Feline Vector-Borne Diseases and Their Possible Association with Hematological Abnormalities in Cats from Midwestern Brazil
Abstract
1. Introduction
2. Materials and Methods
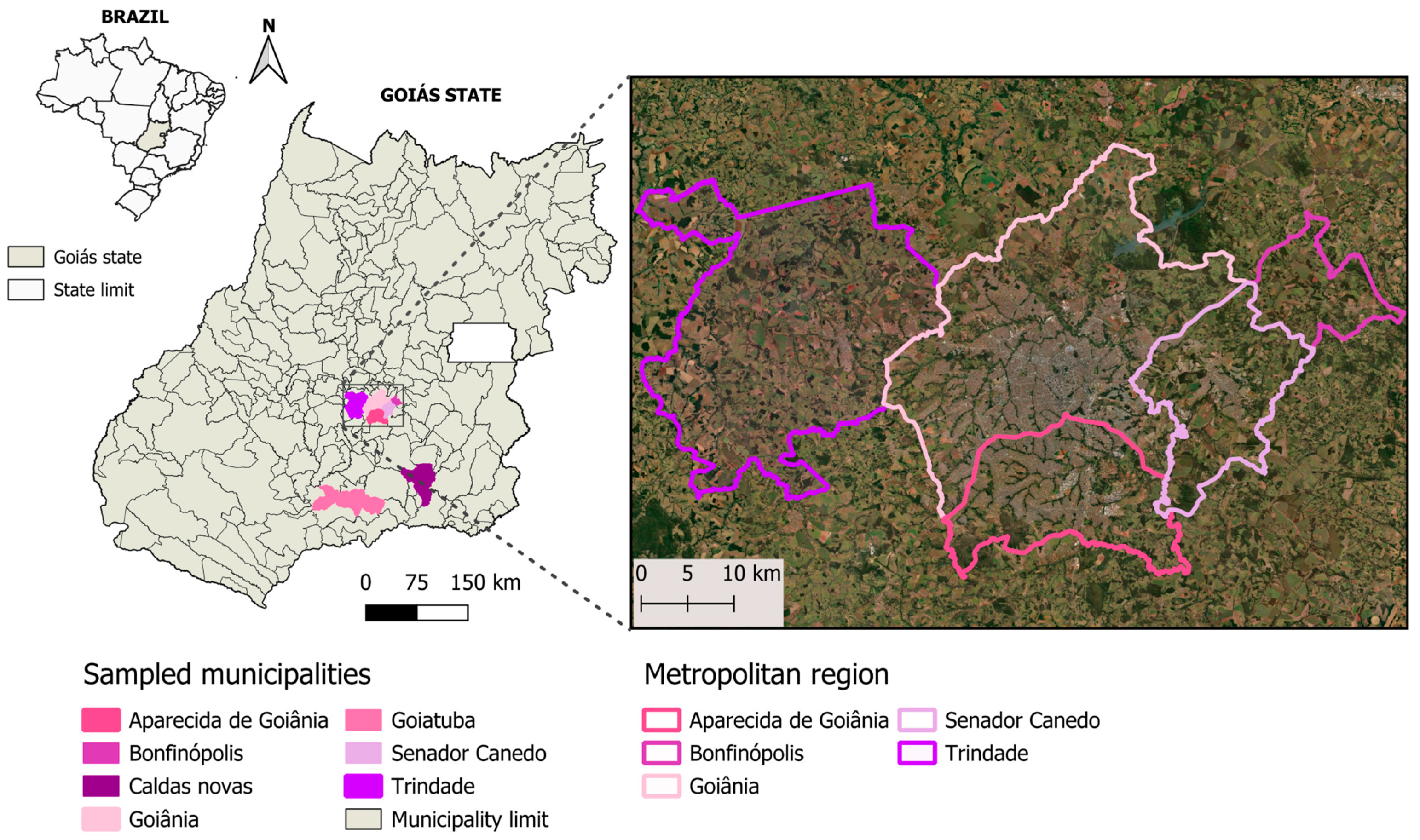
2.1. Study Area
2.2. Sample Collection
2.3. CBC Analysis
2.4. Immunochromatography for FIV and FeLV
2.5. DNA Extraction
2.6. PCR Assays
| Agents (Target Gene) | Primers | Primer Sequences | Product Size (bp) | References |
|---|---|---|---|---|
| Cytauxzoon spp. (18S rRNA) b | Cytaux F | GCGAATCGCATTGCTTTATGCT | 284 | [21] |
| Cytaux R | CCAATTGATACTCCGGAAAGAG | |||
| Ehrlichia spp. (dsb) b | Dsb-330 (F) | GATGATGTCTGAAGATATGAAACAAAT | 409 | [22] |
| Dsb-728 (R) | CTGCTCGTCTATTTTACTTCTTAAAGT | |||
| Mycoplasma spp. (16S rRNA) a | SYBR_For | AGCAATRCCATGTGAACGATGAA | 134 | [20] a |
| SYBR_Rev1 | TGGCACATAGTTWGCTGTCACTT | |||
| Mycoplasma spp. (16S rRNA) b | HBT-F | ATACGGCCCATATTCCTACG | 595–620 | [23] b |
| HBT-R | TGCTCCACCACTTGTTCA |
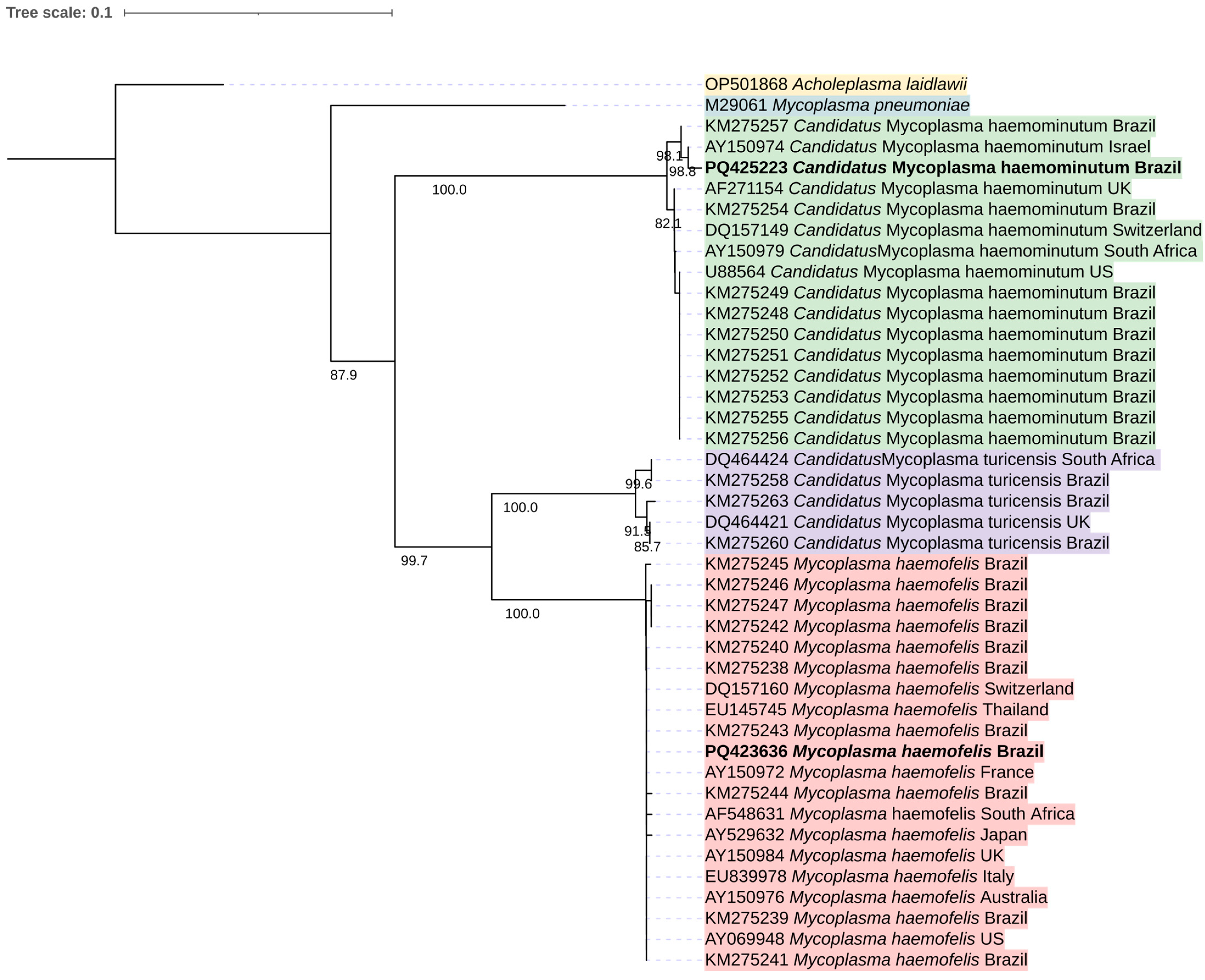
2.7. Partial Mycoplasma spp. 16S rRNA Sequencing and Analysis
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- André, M.R.; Calchi, A.C.; Furquim, M.E.C.; de Andrade, I.; Arantes, P.V.C.; de Melo Lopes, L.C.; Demarchi, I.K.L.D.N.; Figueiredo, M.A.P.; de Paula Lima, C.A.; Machado, R.Z. Molecular detection of tick-borne agents in cats from southeastern and northern Brazil. Pathogens 2022, 11, 106. [Google Scholar] [CrossRef] [PubMed]
- Latrofa, M.S.; Iatta, R.; Toniolo, F.; Furlanello, T.; Ravagnan, S.; Capelli, G.; Schunack, B.; Chomel, B.; Zatelli, A.; Mendoza-Roldan, J.; et al. A molecular survey of vector-borne pathogens and haemoplasmas in owned cats across Italy. Parasite Vectors 2020, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.G.; Krawczak, F.D.S.; de Lima, J.T.R.; Fournier, G.F.D.S.R.; Acosta, I.D.C.L.; Ramirez, D.G.; Marcili, A.; Labruna, M.B.; Gennari, S.M. Occurrence of Ehrlichia canis and Hepatozoon canis and probable exposure to Rickettsia amblyommatis in dogs and cats in Natal, RN. Rev. Bras. Parasitol. Vet. 2019, 28, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Maggi, R.G.; Halls, V.; Krämer, F.; Lappin, M.; Pennisi, M.G.; Peregrine, A.S.; Roura, X.; Schunack, B.; Scorza, V.; Tasker, S.; et al. Vector-borne and other pathogens of potential relevance disseminated by relocated cats. Parasite Vectors 2022, 15, 415. [Google Scholar] [CrossRef] [PubMed]
