In Vitro Assessment of Penicillium expansum Sensitivity to Difenoconazole
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Locations and Fungal Isolation
2.2. Molecular Identification of Fungal Isolates
2.3. Fungicide
2.4. Sensitivity Assay of Mycelial Growth to Difenoconazole
2.5. Spore Germination Assay
2.6. Statistical Analyses
3. Results
3.1. Molecular Identification of Penicillium expansum Strains
3.2. Effect of Difenoconazole on Mycelial Growth of P. expansum
3.3. Effect of Difenoconazole on Spore Germination of P. expansum
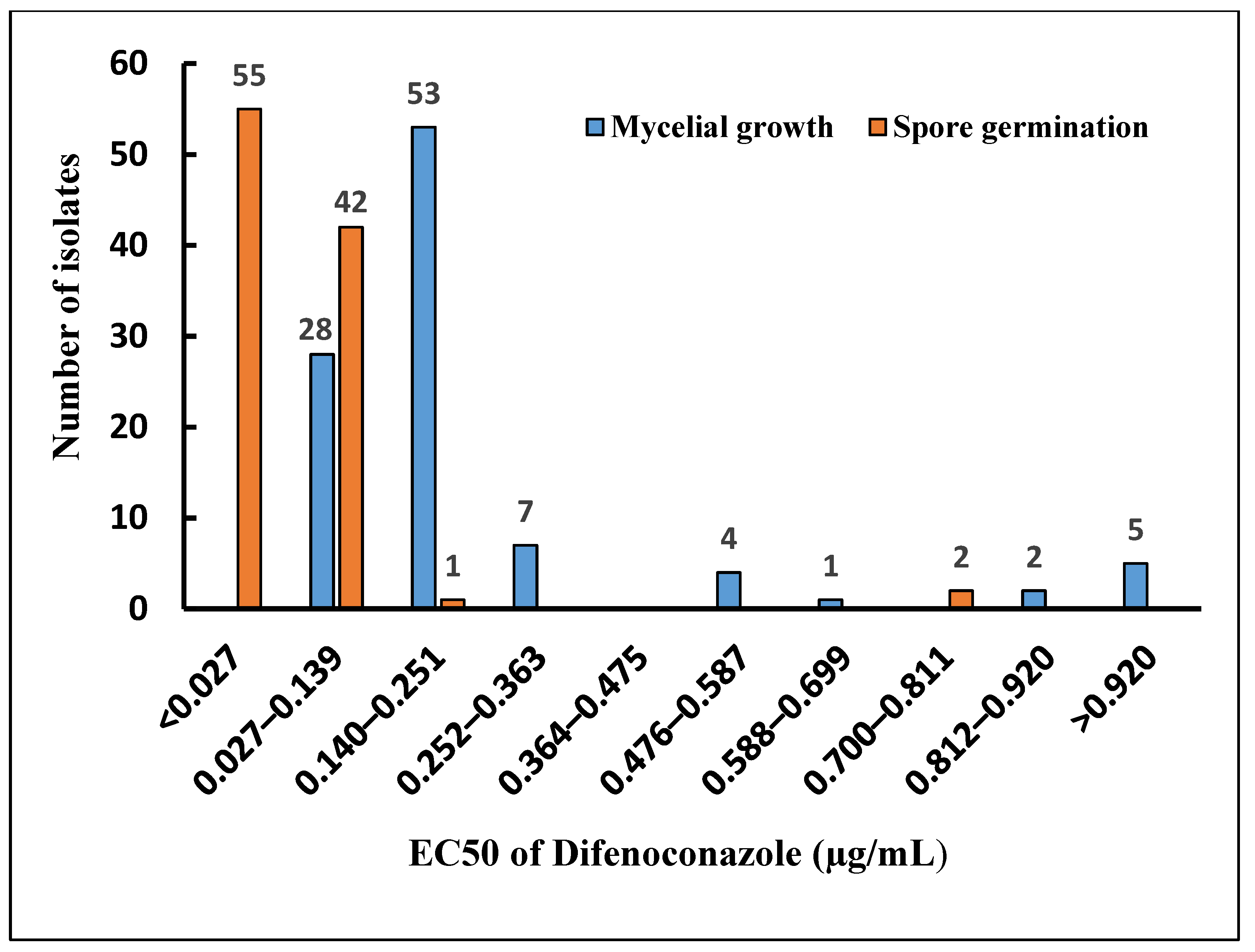
3.4. Analysis of EC50 Values
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, E.; Amiri, A. Selection Pressure Pathways and Mechanisms of Resistance to the Demethylation Inhibitor-Difenoconazole in Penicillium expansum. Front. Microbiol. 2018, 9, 2472. [Google Scholar] [CrossRef] [PubMed]
- Malandrakis, A.A.; Markoglou, A.N.; Konstantinou, S.; Doukas, E.G.; Kalampokis, J.F.; Karaoglanidis, G.S. Molecular Characterization, Fitness and Mycotoxin Production of Benzimidazole-Resistant Isolates of Penicillium Expansum. Int. J. Food Microbiol. 2013, 162, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Luciano-Rosario, D.; Keller, N.P.; Jurick, W.M. Penicillium expansum: Biology, Omics, and Management Tools for a Global Postharvest Pathogen Causing Blue Mould of Pome Fruit. Mol. Plant Pathol. 2020, 21, 1391–1404. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, D.A.; Engle, C.A.; Meyer, F.W.; Watkins, C.B. Penicillium expansum Invades Apples Through Stems During Controlled Atmosphere Storage. Plant Health Prog. 2006, 7, 1. [Google Scholar] [CrossRef]
- Yu, L.; Qiao, N.; Zhao, J.; Zhang, H.; Tian, F.; Zhai, Q.; Chen, W. Postharvest Control of Penicillium expansum in Fruits: A Review. Food Biosci. 2020, 36, 100633. [Google Scholar] [CrossRef]
- Ritieni, A. Patulin in Italian Commercial Apple Products. J. Agric. Food Chem. 2003, 51, 6086–6090. [Google Scholar] [CrossRef]
- Malandrakis, A.A.; Vattis, K.N.; Markoglou, A.N.; Karaoglanidis, G.S. Characterization of Boscalid-Resistance Conferring Mutations in the SdhB Subunit of Respiratory Complex II and Impact on Fitness and Mycotoxin Production in Penicillium Expansum Laboratory Strains. Pestic. Biochem. Physiol. 2017, 138, 97–103. [Google Scholar] [CrossRef]
- Karaoglanidis, G.S.; Markoglou, A.N.; Bardas, G.A.; Doukas, E.G.; Konstantinou, S.; Kalampokis, J.F. Sensitivity of Penicillium expansum Field Isolates to Tebuconazole, Iprodione, Fludioxonil and Cyprodinil and Characterization of Fitness Parameters and Patulin Production. Int. J. Food Microbiol. 2011, 145, 195–204. [Google Scholar] [CrossRef]
- Li, H.X.; Xiao, C.L. Baseline Sensitivities to Fludioxonil and Pyrimethanil in Penicillium expansum Populations from Apple in Washington State. Postharvest Biol. Technol. 2008, 47, 239–245. [Google Scholar] [CrossRef]
- Jurick, W.M.; Macarisin, O.; Gaskins, V.L.; Janisiewicz, W.J.; Peter, K.A.; Cox, K.D. Baseline Sensitivity of Penicillium spp. to Difenoconazole. Plant Dis. 2019, 103, 331–337. [Google Scholar] [CrossRef]
- Birr, T.; Hasler, M.; Verreet, J.A.; Klink, H. Temporal Changes in Sensitivity of Zymoseptoria Tritici Field Populations to Different Fungicidal Modes of Action. Agriculture 2021, 11, 269. [Google Scholar] [CrossRef]
- Yin, Y.N.; Xiao, C.-L. Molecular Characterization and a Multiplex Allele-Specific PCR Method for Detection of Thiabendazole Resistance in Penicillium expansum from Apple. Eur. J. plant Pathol. 2013, 136, 703–713. [Google Scholar] [CrossRef]
