence. Int. J. Surg. 2022, 104, 106750. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.C.; Gottlieb, M.; Montoy, J.C.C.; Rodriguez, R.; Yu, H.; Spatz, E.S.; Chandler, C.W.; Elmore, J.G.; A Hannikainen, P.; Chang, A.M.; et al. Association Between SARS-CoV-2 Variants and Frequency of Acute Symptoms: Analysis of a Multi-institutional Prospective Cohort Study—20 December 2020–20 June 2022. Open Forum Infect. Dis. 2023, 10, ofad275. [Google Scholar] [CrossRef]
- Jin, S.; Lu, X.; Xu, C. COVID-19 induces gastrointestinal symptoms and affects patients’ prognosis. J. Int. Med. Res. 2022, 50, 3000605221129543. [Google Scholar] [CrossRef]
- Galanopoulos, M.; Gkeros, F.; Doukatas, A.; Karianakis, G.; Pontas, C.; Tsoukalas, N.; Viazis, N.; Liatsos, C.; Mantzaris, G.J. COVID-19 pandemic: Pathophysiology and manifestations from the gastrointestinal tract. World J. Gastroenterol. 2020, 26, 4579–4588. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology 2020, 159, 944–955.e8. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.V.; Bhattarai, S.; Rojas-Correa, M.; Purkayastha, A.; Holler, D.; Da Qu, M.; Mitchell, W.G.; Yang, J.; Fountain, S.; Zeamer, A.; et al. The intestinal and oral microbiomes are robust predictors of COVID-19 severity the main predictor of COVID-19-related fatality. medRxiv 2021. [Google Scholar] [CrossRef]
- The COVID-19 Investigation Team. Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID-19) in the United States. Nat. Med. 2020, 26, 861–868. [Google Scholar] [CrossRef]
- Greene, S.K.; Levin-Rector, A.; Kyaw, N.T.T.; Luoma, E.; Amin, H.; McGibbon, E.; Mathes, R.W.; Ahuja, S.D. Comparative hospitalization risk for SARS-CoV-2 Omicron and Delta variant infections, by variant predominance periods and patient-level sequencing results, New York City, August 2021–January 2022. Influenza Other Respir. Viruses 2023, 17, e13062. [Google Scholar] [CrossRef]
- Nyberg, T.; Ferguson, N.M.; Nash, S.G.; Webster, H.H.; Flaxman, S.; Andrews, N.; Hinsley, W.; Bernal, J.L.; Kall, M.; Bhatt, S.; et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: A cohort study. Lancet 2022, 399, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Paredes, M.I.; Lunn, S.M.; Famulare, M.; A Frisbie, L.; Painter, I.; Burstein, R.; Roychoudhury, P.; Xie, H.; Bakhash, S.A.M.; Perez, R.; et al. Associations between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants and risk of coronavirus disease 2019 (COVID-19) hospitalization among confirmed cases in Washington State: A retrospective cohort study. Clin. Infect. Dis. 2022, 75, e536–e544. [Google Scholar] [CrossRef] [PubMed]
- Escalera, A.; Gonzalez-Reiche, A.S.; Aslam, S.; Mena, I.; Laporte, M.; Pearl, R.L.; Fossati, A.; Rathnasinghe, R.; Alshammary, H.; van de Guchte, A.; et al. Mutations in SARS-CoV-2 variants of concern link to increased spike cleavage and virus transmission. Cell Host Microbe 2022, 30, 373–387.e7. [Google Scholar] [CrossRef] [PubMed]