- Morelli, S.; Diakou, A.; Di Cesare, A.; Colombo, M.; Traversa, D. Canine and feline parasitology: Analogies, differences, and relevance for human health. Clin. Microbiol. Rev. 2021, 34, e0026620. [Google Scholar] [CrossRef]
- de Santis, A.G.G.A.; Herrera, H.M.; Sousa, K.C.M.; Gonçalves, L.R.; Denardi, N.C.B.; Domingos, I.H.; Campos, J.B.V.; Machado, R.Z.; André, M.R. Molecular detection of hemotrophic mycoplasmas among domiciled and free-roraming cats in Campo Grande, state of Mato Grosso do Sul, Brazil. Rev. Bras. Parasitol. Vet. 2014, 23, 231–236. [Google Scholar] [CrossRef]
- Tasker, S. Hemotropic Mycoplasma. Vet. Clin. N. Am. Small. Anim. Pract. 2022, 52, 1319–1340. [Google Scholar] [CrossRef]
- Tasker, S.; Hofmann-Lehmann, R.; Belák, S.; Frymus, T.; Addie, D.D.; Pennisi, M.G.; Boucraut-Baralon, C.; Egberink, H.; Hartmann, K.; Hosie, M.J.; et al. Haemoplasmosis in cats: European guidelines from the ABCD on prevention and management. J. Feline Med. Surg. 2018, 20, 256–261. [Google Scholar] [CrossRef]
- Barker, E.N. Update on feline hemoplasmosis. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 733–743. [Google Scholar] [CrossRef]
- Maia, L.M.P.; Cerqueira, A.d.M.F.; Macieira, D.; de Souza, A.M.; Moreira, N.S.; da Silva, A.V.; Messick, J.B.; Ferreira, R.F.; Almosny, N.R.P. Cytauxzoon felis and “Candidatus Mycoplasma haemominutum” coinfection in a brazilian domestic cat (Felis catus). Rev. Bras. Parasitol. Vet. 2013, 22, 289–291. [Google Scholar] [CrossRef]
- Munhoz, A.D.; Simões, I.G.P.C.; Calazans, A.P.F.; Macedo, L.S.; Cruz, R.D.S.; Lacerda, L.C.; Said, R.A.; André, M.R. Hemotropic mycoplasmas in naturally infected cats in northeastern Brazil. Rev. Bras. Parasitol. Vet. 2018, 27, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Aquino, L.C.; Hicks, C.A.E.; Scalon, M.C.; Lima, M.G.d.M.; Lemos, M.d.S.; Paludo, G.R.; Helps, C.R.; Tasker, S. Prevalence and phylogenetic analysis of haemoplasmas from cats infected with multiple species. J. Microbiol. Methods 2014, 107, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Biondo, A.W.; dos Santos, A.P.; Guimarães, A.M.S.; Vieira, R.F.d.C.; Vidotto, O.; Macieira, D.d.B.; Almosny, N.R.P.; Molento, M.B.; Timenetsky, J.; de Morais, H.A.; et al. A review of the occurrence of hemoplasmas (Hemotrophic mycoplasmas) in Brazil. Rev. Bras. Parasitol. Vet. 2009, 18, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lappin, M.R. Update on flea and tick associated diseases of cats. Vet. Parasitol. 