- Samaras, A.; Ntasiou, P.; Myresiotis, C.; Karaoglanidis, G. Multidrug Resistance of Penicillium expansum to Fungicides: Whole Transcriptome Analysis of MDR Strains Reveals Overexpression of Efflux Transporter Genes. Int. J. Food Microbiol. 2020, 335, 108896. [Google Scholar] [CrossRef] [PubMed]
- Khadiri, M.; Boubaker, H.; Laasli, S.-E.; Farhaoui, A.; Ezrari, S.; Radouane, N.; Radi, M.; Askarne, L.; Barka, E.A.; Lahlali, R. Unlocking Nature’s Secrets: Molecular Insights into Postharvest Pathogens Impacting Moroccan Apples and Innovations in the Assessment of Storage Conditions. Plants 2024, 13, 553. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, S.; Karaoglanidis, G.S.; Bardas, G.A.; Minas, I.S.; Doukas, E.; Markoglou, A.N. Postharvest Fruit Rots of Apple in Greece: Pathogen Incidence and Relationships between Fruit Quality Parameters, Cultivar Susceptibility, and Patulin Production. Plant Dis. 2011, 95, 666–672. [Google Scholar] [CrossRef]
- Wenneker, M.; Köhl, J. Postharvest Decay of Apples and Pears in the Netherlands. Acta Hortic. 2014, 1053, 107–112. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Samson, R.A. Polyphasic Taxonomy of Penicillium Subgenus Penicillium. A Guide to Identification of Food and Air-Borne Terverticillate Penicillia and Their Mycotoxins. Stud. Mycol. 2004, 49, 1–174. [Google Scholar]
- Pitt, J.I. A Laboratory Guide to Common Penicillium Species, 3rd ed.; Commonwealth Scientific and Industrial Research Organization, Division of Food Processing: North Ryde, NSW, Australia, 2000; p. 197. ISBN 9780643048379. [Google Scholar]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage; Springer: New York, NY, USA, 2009; Volume 519. [Google Scholar]
- Doyl, J.J.; Doyle, J.L. Isolation of Plant DNA from Fresh Tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Tannous, J.; Atoui, A.; El Khoury, A.; Kantar, S.; Chdid, N.; Oswald, I.P.; Puel, O.; Lteif, R. Development of a Real-Time PCR Assay for Penicillium expansum Quantification and Patulin Estimation in Apples. Food Microbiol. 2015, 50, 28–37. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, B.; Zhu, F.; Fu, Y. Baseline Sensitivity and Fungicidal Action of Propiconazole against Penicillium digitatum. Pestic. Biochem. Physiol. 2021, 172, 104752. [Google Scholar] [CrossRef]
- Boubaker, H.; Saadi, B.; Boudyach, E.H.; Benaoumar, A.A. Sensitivity of Penicillium digitatum and P. Italicum to Imazalil and Thiabendazole in Morocco. Plant Pathol. J. 2009, 8, 152–158. [Google Scholar] [CrossRef]
- El Housni, Z.; Tahiri, A.; Ezrari, S.; Radouane, N.; Ouijja, A. Occurrence of Cercospora beticola Sacc Populations Resistant to Benzimidazole, Demethylation-Inhibiting, and Quinone Outside Inhibitors Fungicides in Morocco. Eur. J. Plant Pathol. 2023, 165, 73–83. [Google Scholar] [CrossRef]
- Qin, G.Z.; Tian, S.P.; Xu, Y.; Wan, Y.K. Enhancement of Biocontrol Efficacy of Antagonistic Yeasts by Salicylic Acid in Sweet Cherry Fruit. Physiol. Mol. Plant Pathol. 2003, 62, 147–154. [Google Scholar] [CrossRef]
- Cabañas, R.; Abarca, M.L.; Bragulat, M.R.; Cabañes, F.J. In Vitro Activity of Imazalil against Penicillium expansum: Comparison of the CLSI M38-A Broth Microdilution Method with Traditional Techniques. Int. J. Food Microbiol. 2009, 129, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Khadiri, M.; Boubaker, H.; Askarne, L.; Ezrari, S.; Radouane, N.; Farhaoui, A.; El Hamss, H.; Tahiri, A.; Barka, E.A.; Lahlali, R. Bacillus cereus B8W8 an Effective Bacterial Antagonist against Major Postharvest Fungal Pathogens of Fruit. Postharvest Biol. Technol. 2023, 200, 112315. [Google Scholar] [CrossRef]