- Zahradník, J.; Marciano, S.; Shemesh, M.; Zoler, E.; Harari, D.; Chiaravalli, J.; Meyer, B.; Rudich, Y.; Li, C.; Marton, I.; et al. SARS-CoV-2 variant prediction and antiviral drug design are enabled by RBD in vitro evolution. Nat. Microbiol. 2021, 6, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Uraki, R.; Kiso, M.; Iida, S.; Imai, M.; Takashita, E.; Kuroda, M.; Halfmann, P.J.; Loeber, S.; Maemura, T.; Yamayoshi, S.; et al. Characterization and antiviral susceptibility of SARS-CoV-2 Omicron BA.2. Nature 2022, 607, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Yamasoba, D.; Kimura, I.; Wang, L.; Kishimoto, M.; Ito, J.; Morioka, Y.; Nao, N.; Nasser, H.; Uriu, K.; et al. Attenuated Fusogenicity and Pathogenicity of SARS-CoV-2 Omicron Variant. Nature 2022, 603, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R. SARS-CoV-2 Omicron Spike Mediated Immune Escape and Tropism Shift. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Vieillard-Baron, A.; Flicoteaux, R.; Salmona, M.; Annane, D.; Ayed, S.; Azoulay, E.; Bellaiche, R.; Beloucif, S.; Berti, E.; Bertier, A.; et al. Epidemiological Characteristics and Severity of Omicron Variant Cases in the APHP Critical Care Units. medRxiv 2022. [Google Scholar] [CrossRef]
- Relan, P.; Motaze, N.V.; Kothari, K.; Askie, L.; Waroux, O.L.P.d.; Van Kerkhove, M.D.; Diaz, J.; Vijayaraghavan, B.K.T. Severity and Outcomes of Omicron Variant of SARS-CoV-2 Compared to Delta Variant and Severity of Omicron Sublineages: A Systematic Review and Metanalysis. BMJ Glob. Health 2023, 8, e012328. [Google Scholar] [CrossRef]
- Bester, J.C. A Clinician’s Obligation to Be Vaccinated: Four Arguments that Establish a Duty for Healthcare Professionals to Be Vaccinated Against COVID-19. J. Bioethical Inq. 2022, 19, 451–465. [Google Scholar] [CrossRef]
- Matsumura, Y.; Yamamoto, M.; Shinohara, K.; Tsuchido, Y.; Yukawa, S.; Noguchi, T.; Ikeda, T.; Nagao, M. High Mortality and Morbidity among Vaccinated Residents Infected with the SARS-CoV-2 Omicron Variant during an Outbreak in a Nursing Home in Kyoto City, Japan. Am. J. Infect. Control 2023, 51, 800–806. [Google Scholar] [CrossRef]
- Ward, T.; Glaser, A.; Overton, C.E.; Carpenter, B.; Gent, N.; Seale, A.C. Replacement Dynamics and the Pathogenesis of the Alpha, Delta and Omicron Variants of SARS-CoV-2. Epidemiol. Infect. 2023, 151, e32. [Google Scholar] [CrossRef] [PubMed]
- Tabatabai, M.; Juarez, P.D.; Matthews-Juarez, P.; Wilus, D.M.; Ramesh, A.; Alcendor, D.J.; Tabatabai, N.; Singh, K.P. An Analysis of COVID-19 Mortality during the Dominancy of Alpha, Delta, and Omicron in the USA. J. Prim. Care Community Health 2023, 14, 21501319231170164. [Google Scholar] [CrossRef] [PubMed]
- Malli, F.; Lampropoulos, I.C.; Perlepe, G.; Papagiannis, D.; Gourgoulianis, K.I. Analysis of SARS-CoV-2 Cases, COVID-19 Outcomes and Vaccinations, during the Different SARS-CoV-2 Variants in Greece. Vaccines 2023, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.A.; Olsen, R.J.; Long, S.W.; Snehal, R.; Davis, J.J.; Saavedra, M.O.; Reppond, K.; Shyer, M.N.; Cambric, J.; Gadd, R.; et al. Signals of Significantly Increased Vaccine Breakthrough, Decreased Hospitalization Rates, and Less Severe Disease in Patients with Coronavirus Disease 2019 Caused by the Omicron Variant of Severe Acute Respiratory Syndrome Coronavirus 2 in Houston, Texas. Am. J. Pathol. 2022, 192, 642–652. [Google Scholar] [CrossRef]
- Johns Hopkins University & Medicine—Coronavirus Resource Center. Comparing Cases, Deaths, and Hospitalizations Indicates Omicron Less Deadly. Available online: https://coronavirus.jhu.edu/pandemic-data-initiative/data-outlook/comparing-cases-deaths-and-hospitalizations-indicates-omicron-less-deadly (accessed on 19 April 2024).