2018, 254, 26–29. [Google Scholar] [CrossRef]
- André, M.R.; Filgueira, K.D.; Calchi, A.C.; de Sousa, K.C.M.; Gonçalves, L.R.; Medeiros, V.B.; Ximenes, P.A.; Lelis, V.C.N.G.; de Meireles, M.V.N.; Machado, R.Z. Co-infection with arthropod-borne pathogens in domestic cats. Rev. Bras. Parasitol. Vet. 2017, 26, 525–531. [Google Scholar] [CrossRef]
- André, M.R.; Herrera, H.M.; de Jesus Fernandes, S.; de Sousa, K.C.M.; Gonçalves, L.R.; Domingos, I.H.; de Macedo, G.C.; Machado, R.Z. Tick-borne agents in domesticated and stray cats from the city of Campo Grande, state of Mato Grosso do Sul, midwestern Brazil. Ticks Tick-Borne Dis. 2015, 6, 779–786. [Google Scholar] [CrossRef]
- André, M.R.; Baccarim Denardi, N.C.; Marques de Sousa, K.C.; Gonçalves, L.R.; Henrique, P.C.; Grosse Rossi Ontivero, C.R.; Lima Gonzalez, I.H.; Cabral Nery, C.V.; Fernandes Chagas, C.R.; Monticelli, C.; et al. Arthropod-borne pathogens circulating in free-roaming domestic cats in a zoo Environment in Brazil. Ticks Tick-Borne Dis. 2014, 5, 545–551. [Google Scholar] [CrossRef]
- Pedrassani, D.; Biolchi, J.; Gonçalves, L.R.; Mendes, N.S.; Zanatto, D.C.d.S.; Calchi, A.C.; Machado, R.Z.; André, M.R. Molecular detection of vector-borne agents in cats in southern Brazil. Rev. Bras. Parasitol. Vet. 2019, 28, 632–643. [Google Scholar] [CrossRef]
- Weiss, D.J.; Wardrop, K.J. (Eds.) Schalm’s Veterinary Hematology, 6th ed.; Blackwell Publishing: Ames, IA, USA, 2010; ISBN 9780470961834. [Google Scholar]
- Willi, B.; Meli, M.L.; Lüthy, R.; Honegger, H.; Wengi, N.; Hoelzle, L.E.; Reusch, C.E.; Lutz, H.; Hofmann-Lehmann, R. Development and application of a universal hemoplasma screening assay based on the SYBR Green PCR principle. J. Clin. Microbiol. 2009, 47, 4049. [Google Scholar] [CrossRef]
- Birkenheuer, A.J.; Marr, H.; Alleman, A.R.; Levy, M.G.; Breitschwerdt, E.B. Development and evaluation of a PCR assay for the detection of Cytauxzoon felis DNA in feline blood samples. Vet. Parasitol. 2006, 137, 144–149. [Google Scholar] [CrossRef]
