Deciphering Immune Modulation in Chickens Co-Infected with ALV-J and CIAV: A Transcriptomic Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Virus and Animals
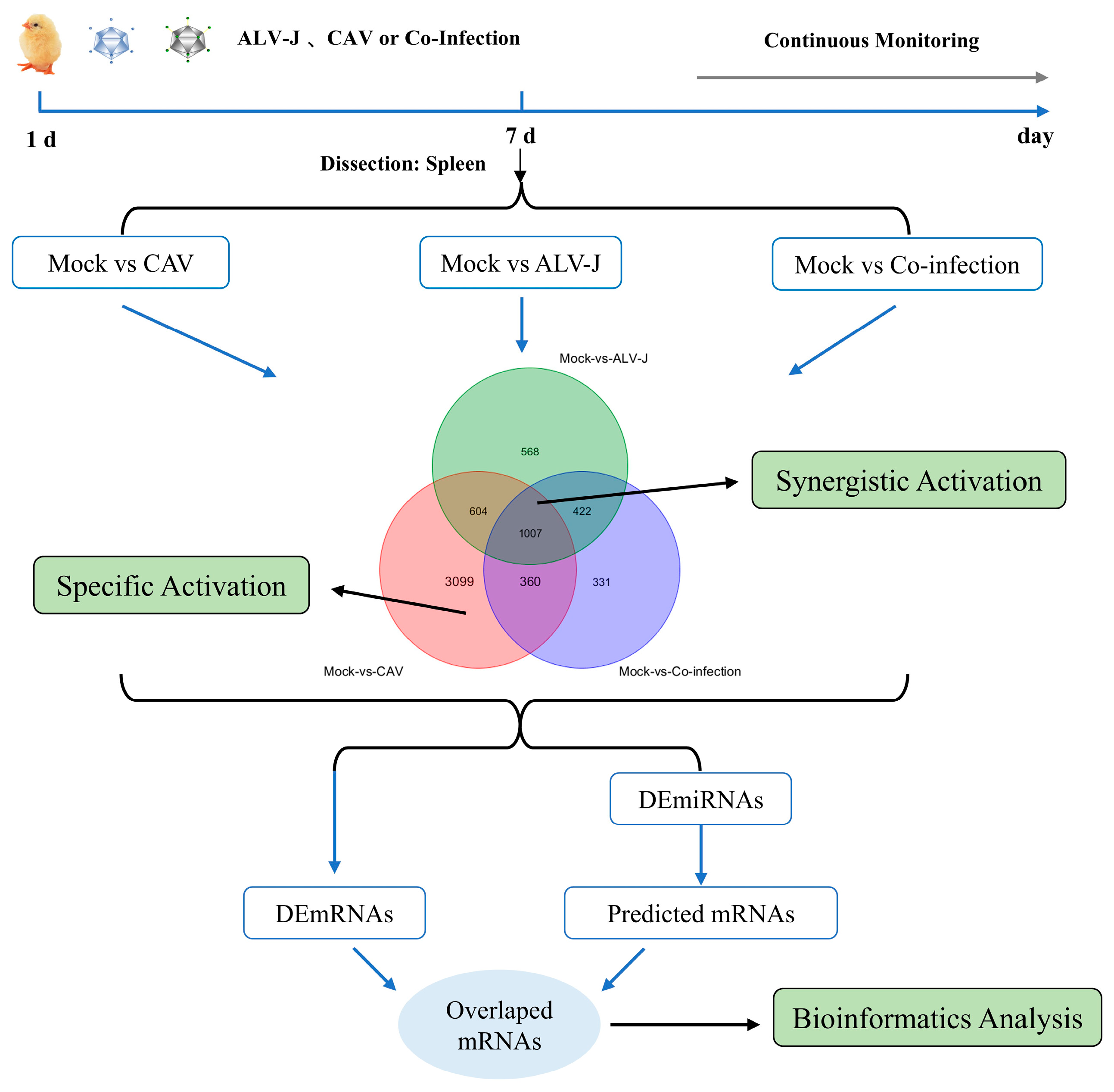
2.3. Animal Experimental Design and Sample Collection
2.4. Differentially Expressed mRNA and miRNA
2.5. MiRNA Target Prediction
2.6. Construction of the miRNA–Target Network
2.7. Visualization of the miRNA–Target Network
2.8. Functional Enrichment Analysis
3. Results
3.1. Identification of Differentially Expressed miRNAs (DEmiRNAs) and mRNAs (DEmRNAs) in the Co-Infection of ALV-J and CIAV
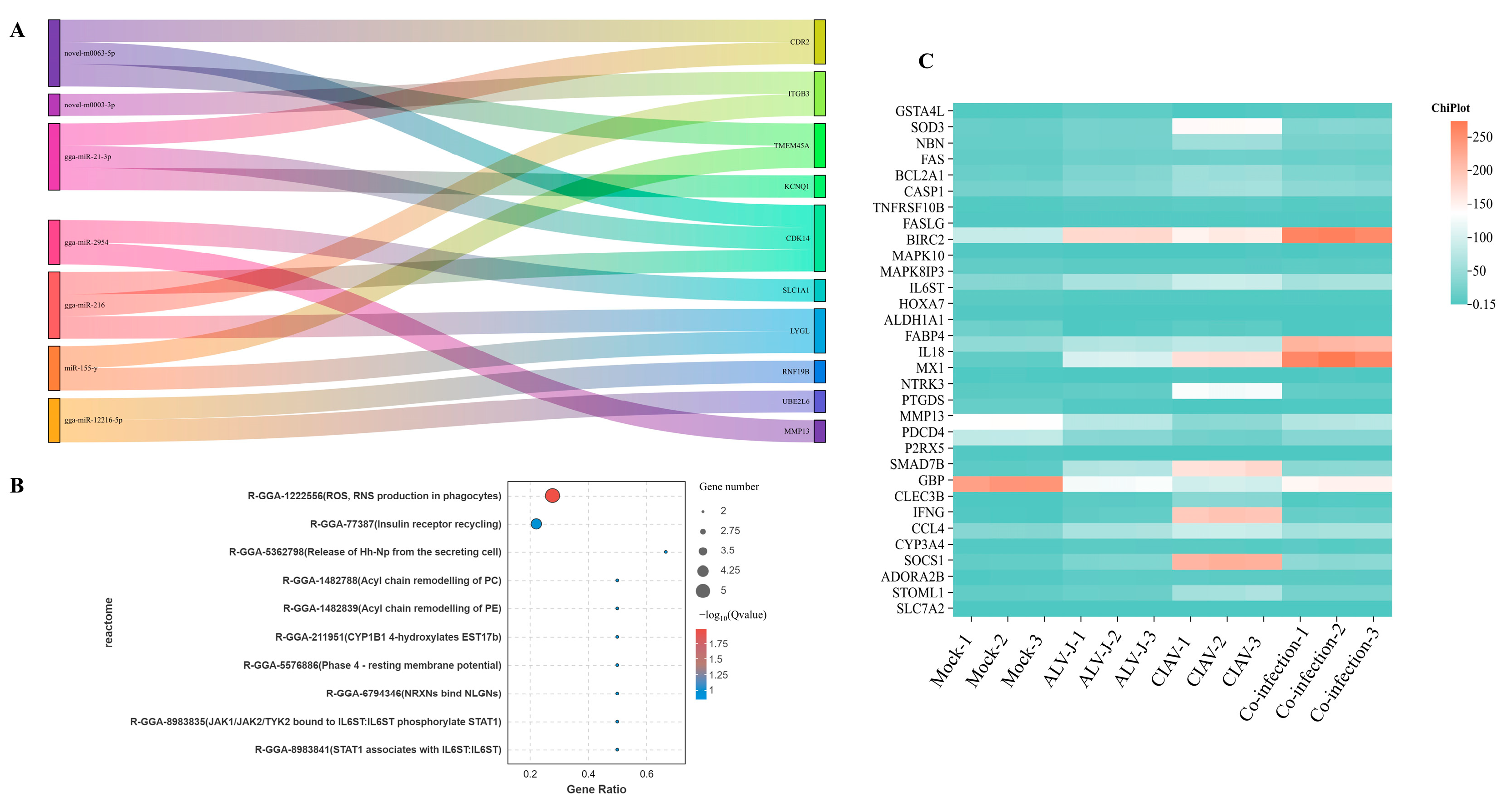
3.2. Biological Functions of DEmRNAs During Co-Infection with Synergistic Activation
3.3. Biological Functions of DEmRNAs During Co-Infection with Specific Activation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hoerr, F.J. Clinical aspects of immunosuppression in poultry. Avian Dis. 2010, 54, 2–15. [Google Scholar] [CrossRef]
- Gimeno, I.M.; Schat, K.A. Virus-Induced Immunosuppression in Chickens. Avian Dis. 2018, 62, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.-P.; Teng, M.; Li, G.-X.; Zhang, W.-K.; Wang, W.-D.; Liu, J.-L.; Li, L.-Y.; Yao, Y.; Nair, V.; Luo, J. Current Epidemiology and Co-Infections of Avian Immunosuppressive and Neoplastic Diseases in Chicken Flocks in Central China. Viruses 2022, 14, 2599. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Ju, S.; Zhao, P.; Li, Y.; Meng, F.; Sun, P.; Cui, Z. Synergetic effects of subgroup J avian leukosis virus and reticuloendotheliosis virus co-infection on growth retardation and immunosuppression in SPF chickens. Vet. Microbiol. 2014, 172, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, S.; Nie, Y.; Li, W.; Li, H.; Zhang, X.; Chen, F.; Xie, Q. Synergistic Immunosuppression of Avian Leukosis Virus Subgroup J and Infectious Bursal Disease Virus Is Responsible for Enhanced Pathogenicity. Viruses 2022, 14, 2312. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, W.; Nie, Y.; Chen, S.; Li, H.; Zhang, X.; Xie, Q.; Chen, W. Synergy of Subgroup J Avian Leukosis Virus and Chicken Infectious Anemia Virus Enhances the Pathogenicity in Chickens. Microorganisms 2024, 12, 740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, L.; Li, T.; Li, L.; Kan, Q.; Yao, X.; Xie, Q.; Wan, Z.; Shao, H.; Qin, A.; et al. Synergistic pathogenesis of chicken infectious anemia virus and J subgroup of avian leukosis virus. Poult. Sci. 2021, 100, 101468. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, Y.; Wang, J.; Wang, Y.; Chang, S.; Zhao, P. Synergistic pathogenicity of vertically transmitted chicken infectious anemia virus and avian leukosis virus subgroup J coinfection in chickens. Poult. Sci. 2024, 103, 103835. [Google Scholar] [CrossRef]
- Paget, C.; Trottein, F. Mechanisms of Bacterial Superinfection Post-influenza: A Role for Unconventional T Cells. Front. Immunol. 2019, 10, 336. [Google Scholar] [CrossRef] [PubMed]
- Semrau, A.; Wibbelt, G.; Hilbe, M.; Lieckfeldt, D.; Hermes, R.; Müller, K.E.; Heckert, H.P.; Hoyer, M.J.; Frölich, K. Experimental superinfection of a Lesser Malayan mousedeer (Tragulus javanicus) persistently infected with bovine viral diarrhea virus. J. Zoo Wildl. Med. 2008, 39, 124–127. [Google Scholar] [CrossRef][Green Version]
- Zhou, J.; Zhou, D.; Zhang, Q.; Zhang, X.; Liu, X.; Ding, L.; Wen, J.; Xu, X.; Cheng, Z. DCLK1 mediated cooperative acceleration of EMT by avian leukosis virus subgroup J and Marek’s disease virus via the Wnt/β-catenin pathway promotes tumor metastasis. J. Virol. 2024, 24, e0111224. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wang, M.; Xu, T.; Zhang, M.; Dai, H.; Wang, C.; Ding, D.; Zhong, Z. Reactive oxygen species-powered cancer immunotherapy: Current status and challenges. J. Control. Release 2023, 356, 623–648. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, B.M.; Banik, B.K.; Borah, P.; Jain, A. Reactive Oxygen Species (ROS): Key Components in Cancer Therapies. Anti-Cancer Agents Med. Chem. 2022, 22, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, Z.; Wang, Z.; Guo, J.; Wen, J. Reactive oxygen species associated immunoregulation post influenza virus infection. Front. Immunol. 2022, 13, 927593. [Google Scholar] [CrossRef]