| Participants <18 Years Old | Total | Non-Omicron Variants (Total) | Non-Omicron Variants | Omicron Variants (Total) | Omicron Variants | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| WTS 3 | Alpha | Delta | Omicron BA.1 | Omicron BA.2 | Omicron BA.4/BA.5 | |||||
| (N = 100) | (N = 34) | (N = 11) | (N = 8) | (N = 15) | (N = 66) | (N = 30) | (N = 16) | (N = 20) | ||
| Severity | ||||||||||
| 0 | 18 (18.0%) | 9 (26.5%) | 5 (45.5%) | 2 (25.0%) | 2 (13.3%) | 9 (13.6%) | 7 (23.3%) | 1 (6.3%) | 1 (5.0%) | N/A 4 |
| 1 | 75 (75.0%) | 22 (64.7%) | 6 (54.5%) | 4 (50.0%) | 12 (80.0%) | 53 80.3%) | 23 (76.7%) | 13 (81.3%) | 17 (85.0%) | |
| 2 | 7 (7.0%) | 3 (8.8%) | 0 (0%) | 2 (25.0%) | 1 (6.7%) | 4 (6.1%) | 0 (0%) | 2 (12.5%) | 2 (10.0%) | |
| 3 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Symptoms | 82 (82.0%) | 25 (73.5%) | 6 (54.5%) | 6 (75.0%) | 13 (86.7%) | 57 (86.4%) | 23 (76.7%) | 15 (93.8%) | 19 (95.0%) | 0.039 |
| Duration of symptoms (days) | 2.00 (2.00) | 2.5 (5.5) | 2.00 (0) | 2.00 (0) | 5.00 (4.00) | 2.00 (2.00) | 2.00 (2.00) | 2.00 (1.00) | 3.00 (2.00) | 0.060 |
| Cough | 36 (36.0%) | 10 (29.4%) | 0 (0%) | 1 (12.5%) | 9 (60.0%) | 26 (39.4%) | 8 (26.7%) | 8 (50.0%) | 10 (50.0%) | 0.007 |
| Duration of cough (days) | 4.00 (4.25) | 5.5 (4.75) | - | 10.0 (0) | 4.00 (5.00) | 4.00 (2.75) | 3.00 (2.50) | 3.50 (1.00) | 6.50 (3.00) | 0.105 |
| Fever | 62 (62.0%) | 21 (61.8%) | 5 (45.5%) | 4 (50.0%) | 12 (80.0%) | 41 (62.1%) | 14 (46.7%) | 14 (87.5%) | 13 (65.0%) | 0.046 |
| Maximum value (°C) | 38.25 (0.68) | 38.00 (0.25) | 38.2 (0.500) | 38.0 (0.150) | 38.0 (0.825) | 38.50 (0.70) | 38.2 (0.500) | 38.6 (0.875) | 38.5 (0.600) | 0.626 |
| Duration of fever (days) | 2.00 (1.00) | 2.00 (1.00) | 2.00 (0) | 2.00 (1.50) | 3.00 (3.00) | 2.00 (1.00) | 1.50 (1.00) | 2.00 (2.00) | 2.00 (1.00) | 0.020 |
| Sore throat (pharyngula) | 21 (21.0%) | 2 (5.9%) | 0 (0%) | 0 (0%) | 2 (13.3%) | 19 (28.8%) | 9 (30.0%) | 2 (12.5%) | 8 (40.0%) | 0.034 |
| Nasal congestion/discharge | 37 (37.0%) | 6 (17.6%) | 1 (9.1%) | 1 (12.5%) | 4 (26.7%) | 31 (47.0%) | 13 (43.3%) | 7 (43.8%) | 11 (55.0%) | 0.074 |
| Duration of nasal congestion (days) | 3.00 (3.00) | 2.00 (2.25) | 5.00 (0) | 2.00 (0) | 2.00 (1.25) | 4.00 (3.00) | 2.00 (3.00) | 4.00 (1.50) | 4.00 (3.00) | 0.247 |
| Headache | 24 (24.0%) | 5 (14.7%) | 1 (9.1%) | 0 (0%) | 4 (26.7%) | 19 (28.8%) | 8 (26.7%) | 6 (37.5%) | 5 (25.0%) | 0.342 |