- Doyle, C.K.; Labruna, M.B.; Breitschwerdt, E.B.; Tang, Y.W.; Corstvet, R.E.; Hegarty, B.C.; Bloch, K.C.; Li, P.; Walker, D.H.; McBride, J.W. Detection of medically important Ehrlichia by quantitative multicolor TaqMan real-time polymerase chain reaction of the Dsb gene. J. Mol. Diagn. 2005, 7, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Criado-Fornelio, A.; Martinez-Marcos, A.; Buling-Saraña, A.; Barba-Carretero, J.C. Presence of Mycoplasma haemofelis, Mycoplasma haemominutum and Piroplasmids in cats from southern Europe: A molecular study. Vet. Microbiol. 2003, 93, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Kocher, T.D.; Thomas, W.K.; Meyer, A.; Edwards, S.V.; Paabo, S.; Villablanca, F.X.; Wilson, A.C. Dynamics of Mitochondrial DNA Evolution in animals: Amplification and sequencing with conserved primers. Proc. Natl. Acad. Sci. USA 1989, 86, 6196. [Google Scholar] [CrossRef]
- Steuber, S.; Abdel-Rady, A.; Clausen, P.H. PCR-RFLP Analysis: A promising technique for host species identification of blood meals from Tsetse flies (Diptera: Glossinidae). Parasitol. Res. 2005, 97, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: Oxford, UK, 2000. [Google Scholar] [CrossRef]
- Stecher, G.; Tamura, K.; Kumar, S. Molecular evolutionary genetics analysis (MEGA) for MacOS. Mol. Biol. Evol. 2020, 37, 1237–1239. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Raimundo, J.M.; Guimarães, A.; Rodrigues, R.B.; Botelho, C.F.M.; Peixoto, M.P.; Pires, M.S.; Machado, C.H.; Santos, H.A.; Massard, C.L.; André, M.R.; et al. Hematological changes associated with hemoplasma infection in cats in Rio de Janeiro, Brazil. Rev. Bras. Parasitol. Vet. 2016, 25, 441–449. [Google Scholar] [CrossRef]
- Maciel, A.d.R.; Biezus, G.; de Cristo, T.G.; Miletti, L.C.; da Costa Maciel, U.; Medeiros, A.L.V.; Xavier, M.G.N.; Casagrande, R.A. Mycoplasma haemofelis infection and its correlation with Feline Leukemia Virus (FeLV) and Feline Immunodeficiency Virus (FIV) in cats in southern Brazil. Comp. Immunol. Microbiol. Infect. Dis. 2023, 93, 101941. [Google Scholar] [CrossRef]