- Fonseka, D.L.; Gudmestad, N.C. Spatial and Temporal Sensitivity of Alternaria Species Associated with Potato Foliar Diseases to Demethylation Inhibiting and Anilino-Pyrimidine Fungicides. Plant Dis. 2016, 100, 1848–1857. [Google Scholar] [CrossRef]
- Cao, X.; Xu, X.; Che, H.; West, J.S.; Luo, D. Distribution and Fungicide Sensitivity of Colletotrichum Species Complexes from Rubber Tree in Hainan, China. Plant Dis. 2017, 101, 1774–1780. [Google Scholar] [CrossRef]
- Ivic, D. Curative and Eradicative Effects of Fungicides. In Fungicides; IntechOpen: Londen, UK, 2010; pp. 3–22. [Google Scholar]
- Darolt, J.C.; da Rocha Neto, A.C.; Di Piero, R.M. Effects of the Protective, Curative, and Eradicative Applications of Chitosan against Penicillium expansum in Apples. Braz. J. Microbiol. 2016, 47, 1014–1019. [Google Scholar] [CrossRef]
- Song, Y.; Li, L.; Li, C.; Lu, Z.; Men, X.; Chen, F. Evaluating the Sensitivity and Efficacy of Fungicides with Different Modes of Action against Botryosphaeria dothidea. Plant Dis. 2018, 102, 1785–1793. [Google Scholar] [CrossRef]
- Nikou, D.; Malandrakis, A.; Konstantakaki, M.; Vontas, J.; Markoglou, A.; Ziogas, B. Molecular Characterization and Detection of Overexpressed C-14 Alpha-Demethylase-Based DMI Resistance in Cercospora beticola Field Isolates. Pestic. Biochem. Physiol. 2009, 95, 18–27. [Google Scholar] [CrossRef]
- Kumar, R.; Mazakova, J.; Ali, A.; Sur, V.P.; Sen, M.K.; Bolton, M.D.; Manasova, M.; Rysanek, P.; Zouhar, M. Characterization of the Molecular Mechanisms of Resistance against DMI Fungicides in Cercospora beticola Populations from the Czech Republic. J. Fungi 2021, 7, 1062. [Google Scholar] [CrossRef]
- Muellender, M.M.; Mahlein, A.; Stammler, G.; Varrelmann, M. Evidence for the Association of Target-site Resistance in Cyp51 with Reduced DMI Sensitivity in European Cercospora beticola Field Isolates. Pest Manag. Sci. 2021, 77, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Spanner, R.; Taliadoros, D.; Richards, J.; Rivera-Varas, V.; Neubauer, J.; Natwick, M.; Hamilton, O.; Vaghefi, N.; Pethybridge, S.; Secor, G.A. Genome-Wide Association and Selective Sweep Studies Reveal the Complex Genetic Architecture of DMI Fungicide Resistance in Cercospora Beticola. Genome Biol. Evol. 2021, 13, evab209. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, G.; Jones, A.L. The 14α-Demethylasse (CYP51A1) Gene Is Overexpressed in Venturia inaequalis Strains Resistant to Myclobutanil. Phytopathology 2001, 91, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Errampalli, D.; Brubacher, N.R.; DeEll, J.R. Sensitivity of Penicillium expansum to Diphenylamine and Thiabendazole and Postharvest Control of Blue Mold with Fludioxonil in “McIntosh” Apples. Postharvest Biol. Technol. 2006, 39, 101–107. [Google Scholar] [CrossRef]
- Caiazzo, R.; Kim, Y.K.; Xiao, C.L. Occurrence and Phenotypes of Pyrimethanil Resistance in Penicillium Expansum from Apple in Washington State. Plant Dis. 2014, 98, 924–928. [Google Scholar] [CrossRef]
- Rocchi, S.; Morin-Crini, N.; Léchenault-Bergerot, C.; Godeau, C.; Fourmentin, M.; Millon, L.; Crini, G. Effet de l’interaction Cyclodextrine-Difénoconazole Sur La Croissance d’une Moisissure Responsable d’infections Fongiques Graves. Environ. Risques Santé 2019, 18, 411–417. [Google Scholar]
- Soro, D.B.; Kouadio, D.L.; Aboua, K.N.; Diarra, M.; Meite, L.; Traore, K.S. Dégradation Photocatalytique Du Thiabendazole En Solution Aqueuse. Afr. Sci. 2018, 14, 55–63. [Google Scholar]