| Headache intensity | ||||||||||
| Severe | 10 (10.0%) | 5 (14.7%) | 1 (9.1%) | 0 (0%) | 4 (26.7%) | 5 (76%) | 2 (6.7%) | 2 (12.5%) | 1 (5.0%) | 0.144 |
| Mild | 14 (14.0%) | 0 (0.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 14 (21.2%) | 6 (20.0%) | 4 (25.0%) | 4 (20.0%) | |
| Fatigue | 30 (30.0%) | 7 (20.6%) | 3 (27.3%) | 0 (0%) | 4 (26.7%) | 23 (34.8%) | 13 (43.3%) | 5 (31.3%) | 5 (25.0%) | 0.275 |
| Taste disorders | 11 (11.0%) | 7 (20.6%) | 1 (9.1%) | 1 (12.5%) | 5 (33.3%) | 4 (6.1%) | 2 (6.7%) | 2 (12.5%) | 0 (0%) | 0.056 |
| Duration of taste disorders | 4.00 (4.00) | 7.00 (5.50) | 2.00 (0) | 4.00 (0) | 7.00 (3.00) | 3.50 (1.00) | 3.00 (0) | 4.00 (0) | - | 0.383 |
| Smell disorders | 9 (9.0%) | 7 (20.6%) | 2 (18.2%) | 0 (0%) | 5 (33.3%) | 2 (3.0%) | 1 (3.3%) | 1 (6.3%) | 0 (0%) | 0.007 |
| Duration of smell disorders (days) | 7.00 (8.00) | 7.00 (7.50) | 16.0 (14.0) | - | 7.00 (3.00) | 2.00 (1.00) | 1.00 (0) | 3.00 (0) | - | 0.525 |
| GI 5 symptoms | 8 (8.0%) | 1 (2.9%) | 0 (0%) | 0 (0%) | 1 (6.7%) | 7 (10.6%) | 3 (10.0%) | 1 (6.3%) | 3 (15.0%) | 0.662 |
| Appetite disorders | ||||||||||
| No | 96 (96.0%) | 32 (94.1%) | 11 (100%) | 7 (87.5%) | 14 (93.3%) | 64 (97.0%) | 30 (100%) | 16 (100%) | 18 (90.0%) | 0.371 |
| Increased | 2 (2.0%) | 1 (2.9%) | 0 (0%) | 1 (12.5%) | 0 (0%) | 1 (1.5%) | 0 (0%) | 0 (0%) | 1 (5.0%) | |
| Decreased | 2 (2.0%) | 1 (2.9%) | 0 (0%) | 0 (0%) | 1 (6.7%) | 1 (1.5%) | 0 (0%) | 0 (0%) | 1 (5.0%) | |
| Dyspnea | 1 | 0 (0.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1.5%) | 0 (0%) | 0 (0%) | 1 (5.0%) | 0.544 |
| Participants ≥18 Years Old | Total | Non-Omicron Variants (Total) | Non-Omicron Variants | Omicron Variants (Total) | Omicron Variants | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| WTS 3 | Alpha | Delta | Omicron BA.1 | Omicron BA.2 | Omicron BA.4/BA.5 | |||||
| (N = 813) | (N = 474) | (N = 176) | (N = 156) | (N = 142) | (N = 339) | (N = 142) | (N = 109) | (N = 88) | ||
| Severity | ||||||||||
| 0 | 51 (6.3%) | 26 (5.5%) | 11 (6.3%) | 7 (4.5%) | 8 (5.6%) | 25 (7.4%) | 11 (7.7%) | 9 (8.3%) | 5 (5.7%) | <0.001 |
| 1 | 577 (71.0%) | 302 (63.7%) | 129 (73.3%) | 90 (57.7%) | 83 (58.5%) | 275 (81.1%) | 117 (82.4%) | 88 (80.7%) | 70 (79.5%) | |