- Ider, M.; Ceylan, C.; Naseri, A.; Ceylan, O.; Durgut, M.K.; Ok, M.; Iyigun, S.S.; Erol, B.B.; Sahin, H.B.; Kilickaya, M.C. Evaluation of endothelial glycocalyx injury biomarkers in feline hemotropic mycoplasmosis. Sci. Rep. 2024, 14, 12931. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, R.A.; Hart, B.L. Grooming and control of fleas in cats. Appl. Anim. Behav. Sci. 2000, 68, 141–150. [Google Scholar] [CrossRef]
- Richards, S.L.; Langley, R.; Apperson, C.S.; Watson, E. Do tick attachment times vary between different tick-pathogen systems? Environments 2017, 4, 37. [Google Scholar] [CrossRef]
- Tahir, D.; Meyer, L.; Fourie, J.; Jongejan, F.; Mather, T.; Choumet, V.; Blagburn, B.; Straubinger, R.K.; Varloud, M. Interrupted blood feeding in ticks: Causes and consequences. Microorganisms 2020, 8, 910. [Google Scholar] [CrossRef]
- Thomas, J.E.; Ohmes, C.M.; Payton, M.E.; Hostetler, J.A.; Reichard, M.V. Minimum transmission time of Cytauxzoon felis by Amblyomma americanum to domestic cats in relation to duration of infestation, and investigation of ingestion of infected ticks as a potential route of transmission. J. Feline Med. Surg. 2017, 20, 67–72. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, C.M.; Yang, S.; Duarte, M.A.; Figueiredo, D.M.; do Rosario Batista, L.M.; Marr, H.; McManus, C.M.; André, M.R.; Birkenheuer, A.J.; Paludo, G.R. Piroplasmid infection is not associated with clinicopathological and laboratory abnormalities in cats from midwestern Brazil. Parasitol. Res. 2022, 121, 2561–2570. [Google Scholar] [CrossRef]
- Duarte, M.A.; de Oliveira, C.M.; Honorato, S.M.; do Rosario Batista, L.M.; Mendonça, J.T.; de Sousa, D.E.R.; Hirano, L.Q.L.; André, M.R.; de Castro, M.B.; Paludo, G.R. Cytauxzoon brasiliensis Sp. Nov. (Apicomplexa: Theileriidae), a new species infecting a little-spotted-cat (Leopardus tigrinus) (Carnivora: Felidae) from Brazil. Syst. Parasitol. 2024, 101, 53. [Google Scholar] [CrossRef] [PubMed]
- de Paula, L.G.F.; Weck, B.C.; Neves, L.C.; Paula, W.V.d.F.; Araújo, L.B.d.M.; Martins, D.B.; Peres, P.C.d.O.; Labruna, M.B.; Krawczak, F.d.S. Natural infection and molecular detection of Cytauxzoon felis in a free-ranging Puma concolor in the State of Goiás, Brazil. Ciência Rural 2022, 52, e20210577. [Google Scholar] [CrossRef]
- de Sousa, K.C.M.; Fernandes, M.P.; Herrera, H.M.; Freschi, C.R.; Machado, R.Z.; André, M.R. Diversity of piroplasmids among wild and domestic mammals and ectoparasites in Pantanal wetland, Brazil. Ticks Tick-Borne Dis. 2018, 9, 245–253. [Google Scholar] [CrossRef]