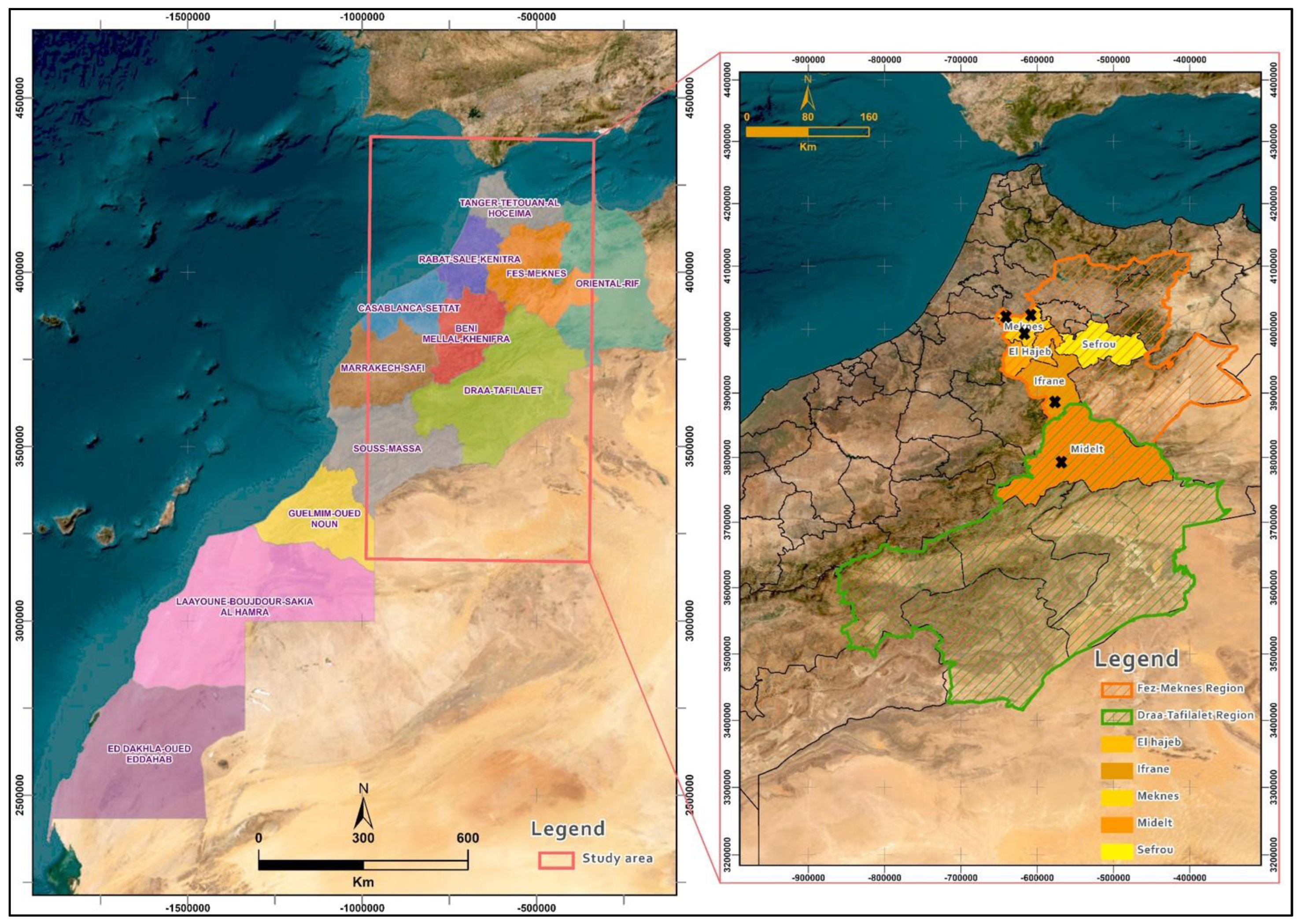


| Isolate | Year | Cultivar or Source | GPS Coordinates of Storage Stations | PAD |
|---|---|---|---|---|
| IT2 | 2020 | SD | N 33°58′8.54976″; W 5°15′48.798″ | Meknes |
| IT3 | 2020 | GD | N 33°58′8.54976″; W 5°15′48.798″ | Meknes |
| IT4 | 2020 | GD | N 33°58′8.54976″; W 5°15′48.798″ | Meknes |
| IT5 | 2020 | GD | N 33°58′8.54976″; W 5°15′48.798″ | Meknes |
| IT9 | 2020 | GD | N 33°58′8.54976″; W 5°15′48.798″ | Meknes |
| IT10 | 2020 | GD | N 33°58′8.54976″; W 5°15′48.798″ | Meknes |
| T5a | 2020 | GD | N 33°22′29.50392″; W 5°22′38.24184″ | Ifrane |
| T10 | 2020 | GD | N 33°22′29.50392″; W 5°22′38.24184″ | Ifrane |
| T12b | 2020 | GD | N 33°22′29.50392″; W 5°22′38.24184″ | Ifrane |
| M7a | 2020 | F | N 33°24′52.4106″; W 5°17′40.58628″ | Ifrane |
| M11 | 2020 | SD | N 33°24′52.4106″; W 5°17′40.58628″ | Ifrane |
| MA2 | 2020 | GD | N 33°25′31.72944″; W 5°14′37.7826″ | Ifrane |
| MA5 | 2020 | SD | N 33°25′31.72944″; W 5°14′37.7826″ | Ifrane |
| MA6 | 2020 | SD | N 33°25′31.72944″; W 5°14′37.7826″ | Ifrane |
| A11 | 2020 | SD | N 33°25′31.72944″; W 5°14′37.7826″ | Ifrane |
| S2 | 2020 | GD | N 33°21′49.25448″; W 5°22′5.33568″ | Ifrane |
| S3 | 2020 | GD | N 33°21′49.25448″; W 5°22′5.33568″ | Ifrane |
| S5 | 2020 | F | N 33°21′49.25448″; W 5°22′5.33568″ | Ifrane |
| BNS2 | 2020 | GD | N 33°53′54.5208″; W 5°26′47.76864″ | Meknes |
| FMB8 | 2020 | GD | N 33°41′10.21704″; W 5°30′7.88904″ | El Hajeb |
| Bs2 | 2020 | GD | N 33°49′37.4″; W 5°28′2.9″ | Meknes |
| Bs3 | 2020 | GD | N 33°49′37.4″; W 5°28′2.9″ | Meknes |
| Bs(AS3) | 2020 | CRA | N 33°49′37.4″; W 5°28′2.9″ | Meknes |
| Bs(AS7) | 2020 | CRA | N 33°49′37.4″; W 5°28′2.9″ | Meknes |
| V5 | 2021 | GD | N 33°45′35.1″; W 5°19′37.1″ | El Hajeb |
| V6b | 2021 | GD | N 33°45′35.1″; W 5°19′37.1″ | El Hajeb |
| V7 | 2021 | GD | N 33°45′35.1″; W 5°19′37.1″ | El Hajeb |
| V10 | 2021 | GD | N 33°45′35.1″; W 5°19′37.1″ | El Hajeb |
| Tg10 | 2021 | GD | N 33°46′16.41″; W 5°20′42.0972″ | El Hajeb |
| Tg11 | 2021 | GD | N 33°46′16.41″; W 5°20′42.0972″ | El Hajeb |
| Ag3 | 2021 | GD | N 33°49′46.5456″; W 4°59′7.5876″ | Sefrou |
| Ag6 | 2021 | GD | N 33°49′46.5456″; W 4°59′7.5876″ | Sefrou |
| Ag8 | 2021 | GD | N 33°49′46.5456″; W 4°59′7.5876″ | Sefrou |
| AO1 | 2021 | GD | N 33°45′50.3568″; W 5°0′53.5644″ | Sefrou |
| AO3 | 2021 | GD | N 33°45′50.3568″; W 5°0′53.5644″ | Sefrou |
| AO6 | 2021 | GD | N 33°45′50.3568″; W 5°0′53.5644″ | Sefrou |
| At1 | 2021 | GD | N 33°46′28.4844″; W 5°1′20.3916″ | Sefrou |
| At2 | 2021 | GD | N 33°46′28.4844″; W 5°1′20.3916″ | Sefrou |
| At3 | 2021 | GD | N 33°46′28.4844″; W 5°1′20.3916″ | Sefrou |
| CH2 | 2021 | SD | N 33°42′42.588″; W 5°2′29.6484″ | Sefrou |
| CH4 | 2021 | GD | N 33°42′42.588″; W 5°2′29.6484″ | Sefrou |
| Tl1 | 2021 | GD | N 32°49′3.4032″; W 4°57′34.9056″ | Midelt |
| Tl2 | 2021 | GD | N 32°49′3.4032″; W 4°57′34.9056″ | Midelt |
| Tl3 | 2021 | GD | N 32°49′3.4032″; W 4°57′34.9056″ | Midelt |
| Tl4 | 2021 | GD | N 32°49′3.4032″; W 4°57′34.9056″ | Midelt |
| Tl5 | 2021 | GD | N 32°49′3.4032″; W 4°57′34.9056″ | Midelt |
| Tl6 | 2021 | GD | N 32°49′3.4032″; W 4°57′34.9056″ | Midelt |
| TM1 | 2021 | GD | N 32°59′49.1496″; W 4°52′24.3228″ | Midelt |
| TM4 | 2021 | DG | N 32°59′49.1496″; W 4°52′24.3228″ | Midelt |
| TM6 | 2021 | GD | N 32°59′49.1496″; W 4°52′24.3228″ | Midelt |
| TM7 | 2021 | GD | N 32°59′49.1496″; W 4°52′24.3228″ | Midelt |
| Aby4 | 2021 | GD | N 32°45′2.2896″; W 5°1′45.1776″ | Midelt |