| 2 | 148 (18.2%) | 115 (24.3%) | 30 (17.0%) | 47 (30.1%) | 38 (26.8%) | 33 (9.7%) | 11 (7.7%) | 11 (10.1%) | 11 (12.5%) | |
| 3 | 37 (4.6%) | 31 (6.5%) | 6 (3.4%) | 12 (7.7%) | 13 (9.2%) | 6 (1.8%) | 3 (2.1%) | 1 (0.9%) | 2 (2.3%) | |
| Symptoms | 762 (93.7%) | 448 (94.5%) | 165 (93.8%) | 149 (95.5%) | 134 (94.4%) | 314 (92.6%) | 131 (92.3%) | 100 (91.7%) | 83 (94.3%) | 0.813 |
| Duration of symptoms (days) | 5.00 (7.00) | 7.00 (7.00) | 6.00 (7.00) | 10.0 (10.0) | 7.00 (6.75) | 5.00 (3.00) | 5.00 (4.00) | 5.00 (3.00) | 4.00 (3.00) | <0.001 |
| Cough | 328 (40.3%) | 184 (38.8%) | 70 (39.8%) | 58 (37.2%) | 56 (39.4%) | 144 (42.5%) | 58 (40.8%) | 47 (43.1%) | 39 (44.3%) | 0.894 |
| Duration of cough (days) | 7.00 (6.00) | 7.00 (5.00) | 7.00 (8.75) | 7.00 (5.75) | 7.00 (5.00) | 6.00 (6.00) | 7.00 (5.00) | 5.00 (4.50) | 6.00 (8.00) | 0.187 |
| Fever | 607 (74.7%) | 381 (80.4%) | 144 (81.8%) | 124 (79.5%) | 113 (79.6%) | 226 (66.7%) | 92 (64.8%) | 71 (65.1%) | 63 (71.6%) | <0.001 |
| Maximum value (°C) | 38.0 (0.80) | 38.0 (0.50) | 38.0 (0.80) | 38.0 (0.70) | 38.3 (0.80) | 38.0 (1.05) | 38.0 (0.90) | 38.0 (1.00) | 38.5 (1.00) | <0.001 |
| Duration of fever (days) | 3.00 (2.00) | 3.00 (4.00) | 3.00 (4.25) | 3.00 (4.00) | 3.00 (3.00) | 2.00 (1.00) | 2.00 (1.25) | 2.00 (2.00) | 2.00 (1.00) | <0.001 |
| Sore throat (pharyngula | 237 (29.2%) | 98 (20.7%) | 42 (23.9%) | 29 (18.6%) | 27 (19.0%) | 139 (41.0%) | 64 (45.1%) | 40 (36.7%) | 35 (39.8%) | <0.001 |
| Nasal congestion/discharge | 269 (33.1%) | 101 (21.3%) | 42 (23.9%) | 29 (18.6%) | 30 (21.1%) | 168 (49.6%) | 72 (50.7%) | 55 (50.5%) | 41 (46.6%) | <0.001 |
| Duration of nasal congestion(days) | 5.00 (4.00) | 5.00 (5.00) | 5.00 (3.00) | 4.00 (7.00) | 5.00 (7.00) | 5.00 (3.00) | 5.00 (3.00) | 5.00 (4.00) | 5.00 (3.00) | 0.211 |
| Headache | 337 (41.5%) | 179 (37.8%) | 89 (50.6%) | 49 (31.4%) | 41 (28.9%) | 158 (46.6%) | 65 (45.8%) | 45 (41.3%) | 48 (54.5%) | <0.001 |
| Headache intensity | ||||||||||
| Severe | 253 (31.1%) | 150 (31.6%) | 74 (42.0%) | 38 (24.4%) | 38 (26.8%) | 103 (30.4%) | 39 (27.5%) | 25 (22.9%) | 39 (44.3%) | <0.001 |
| Mild | 84 (10.3%) | 29 (6.1%) | 15 (8.5%) | 11 (7.1%) | 3 (2.1%) | 55 (16.2%) | 26 (18.3%) | 20 (18.3%) | 9 (10.2%) | |
| Fatigue | 466 (57.3%) | 255 (53.8%) | 98 (55.7%) | 83 (53.2%) | 74 (52.1%) | 211 (62.2%) | 75 (52.8%) | 68 (62.4%) | 68 (77.3%) | 0.002 |