- Fagundes-Moreira, R.; Souza, U.A.; May-Junior, J.A.; Baggio-Souza, V.; Berger, L.; Wagner, P.G.C.; Mazim, F.D.; Peters, F.B.; Favarini, M.O.; Tortato, M.A.; et al. Epidemiological compatibility of Amblyomma sculptum as possible vector and Panthera onca as reservoir of Cytauxzoon spp. in midwestern Brazil. Ticks Tick-Borne Dis. 2022, 13, 102021. [Google Scholar] [CrossRef]
- Malheiros, J.; Costa, M.M.; do Amaral, R.B.; de Sousa, K.C.M.; André, M.R.; Machado, R.Z.; Vieira, M.I.B. Identification of vector-borne pathogens in dogs and cats from southern Brazil. Ticks Tick-Borne Dis. 2016, 7, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Saz, S.; Martínez, M.; Nijhof, A.M.; Gerst, B.; Gentil, M.; Müller, E.; Fernández, A.; González, A.; Yusuf, M.S.M.; Greco, G.; et al. Molecular survey on vector-borne pathogens in clinically healthy stray cats in Zaragoza (Spain). Parasite Vectors 2023, 16, 428. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.; Ramos, C.; Coimbra, M.; Bastos, F.; Martins, Â.; Pinto, P.; Nunes, M.; Vieira, M.L.; Cardoso, L.; Campino, L. Bacterial and Protozoal Agents of feline vector-borne diseases in domestic and stray cats from southern Portugal. Parasite Vectors 2014, 7, 115. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Englert, T.; Stuetzer, B.; Hawley, J.R.; Lappin, M.R.; Hartmann, K. Prevalence of selected rickettsial infections in cats in southern Germany. Comp. Immunol. Microbiol. Infect. Dis. 2015, 42, 33–36. [Google Scholar] [CrossRef]
- Paula, W.V.d.F.; Taques, Í.I.G.G.; Miranda, V.C.; Barreto, A.L.G.; de Paula, L.G.F.; Martins, D.B.; Damasceno, A.D.; Muñoz-Leal, S.; Sevá, A.d.P.; Dantas-Torres, F.; et al. Seroprevalence and hematological abnormalities associated with Ehrlichia canis in dogs referred to a veterinary teaching hospital in central-western Brazil. Ciência Rural 2021, 52, e20201131. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Figueredo, L.A.; Sales, K.G.D.S.; Miranda, D.E.D.O.; Alexandre, J.L.D.A.; Da Silva, Y.Y.; Da Silva, L.G.; Valle, G.R.; Ribeiro, V.M.; Otranto, D.; et al. Prevalence and incidence of vector-borne pathogens in unprotected dogs in two brazilian regions. Parasite Vectors 2020, 13, 195. [Google Scholar] [CrossRef]