| Ml4 | 2021 | GD | N 32°44′56.5944″; W 5°1′45.3864″ | Midelt |
| HM1 | 2021 | GD | N 32°54′7.4664″; W 4°58′12.5256″ | Midelt |
| HM3a | 2021 | GD | N 32°54′7.4664″; W 4°58′12.5256″ | Midelt |
| AH1 | 2021 | GD | N 32°46′3.4356″; W 5°0′8.136″ | Midelt |
| AH2 | 2021 | GD | N 32°46′3.4356″; W 5°0′8.136″ | Midelt |
| AH3 | 2021 | GD | N 32°46′3.4356″; W 5°0′8.136″ | Midelt |
| AH4 | 2021 | GD | N 32°46′3.4356″; W 5°0′8.136″ | Midelt |
| BK3 | 2021 | GD | N 32°46′12.5688″; W 4°59′56.4684″ | Midelt |
| BK5 | 2021 | F | N 32°46′12.5688″; W 4°59′56.4684″ | Midelt |
| BK7 | 2021 | GD | N 32°46′12.5688″; W 4°59′56.4684″ | Midelt |
| ASL1 | 2021 | GD | N 32°43′16.4028″; W 5°3′13.3488″ | Midelt |
| ASL4 | 2021 | GD | N 32°43′16.4028″; W 5°3′13.3488″ | Midelt |
| ASL6 | 2021 | GD | N 32°43′16.4028″; W 5°3′13.3488″ | Midelt |
| DA2 | 2021 | DG | N 32°40′27.5052″; W 5°15′30.9204″ | Midelt |
| DA3 | 2021 | GD | N 32°40′27.5052″; W 5°15′30.9204″ | Midelt |
| DA5 | 2021 | GD | N 32°40′27.5052″; W 5°15′30.9204″ | Midelt |
| DA7 | 2021 | GD | N 32°40′27.5052″; W 5°15′30.9204″ | Midelt |
| DA8 | 2021 | F | N 32°40′27.5052″; W 5°15′30.9204″ | Midelt |
| PR2 | 2021 | SD | N 33°47′43.5336″; W 5°29′41.226″ | Meknes |
| DN2 | 2021 | SD | N 33°44′28.5324″; W 5°28′21.0756″ | Meknes |
| DN3 | 2021 | F | N 33°44′28.5324″; W 5°28′21.0756″ | Meknes |
| DN9 | 2021 | SD | N 33°44′28.5324″; W 5°28′21.0756″ | Meknes |
| DN10 | 2021 | GD | N 33°44′28.5324″; W 5°28′21.0756″ | Meknes |
| AML8 | 2021 | GD | N 33°48′28.6812″; W 5°22′35.2884″ | El Hajeb |
| OS14 | 2021 | GD | N 33°55′35.526″; W 4°54′1.9584″ | Sefrou |
| OS15 | 2021 | DG | N 33°55′35.526″; W 4°54′1.9584″ | Sefrou |
| CB1 | 2021 | GD | N 33°51′36.2484″; W 4°29′56.94″ | Sefrou |
| ZA1 | 2021 | GD | N 33°49′9.39″; W 4°45′58.9068″ | Sefrou |
| ZA2 | 2021 | GD | N 33°49′9.39″; W 4°45′58.9068″ | Sefrou |
| ZA3 | 2021 | GD | N 33°49′9.39″; W 4°45′58.9068″ | Sefrou |
| DKS2b | 2022 | SD | N 33°49′39.2592″; W 5°31′8.1372″ | Meknes |
| AM2 | 2022 | GD | N 33°45′49.4352″; W 5°20′15.2448″ | El Hajeb |
| LM1 | 2022 | GD | N 33°46′38.1036″; W 5°22′54.2928″ | El Hajeb |
| FN1 | 2022 | GD | N 33°58′4.116″; W 5°14′15.8928″ | Meknes |
| FN8 | 2022 | GD | N 33°58′4.116″; W 5°14′15.8928″ | Meknes |
| MY8 | 2022 | GD | N 33°59′36.8088″; W 5°12′2.4948″ | Meknes |
| MY10 | 2022 | SD | N 33°59′36.8088″; W 5°12′2.4948″ | Meknes |
| BNS3 | 2022 | SD | N 33°53′54.5208″; W 5°26′47.76864″ | Meknes |
| BNS4 | 2022 | GD | N 33°53′54.5208″; W 5°26′47.76864″ | Meknes |
| BNS5 | 2022 | SD | N 33°53′54.5208″; W 5°26′47.76864″ | Meknes |
| BNS8 | 2022 | SD | N 33°53′54.5208″; W 5°26′47.76864″ | Meknes |
| BNS25 | 2022 | GD | N 33°53′54.5208″; W 5°26′47.76864″ | Meknes |
| BNS32 | 2022 | GD | N 33°53′54.5208″; W 5°26′47.76864″ | Meknes |
| AML13 | 2022 | GD | N 33°48′28.6812″; W 5°22′35.2884″ | El Hajeb |
| AML17 | 2022 | GD | N 33°48′28.6812″; W 5°22′35.2884″ | El Hajeb |
| AML18 | 2022 | GD | N 33°48′28.6812″; W 5°22′35.2884″ | El Hajeb |
| AML25b | 2022 | GD | N 33°48′28.6812″; W 5°22′35.2884″ | El Hajeb |
| TG3 | 2022 | GD | N 33°46′16.41″; W 5°20′42.0972″ | El Hajeb |
| Isolate | PAD | PI (MG) | PI (SG) | EC50 (MG) µg/mL | EC50 (SG) µg/mL | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.01 µg/mL | 0.5 µg/mL | 1 µg/mL | 5 µg/mL | 0.01 µg/mL | 0.5 µg/mL | 1 µg/mL | 5 µg/mL | ||||
| IT2 | Meknes | 18.25 | 59.10 | 69.96 | 100 | 33.96 | 100 | 100 | 100 | 0.158 | 0.027 |
| IT3 | Meknes | 19.53 | 63.23 | 68.81 | 100 | 50.93 | 95.06 | 100 | 100 | 0.143 | 0.009 |