- Foo, J.; Bellot, G.; Pervaiz, S.; Alonso, S. Mitochondria-mediated oxidative stress during viral infection. Trends Microbiol. 2022, 7, 679–692. [Google Scholar] [CrossRef]
- Kim, S.; Park, J.; Han, J.; Jang, K.L. Hepatitis B Virus X Protein Induces Reactive Oxygen Species Generation via Activation of p53 in Human Hepatoma Cells. Biomolecules 2024, 14, 1201. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Han, J.; Jang, K.L. Reactive Oxygen Species Induction by Hepatitis B Virus: Implications for Viral Replication in p53-Positive Human Hepatoma Cells. Int. J. Mol. Sci. 2024, 25, 6606. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Y.; Zhu, Y.; Li, N.; Li, W.; Shang, C.; Song, G.; Li, S.; Cong, J.; Li, T.; et al. Apoptin induces pyroptosis of colorectal cancer cells via the GSDME-dependent pathway. Int. J. Biol. Sci. 2022, 18, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, K.; Chawla, A. Metabolic regulation of immune responses. Annu. Rev. Immunol. 2014, 32, 609–634. [Google Scholar] [CrossRef]
- Van den Bossche, J.; Baardman, J.; Otto, N.A.; van der Velden, S.; Neele, A.E.; van den Berg, S.M.; Luque-Martin, R.; Chen, H.J.; Boshuizen, M.C.; Ahmed, M.; et al. Mitochondrial Dysfunction Prevents Repolarization of Inflammatory Macrophages. Cell Rep. 2016, 17, 684–696. [Google Scholar] [CrossRef]
- Li, S. Regulation of Ribosomal Proteins on Viral Infection. Cells 2019, 8, 508. [Google Scholar] [CrossRef] [PubMed]
- Rawlinson, S.M.; Zhao, T.; Ardipradja, K.; Zhang, Y.; Veugelers, P.F.; Harper, J.A.; David, C.T.; Sundaramoorthy, V.; Moseley, G.W. Henipaviruses and lyssaviruses target nucleolar treacle protein and regulate ribosomal RNA synthesis. Traffic 2023, 24, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Bianco, C.; Mohr, I. Ribosome biogenesis restricts innate immune responses to virus infection and DNA. Elife 2019, 8, e49551. [Google Scholar] [CrossRef]