- Moraes-Filho, J.; Krawczak, F.S.; Costa, F.B.; Soares, J.F.; Labruna, M.B. Comparative evaluation of the vector competence of four south american populations of the Rhipicephalus sanguineus group for the bacterium Ehrlichia canis, the agent of canine monocytic ehrlichiosis. PLoS ONE 2015, 10, e0139386. [Google Scholar] [CrossRef]
| Age Groups | n | % |
|---|---|---|
| Kitten (<1 year) | 6 | 4.4 |
| Young adult (1–6 years) | 54 | 40 |
| Adult (7–9 years) | 11 | 8.2 |
| Senior (>9 years) | 22 | 16.3 |
| Unknown | 42 | 31.1 |
| Cat Number | Mycoplasma qPCR Positivity | Cytauxzoon cPCR Positivity | FIV Seropositivity | FELV Seropositivity |
|---|---|---|---|---|
| 1 | + | − | − | − |
| 2 | + | − | − | − |
| 3 | + | − | + | + |
| 4 | + | − | nt | nt |
| 5 | + | − | + | + |
| 6 | + | − | nt | nt |
| 7 | + | − | − | − |
| 8 | + | − | nt | nt |
| 9 | + | − | nt | nt |
| 10 | + | − | nt | nt |
| 11 | + | − | − | − |
| 12 | + | + | nt | nt |
| 13 | + | − | nt | nt |
| 14 | + | + | − | − |
| 15 | + | − | nt | nt |
| 16 | + | − | nt | nt |
| 17 | + | − | − | − |
| 18 | + | − | nt | nt |
| 19 | + | − | − | − |
| 20 | + | − | nt | nt |
| 21 | + | − | − | + |
| 22 | + | − | nt | nt |
| 23 | + | − | nt | nt |
| 24 | + | − | nt | nt |
| 25 | + | − | − | − |
| 26 | + | − | − | − |
| 27 | + | − | − | + |
| 28 | + | − | nt | nt |
| CBC Abnormalities | n | % | Mycoplasma-Positive | FIV/FELV- Positive | Mycoplasma- and FIV/FELV-Positive | |||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Anemia | 21/135 | 15.5 | 6/21 | 28.6 | 4/21 | 19.0 | 1/21 | 4.8 |
| Thrombocytopenia | 71/135 | 52.6 | 19/71 | 26.8 | 6/71 | 8.4 | 3/71 | 4.2 |
| Thrombocytosis | 3/135 | 2.2 | 0/3 | 0 | 0/3 | 0 | 0/3 | 0 |
| Leukopenia | 11/135 | 8.1 | 1/11 | 9.1 | 0/11 | 0 | 0/11 | 0 |
| Leukocytosis | 30/135 | 22.2 | 10/30 | 33.3 | 1/30 | 3.3 | 1/30 | 3.3 |
| Anemia + thrombocytopenia | 10/135 | 7.4 | 4/10 | 40 | 3/10 | 30 | 1/10 | 10 |
| Anemia + thrombocytosis | 2/135 | 1.5 | 0/2 | 0 | 0/2 | 0 | 0/2 | 0 |
| Anemia + leukopenia | 2/135 | 1.5 | 0/2 | 0 | 0/2 | 0 | 0/2 | 0 |
| Anemia + leukocytosis | 5/135 | 3.7 | 3/5 | 60 | 1/5 | 20 | 1/5 | 20 |
| Thrombocytopenia + leukopenia | 3/135 | 2.2 | 0/3 | 0 | 0/3 | 0 | 0/3 | 0 |