| IT4 | Meknes | 21.18 | 62.52 | 71.94 | 100 | 47.95 | 86.30 | 100 | 100 | 0.130 | 0.013 |
| IT5 | Meknes | 20.99 | 58.57 | 69.83 | 100 | 42.81 | 95.89 | 100 | 100 | 0.145 | 0.017 |
| IT9 | Meknes | 16.87 | 60.21 | 67.17 | 78.35 | 49.31 | 95.14 | 100 | 100 | 0.208 | 0.011 |
| IT10 | Meknes | 20.29 | 65.29 | 68.72 | 81.71 | 23.51 | 40.73 | 88.08 | 100 | 0.155 | 0.076 |
| T5a | Ifrane | 9.89 | 52.44 | 60.11 | 100 | 45.45 | 82.21 | 100 | 100 | 0.262 | 0.017 |
| T10 | Ifrane | 19.63 | 68.12 | 77.45 | 100 | 57.25 | 93.89 | 100 | 100 | 0.114 | 0.005 |
| T12b | Ifrane | 10.47 | 67.97 | 78.22 | 83.52 | 33.33 | 94.10 | 100 | 100 | 0.178 | 0.030 |
| M7a | Ifrane | 18.79 | 49.85 | 56.67 | 78.29 | 32.21 | 54.70 | 74.83 | 91.95 | 0.895 | 0.095 |
| M11 | Ifrane | 31.94 | 44.66 | 49.65 | 72.86 | 51.82 | 72.12 | 79.09 | 100 | 1.442 | 0.011 |
| MA2 | Ifrane | 12.73 | 63.75 | 73.29 | 100 | 35.76 | 78.79 | 100 | 100 | 0.167 | 0.033 |
| MA5 | Ifrane | 12.10 | 60.56 | 68.88 | 83.20 | 38.64 | 96.27 | 100 | 100 | 0.223 | 0.022 |
| MA6 | Ifrane | 18.90 | 58.61 | 65.98 | 79.43 | 40.40 | 100 | 100 | 100 | 0.200 | 0.019 |
| MA11 | Ifrane | 11.65 | 60.69 | 68.38 | 82.96 | 58.62 | 96.26 | 100 | 100 | 0.228 | 0.004 |
| S2 | Ifrane | 14.09 | 70.56 | 73.88 | 100 | 56.89 | 94.70 | 100 | 100 | 0.141 | 0.005 |
| S3 | Ifrane | 6.21 | 62.92 | 69.30 | 100 | 53.57 | 95.71 | 100 | 100 | 0.217 | 0.007 |
| S5 | Ifrane | 10.97 | 54.51 | 64.26 | 75.48 | 43.66 | 96.83 | 100 | 100 | 0.301 | 0.016 |
| BNS2 | Meknes | 15.09 | 62.67 | 69.67 | 100 | 22.22 | 81.25 | 88.89 | 100 | 0.166 | 0.068 |
| FMB8 | El Hajeb | 14.05 | 57.25 | 65.39 | 82.22 | 50.00 | 95.33 | 100 | 100 | 0.238 | 0.010 |
| Bs2 | Meknes | 9.71 | 62.96 | 67.25 | 100 | 61.72 | 95.70 | 100 | 100 | 0.202 | 0.003 |
| Bs3 | Meknes | 13.50 | 40.38 | 51.30 | 79.25 | 43.94 | 96.21 | 100 | 100 | 0.894 | 0.016 |
| Bs(AS3) | Meknes | 16.52 | 37.97 | 49.79 | 71.50 | 63.75 | 95.83 | 100 | 100 | 0.994 | 0.002 |
| Bs(AS7) | Meknes | 11.08 | 40.30 | 47.17 | 69.94 | 60.63 | 96.56 | 100 | 100 | 1.673 | 0.003 |
| V5 | El Hajeb | 18.26 | 68.27 | 73.73 | 100 | 64.52 | 95.56 | 100 | 100 | 0.127 | 0.002 |
| V6b | El Hajeb | 8.26 | 62.23 | 70.68 | 100 | 69.32 | 94.89 | 100 | 100 | 0.204 | 0.0006 |
| V7 | El Hajeb | 26.10 | 63.10 | 70.65 | 83.62 | 58.87 | 75.81 | 82.66 | 100 | 0.121 | 0.004 |
| V10 | El Hajeb | 19.05 | 69.83 | 81.06 | 100 | 56.06 | 90.91 | 100 | 100 | 0.107 | 0.006 |
| Tg10 | El Hajeb | 8.82 | 65.62 | 72.54 | 100 | 44.74 | 94.41 | 100 | 100 | 0.185 | 0.015 |
| Tg11 | El Hajeb | 4.50 | 54.89 | 63.59 | 100 | 17.65 | 94.49 | 100 | 100 | 0.277 | 0.056 |
| Ag3 | Sefrou | 8.53 | 59.05 | 66.10 | 100 | 80.92 | 98.03 | 100 | 100 | 0.226 | 0.013 |
| Ag6 | Sefrou | 9.24 | 73.42 | 79.19 | 100 | 27.27 | 96.59 | 100 | 100 | 0.147 | 0.038 |
| Ag8 | Sefrou | 4.49 | 64.90 | 73.16 | 100 | 53.85 | 92.31 | 100 | 100 | 0.210 | 0.007 |
| AO1 | Sefrou | 3.90 | 68.42 | 74.46 | 100 | 30.77 | 96.63 | 100 | 100 | 0.198 | 0.033 |
| AO3 | Sefrou | 4.82 | 58.61 | 68.27 | 84.41 | 40.00 | 95.63 | 100 | 100 | 0.283 | 0.021 |
| AO6 | Sefrou | 13.87 | 59.86 | 66.09 | 77.21 | 68.42 | 73.68 | 94.41 | 100 | 0.238 | 0.0002 |
| At1 | Sefrou | 10.39 | 76.31 | 81.06 | 88.07 | 18.27 | 92.31 | 97.60 | 100 | 0.143 | 0.058 |
| At2 | Sefrou | 26.72 | 81.71 | 86.43 | 100 | 61.67 | 100 | 100 | 100 | 0.054 | 0.003 |
| At3 | Sefrou | 16.61 | 80.61 | 84.61 | 100 | 7.14 | 94.20 | 100 | 100 | 0.092 | 0.075 |
| CH2 | Sefrou | 20.06 | 72.91 | 78.62 | 84.09 | 28.57 | 96.43 | 100 | 100 | 0.111 | 0.036 |
| CH4 | Sefrou | 12.79 | 68.76 | 75.54 | 83.83 | 16.52 | 87.50 | 92.86 | 100 | 0.169 | 0.069 |