| Taste disorders | 209 (25.7%) | 164 (34.6%) | 79 (44.9%) | 48 (30.8%) | 37 (26.1%) | 45 (13.3%) | 18 (12.7%) | 11 (10.1%) | 16 (18.2%) | <0.001 |
| Duration of taste disorders (days) | 7.00 (9.00) | 8.00 (7.00) | 7.00 (3.75) | 7.00 (7.00) | 12.5 (9.25) | 5.00 (7.00) | 5.00 (5.25) | 4.00 (3.00) | 5.00 (12.3) | <0.001 |
| Smell disorders | 183 (22.5%) | 146 (30.8%) | 70 (39.8%) | 39 (25.0%) | 37 (26.1%) | 37 (10.9%) | 17 (12.0%) | 8 (7.3%) | 12 (13.6%) | <0.001 |
| Duration of smell disorders (days) | 7.00 (8.00) | 7.00 (7.25) | 7.00 (4.00) | 7.00 (6.00) | 12.5 (9.25) | 5.00 (7.00) | 5.00 (3.00) | 4.50 (6.25) | 4.50 (8.75) | <0.001 |
| GI 4 symptoms | 152 (18.7%) | 96 (20.3%) | 35 (19.9%) | 49 (31.4%) | 12 (8.5%) | 56 (16.5%) | 20 (14.1%) | 11 (10.1%) | 25 (28.4%) | <0.001 |
| Appetite disorders | ||||||||||
| No | 746 (91.8%) | 433 (91.4%) | 169 (96.0%) | 125 (80.1%) | 139 (97.9%) | 313 (92.3%) | 135 (95.1%) | 102 (93.6%) | 76 (86.4%) | <0.001 |
| Increased | 11 (1.4%) | 7 (1.5%) | 1 (0.6%) | 6 (3.8%) | 0 (0%) | 4 (1.2%) | 3 (2.1%) | 1 (0.9%) | 0 (0%) | |
| Decreased | 56 (6.9%) | 34 (7.2%) | 6 (3.4%) | 25 (16.0%) | 3 (2.1%) | 22 (6.5%) | 4 (2.8%) | 6 (5.5%) | 12 (13.6%) | |
| Dyspnea | 138 (17.0%) | 111 (23.4%) | 35 (19.9%) | 37 (23.7%) | 39 (27.5%) | 27 (8.0%) | 12 (8.5%) | 6 (5.5%) | 9 (10.2%) | <0.001 |
| Participants ≥18 Years Old | Total | Non-Omicron Variants (Total) | Non-Omicron Variants | Omicron Variants (Total) | Omicron Variants | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| WTS 3 | Alpha | Delta | BA.1 | BA.2 | BA.4/BA.5 | |||||
| 813 | 474 | (N = 176) | (N = 156) | (N = 142) | 339 | (N = 142) | (N = 109) | (N = 88) | ||
| Hospitalization because of COVID-19 4 | 172 (21.2%) | 145 (30.6%) | 36 (20.5%) | 58 (37.2%) | 51 (35.9%) | 27 (8.0%) | 14 (9.9%) | 12 (11.0%) | 13 (14.8%) | <0.001 |
| Duration of hospitalization (days) | 6.00 (6.00) | 6.00 (6.00) | 7.00 (5.75) | 6.00 (7.50) | 6.00 (5.00) | 5.00 (5.00) | 6.50 (8.25) | 4.00 (2.25) | 3.00 (2.00) | 0.084 |
| Diagnosis of pneumonia | 123 (15.1%) | 109 (23.0%) | 29 (16.5%) | 39 (25.0%) | 41 (28.9%) | 14 (4.1%) | 9 (6.3%) | 3 (2.8%) | 2 (2.3%) | <0.001 |
| HDU 5 | 15 (1.5%) | 13 (2.7%) | 5 (2.8%) | 3 (1.9%) | 5 (3.5%) | 2 (0.6%) | 1 (0.7%) | 1 (0.9%) | 0 (0%) | 0.272 |
| Duration in HDU (days) | 9.00 (5.00) | 9.00 (3.00) | 6.00 (3.00) | 9.00 (4.00) | 9.00 (6.00) | 27.5 (12.50) | 40.0 (0) | 15.0 (0) | - | 0.255 |