- Lee, A.J.; Feng, E.; Chew, M.V.; Balint, E.; Poznanski, S.M.; Giles, E.; Zhang, A.; Marzok, A.; Revill, S.D.; Vahedi, F.; et al. Type I interferon regulates proteolysis by macrophages to prevent immunopathology following viral infection. PLoS Pathog. 2022, 18, e1010471. [Google Scholar] [CrossRef] [PubMed]
- Insua, J.L.; Llobet, E.; Moranta, D.; Pérez-Gutiérrez, C.; Tomás, A.; Garmendia, J.; Bengoechea, J.A. Modeling Klebsiella pneumoniae pathogenesis by infection of the wax moth Galleria mellonella. Infect. Immun. 2013, 81, 3552–3565. [Google Scholar] [CrossRef]
- Beura, L.K.; Dinh, P.X.; Osorio, F.A.; Pattnaik, A.K. Cellular poly(c) binding proteins 1 and 2 interact with porcine reproductive and respiratory syndrome virus nonstructural protein 1β and support viral replication. J. Virol. 2011, 85, 12939–12949. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Xu, H.; Li, W.; Nie, Y.; Xie, Q.; Chen, W. Deciphering Immune Modulation in Chickens Co-Infected with ALV-J and CIAV: A Transcriptomic Approach. Microorganisms 2024, 12, 2453. https://doi.org/10.3390/microorganisms12122453
Chen S, Xu H, Li W, Nie Y, Xie Q, Chen W. Deciphering Immune Modulation in Chickens Co-Infected with ALV-J and CIAV: A Transcriptomic Approach. Microorganisms. 2024; 12(12):2453. https://doi.org/10.3390/microorganisms12122453
Chicago/Turabian StyleChen, Sheng, Huijuan Xu, Wenxue Li, Yu Nie, Qingmei Xie, and Weiguo Chen. 2024. "Deciphering Immune Modulation in Chickens Co-Infected with ALV-J and CIAV: A Transcriptomic Approach" Microorganisms 12, no. 12: 2453. https://doi.org/10.3390/microorganisms12122453
APA StyleChen, S., Xu, H., Li, W., Nie, Y., Xie, Q., & Chen, W. (2024). Deciphering Immune Modulation in Chickens Co-Infected with ALV-J and CIAV: A Transcriptomic Approach. Microorganisms, 12(12), 2453. https://doi.org/10.3390/microorganisms12122453