| Tl1 | Midelt | 10.55 | 50.47 | 63.14 | 100 | 26.67 | 80.00 | 93.33 | 100 | 0.498 | 0.054 |
| Tl2 | Midelt | 18.45 | 68.28 | 72.87 | 79.50 | 22.29 | 40.29 | 58.57 | 95.43 | 0.149 | 0.712 |
| Tl3 | Midelt | 16.53 | 70.85 | 75.03 | 100 | 45.00 | 86.67 | 100 | 100 | 0.126 | 0.017 |
| Tl4 | Midelt | 10.07 | 55.85 | 64.02 | 100 | 57.50 | 83.33 | 90.00 | 94.17 | 0.234 | 0.003 |
| Tl5 | Midelt | 11.14 | 67.72 | 72.31 | 100 | 55.00 | 76.67 | 93.33 | 100 | 0.167 | 0.005 |
| Tl6 | Midelt | 10.58 | 55.17 | 61.27 | 100 | 58.18 | 72.27 | 92.73 | 100 | 0.243 | 0.003 |
| TM1 | Midelt | 4.48 | 69.01 | 73.58 | 82.98 | 23.21 | 69.64 | 91.96 | 100 | 0.227 | 0.076 |
| TM4 | Midelt | 2.70 | 67.03 | 73.93 | 83.96 | 57.14 | 82.14 | 92.86 | 100 | 0.242 | 0.003 |
| TM6 | Midelt | 3.01 | 72.76 | 77.20 | 100 | 55.42 | 93.33 | 100 | 100 | 0.184 | 0.004 |
| TM7 | Midelt | 5.67 | 57.45 | 64.56 | 82.73 | 24.04 | 77.88 | 92.79 | 100 | 0.302 | 0.063 |
| Aby4 | Midelt | 10.30 | 46.95 | 72.34 | 100 | 31.58 | 94.74 | 100 | 100 | 0.520 | 0.057 |
| Ml4 | Midelt | 6.36 | 66.98 | 81.31 | 100 | 17.65 | 66.91 | 96.69 | 100 | 0.173 | 0.095 |
| HM1 | Midelt | 10.45 | 40.87 | 74.00 | 100 | 16.67 | 88.89 | 94.44 | 100 | 0.563 | 0.077 |
| HM3a | Midelt | 9.28 | 51.60 | 69.62 | 100 | 6.67 | 86.67 | 96.25 | 100 | 0.358 | 0.088 |
| AH1 | Midelt | 10.90 | 77.02 | 83.37 | 100 | 56.25 | 100 | 100 | 100 | 0.124 | 0.004 |
| AH2 | Midelt | 4.49 | 74.34 | 81.21 | 100 | 29.03 | 96.77 | 100 | 100 | 0.163 | 0.035 |
| AH3 | Midelt | 5.97 | 73.32 | 78.95 | 100 | 48.96 | 69.44 | 83.33 | 100 | 0.164 | 0.012 |
| AH4 | Midelt | 6.48 | 70.59 | 76.36 | 100 | 32.41 | 85.86 | 94.48 | 100 | 0.174 | 0.036 |
| BK3 | Midelt | 10.15 | 63.65 | 70.66 | 85.80 | 38.46 | 93.75 | 100 | 100 | 0.207 | 0.023 |
| BK5 | Midelt | 9.96 | 64.51 | 72.67 | 100 | 18.75 | 69.64 | 76.79 | 100 | 0.178 | 0.116 |
| BK7 | Midelt | 10.43 | 63.39 | 74.21 | 82.72 | 58.33 | 96.35 | 100 | 100 | 0.216 | 0.004 |
| ASL1 | Midelt | 11.66 | 76.39 | 80.02 | 100 | 35.71 | 79.93 | 87.07 | 100 | 0.128 | 0.033 |
| ASL4 | Midelt | 11.11 | 75.80 | 79.23 | 100 | 18.52 | 92.13 | 100 | 100 | 0.133 | 0.056 |
| ASL6 | Midelt | 9.57 | 79.54 | 83.34 | 100 | 38.46 | 69.23 | 94.23 | 100 | 0.124 | 0.033 |
| DA2 | Midelt | 20.99 | 63.22 | 67.98 | 85.96 | 50.96 | 95.67 | 100 | 100 | 0.153 | 0.009 |
| DA3 | Midelt | 6.67 | 62.73 | 71.38 | 81.70 | 23.53 | 98.90 | 100 | 100 | 0.247 | 0.043 |
| DA5 | Midelt | 8.88 | 62.02 | 69.71 | 84.03 | 36.96 | 99.01 | 100 | 100 | 0.235 | 0.024 |
| DA7 | Midelt | 13.85 | 26.73 | 59.94 | 82.84 | 47.50 | 66.67 | 93.33 | 100 | 0.971 | 0.011 |
| DA8 | Midelt | 3.70 | 60.09 | 70.66 | 85.32 | 47.06 | 93.75 | 100 | 100 | 0.272 | 0.013 |
| PR2 | Meknes | 19.22 | 68.95 | 74.87 | 100 | 6.67 | 97.08 | 100 | 100 | 0.119 | 0.074 |
| DN2 | Meknes | 15.59 | 66.62 | 71.13 | 81.36 | 40.23 | 78.43 | 91.25 | 100 | 0.175 | 0.025 |
| DN3 | Meknes | 19.05 | 63.28 | 69.50 | 79.36 | 25.50 | 85.91 | 93.62 | 96.64 | 0.171 | 0.059 |
| DN9 | Meknes | 2.42 | 26.13 | 38.68 | 71.82 | 15.38 | 33.17 | 61.54 | 100 | 1.582 | 0.787 |
| DN10 | Meknes | 9.54 | 65.28 | 77.49 | 100 | 13.39 | 92.86 | 100 | 100 | 0.170 | 0.065 |
| AML8 | El Hajeb | 20.94 | 69.28 | 76.51 | 100 | 73.33 | 96.25 | 100 | 100 | 0.108 | 0.0002 |
| OS14 | Sefrou | 15.87 | 74.83 | 80.68 | 86.51 | 7.25 | 55.07 | 78.26 | 100 | 0.121 | 0.168 |
| OS15 | Sefrou | 9.95 | 50.67 | 63.11 | 74.68 | 25.00 | 68.75 | 90.63 | 100 | 0.495 | 0.073 |
| CB1 | Sefrou | 9.17 | 82.09 | 86.18 | 100 | 55.15 | 84.19 | 100 | 100 | 0.116 | 0.004 |