| Hospitalization in ICU 6 | 16 (2.0%) | 15 (3.2%) | 5 (2.8%) | 4 (2.6%) | 6 (4.2%) | 1 (0.3%) | 1 (0.7%) | 0 (0%) | 0 (0%) | 0.079 |
| Duration in ICU (days) | 11.50 (14.75) | 10.00 (14.00) | 10.0 (4.00) | 5.50 (8.00) | 21.0 (6.00) | 36.0 (0) | 36.0 (0) | - | - | 0.247 |
| Intubation | 17 (2.1%) | 15 (3.2%) | 5 (2.8%) | 4 (2.6%) | 6 (4.2%) | 2 (0.6%) | 1 (0.7%) | 1 (0.9%) | 0 (0%) | 0.170 |
| Duration of intubation (days) | 9.00 (7.00) | 9.00 (6.50) | 9.00 (4.00) | 7.50 (5.50) | 9.00 (8.75) | 11.50 (1.50) | 10.0 (0) | 13.0 (0) | - | 0.835 |
| Death | 35 (4.3%) | 29 (6.1%) | 5 (2.8%) | 11 (7.1%) | 13 (9.2%) | 6 (1.8%) | 3 (2.1%) | 1 (0.9%) | 2 (2.3%) | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voulgaridi, I.; Bogogiannidou, Z.; Dadouli, K.; Galanopoulos, A.P.; Kyritsi, M.A.; Vontas, A.; Matziri, A.; Kola, K.; Vachtsioli, E.; Anagnostopoulos, L.; et al. The Clinical Anatomy of SARS-CoV-2 Variants of Concern in Central Greece During October 2020–July 2022. Microorganisms 2024, 12, 2573. https://doi.org/10.3390/microorganisms12122573
Voulgaridi I, Bogogiannidou Z, Dadouli K, Galanopoulos AP, Kyritsi MA, Vontas A, Matziri A, Kola K, Vachtsioli E, Anagnostopoulos L, et al. The Clinical Anatomy of SARS-CoV-2 Variants of Concern in Central Greece During October 2020–July 2022. Microorganisms. 2024; 12(12):2573. https://doi.org/10.3390/microorganisms12122573
Chicago/Turabian StyleVoulgaridi, Ioanna, Zacharoula Bogogiannidou, Katerina Dadouli, Achilleas P. Galanopoulos, Maria A. Kyritsi, Alexandros Vontas, Alexia Matziri, Konstantina Kola, Evangelia Vachtsioli, Lemonia Anagnostopoulos, and et al. 2024. "The Clinical Anatomy of SARS-CoV-2 Variants of Concern in Central Greece During October 2020–July 2022" Microorganisms 12, no. 12: 2573. https://doi.org/10.3390/microorganisms12122573
APA StyleVoulgaridi, I., Bogogiannidou, Z., Dadouli, K., Galanopoulos, A. P., Kyritsi, M. A., Vontas, A., Matziri, A., Kola, K., Vachtsioli, E., Anagnostopoulos, L., Tsispara, A., Oikonomou, K. G., Babalis, D., Petinaki, E., Tseroni, M., Kalala, F., Speletas, M., Mouchtouri, V. A., & Hadjichristodoulou, C. (2024). The Clinical Anatomy of SARS-CoV-2 Variants of Concern in Central Greece During October 2020–July 2022. Microorganisms, 12(12), 2573. https://doi.org/10.3390/microorganisms12122573