| Thrombocytopenia + leukocytosis | 15/135 | 11.1 | 8/15 | 53.3 | 1/15 | 6.7 | 1/15 | 6.7 |
| Thrombocytosis + leukopenia | 1/135 | 0.7 | 0/1 | 0 | 0/1 | 0 | 0/1 | 0 |
| CBC Abnormalities | Present? | Mycoplasma qPCR | Chi-Squared Test | p-Value | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| Anemia | Yes | 6 | 15 | 0.928 | 0.3355 |
| No | 22 | 92 | |||
| Thrombocytopenia | Yes | 19 | 52 | 3.301 | 0.0692 |
| No | 9 | 55 | |||
| Leukocytosis | Yes | 10 | 20 | 3.721 | 0.0537 |
| No | 18 | 87 | |||
| Thrombocytopenia + leukocytosis | Yes | 8 | 7 | 10.905 | 0.0010 |
| No | 20 | 100 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, S.F.; Pádua, G.T.; Paula, W.V.d.F.; Tavares, M.A.; Neves, L.C.; Pereira, B.G.; Santos, R.A.; dos Santos, G.C.; Cardoso, E.R.N.; Qualhato, A.F.; et al. Feline Vector-Borne Diseases and Their Possible Association with Hematological Abnormalities in Cats from Midwestern Brazil. Microorganisms 2024, 12, 2171. https://doi.org/10.3390/microorganisms12112171
Carvalho SF, Pádua GT, Paula WVdF, Tavares MA, Neves LC, Pereira BG, Santos RA, dos Santos GC, Cardoso ERN, Qualhato AF, et al. Feline Vector-Borne Diseases and Their Possible Association with Hematological Abnormalities in Cats from Midwestern Brazil. Microorganisms. 2024; 12(11):2171. https://doi.org/10.3390/microorganisms12112171
Chicago/Turabian StyleCarvalho, Stephani Félix, Gracielle Teles Pádua, Warley Vieira de Freitas Paula, Mariana Avelar Tavares, Lucianne Cardoso Neves, Brenda Gomes Pereira, Rayane Almeida Santos, Gabriel Cândido dos Santos, Ennya Rafaella Neves Cardoso, Andriele Ferreira Qualhato, and et al. 2024. "Feline Vector-Borne Diseases and Their Possible Association with Hematological Abnormalities in Cats from Midwestern Brazil" Microorganisms 12, no. 11: 2171. https://doi.org/10.3390/microorganisms12112171
APA StyleCarvalho, S. F., Pádua, G. T., Paula, W. V. d. F., Tavares, M. A., Neves, L. C., Pereira, B. G., Santos, R. A., dos Santos, G. C., Cardoso, E. R. N., Qualhato, A. F., Bittencourt, R. B. M., de Lima, N. J., Martins, D. B., Dantas-Torres, F., & Krawczak, F. d. S. (2024). Feline Vector-Borne Diseases and Their Possible Association with Hematological Abnormalities in Cats from Midwestern Brazil. Microorganisms, 12(11), 2171. https://doi.org/10.3390/microorganisms12112171