| ZA1 | Sefrou | 15.27 | 100 | 100 | 100 | 36.08 | 94.94 | 100 | 100 | 0.050 | 0.026 |
| ZA2 | Sefrou | 10.87 | 68.30 | 74.96 | 86.06 | 26.67 | 60.00 | 75.83 | 85.00 | 0.180 | 0.116 |
| ZA3 | Sefrou | 12.33 | 35.67 | 73.89 | 100 | 48.57 | 77.14 | 82.86 | 88.57 | 0.684 | 0.012 |
| DKS2b | Meknes | 20.43 | 68.51 | 74.88 | 100 | 54.23 | 90.91 | 100 | 100 | 0.114 | 0.007 |
| AM2 | El Hajeb | 13.80 | 65.34 | 76.06 | 100 | 51.98 | 89.83 | 96.89 | 100 | 0.151 | 0.008 |
| LM1 | El Hajeb | 42.95 | 71.75 | 81.04 | 100 | 49.82 | 90.88 | 96.49 | 100 | 0.027 | 0.010 |
| FN1 | Meknes | 11.48 | 66.92 | 69.02 | 80.64 | 39.94 | 77.67 | 97.48 | 100 | 0.207 | 0.029 |
| FN8 | Meknes | 13.99 | 75.89 | 79.28 | 86.35 | 22.49 | 71.01 | 93.20 | 100 | 0.131 | 0.076 |
| MY8 | Meknes | 14.73 | 61.14 | 67.60 | 82.96 | 25.42 | 63.05 | 88.14 | 100 | 0.207 | 0.102 |
| MY10 | Meknes | 15.23 | 75.73 | 78.74 | 84.14 | 23.36 | 81.58 | 94.41 | 100 | 0.128 | 0.057 |
| BNS3 | Meknes | 18.45 | 64.33 | 72.48 | 84.31 | 31.82 | 87.34 | 100 | 100 | 0.156 | 0.037 |
| BNS4 | Meknes | 24.49 | 61.97 | 69.39 | 83.02 | 43.73 | 92.28 | 100 | 100 | 0.139 | 0.017 |
| BNS5 | Meknes | 22.03 | 71.43 | 81.00 | 100 | 50.32 | 82.28 | 93.67 | 95.89 | 0.092 | 0.010 |
| BNS8 | Meknes | 19.20 | 69.03 | 77.22 | 85.45 | 21.82 | 75.57 | 84.04 | 100 | 0.127 | 0.081 |
| BNS25 | Meknes | 18.02 | 64.65 | 72.33 | 77.06 | 18.69 | 93.77 | 97.38 | 100 | 0.170 | 0.056 |
| BNS32 | Meknes | 14.74 | 65.67 | 73.75 | 82.03 | 37.17 | 96.17 | 100 | 100 | 0.177 | 0.024 |
| AML13 | El Hajeb | 27.04 | 60.23 | 70.67 | 100 | 26.02 | 82.45 | 95.30 | 97.81 | 0.110 | 0.062 |
| AML17 | El Hajeb | 19.45 | 62.96 | 77.43 | 82.36 | 23.05 | 90.91 | 100 | 100 | 0.147 | 0.049 |
| AML18 | El Hajeb | 23.22 | 72.19 | 81.46 | 100 | 19.75 | 85.03 | 100 | 100 | 0.087 | 0.059 |
| AML25b | El Hajeb | 19.00 | 63.92 | 74.58 | 100 | 44.05 | 76.79 | 88.99 | 95.83 | 0.132 | 0.018 |
| TG3 | El Hajeb | 10.81 | 69.92 | 79.17 | 100 | 44.41 | 91.78 | 96.38 | 100 | 0.148 | 0.016 |
| EC50 (µg/mL) | Mycelial Growth | Spore Germination | Reference |
|---|---|---|---|
| Mean | 0.263 | 0.048 | This study |
| Min | 0.027 | 0.0002 | |
| Max | 1.673 | 0.787 | |
| VF * | 62.507 | 4113.835 | |
| Mean | 0.18 | 0.32 | [1] |
| Min | 0.13 | 0.19 | |
| Max | 0.29 | 0.37 | |
| VF * | 2.23 | 1.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khadiri, M.; Boubaker, H.; Farhaoui, A.; Ezrari, S.; Radi, M.; Ezzouggari, R.; Mokrini, F.; Barka, E.A.; Lahlali, R. In Vitro Assessment of Penicillium expansum Sensitivity to Difenoconazole. Microorganisms 2024, 12, 2169. https://doi.org/10.3390/microorganisms12112169
Khadiri M, Boubaker H, Farhaoui A, Ezrari S, Radi M, Ezzouggari R, Mokrini F, Barka EA, Lahlali R. In Vitro Assessment of Penicillium expansum Sensitivity to Difenoconazole. Microorganisms. 2024; 12(11):2169. https://doi.org/10.3390/microorganisms12112169
Chicago/Turabian StyleKhadiri, Mohammed, Hassan Boubaker, Abdelaaziz Farhaoui, Said Ezrari, Mohammed Radi, Rachid Ezzouggari, Fouad Mokrini, Essaid Ait Barka, and Rachid Lahlali. 2024. "In Vitro Assessment of Penicillium expansum Sensitivity to Difenoconazole" Microorganisms 12, no. 11: 2169. https://doi.org/10.3390/microorganisms12112169
APA StyleKhadiri, M., Boubaker, H., Farhaoui, A., Ezrari, S., Radi, M., Ezzouggari, R., Mokrini, F., Barka, E. A., & Lahlali, R. (2024). In Vitro Assessment of Penicillium expansum Sensitivity to Difenoconazole. Microorganisms, 12(11), 2169. https://doi.org/10.3390/microorganisms12112169











