Effects of Free or Immobilized Pediococcus acidilactici ORE5 on Corinthian Currants on Gut Microbiome of Streptozotocin-Induced Diabetic Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Strain
2.2. Immobilization of Cells on Corinthian Currants and Freeze-Drying
2.3. Scanning Electron Microscopy
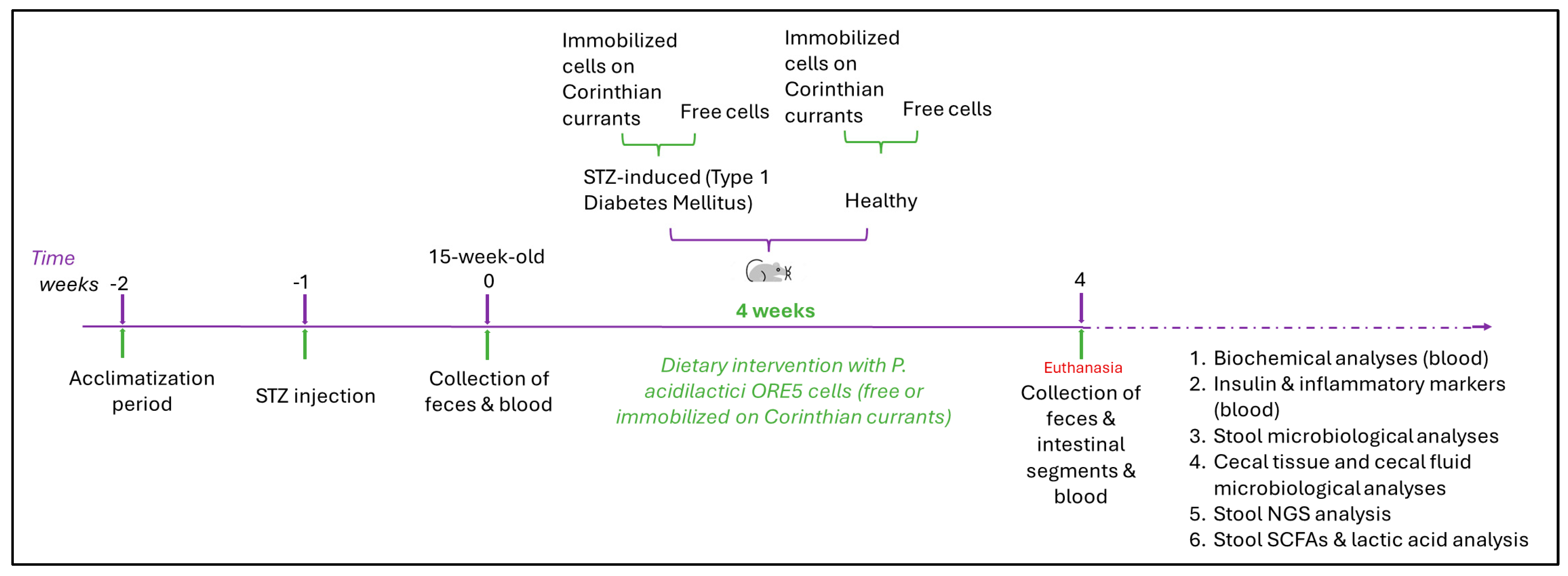
2.4. In Vivo Study Design
2.4.1. STZ-Induced Animal Model
2.4.2. Dietary Intervention with Probiotics
2.4.3. Sample Collection
2.5. Sample Analyses
2.5.1. Blood Analyses
2.5.2. Microbiological Analyses
Determination of P. acidilactici ORE5 Levels
Stool and Tissue Microbiota Analyses
2.5.3. DNA Extraction, PCR Amplification and 16S rRNA Gene Sequencing
2.6. Stool SCFAs and Lactic Acid Profile
2.7. Statistical Analysis
3. Results and Discussion
3.1. Immobilized P. acidilactici ORE5 Cells on Corinthian Currant
3.2. Body Weight, Biochemical Profile and Inflammatory Factors
3.3. Fecal and Intestinal Microbiota Analysis
3.4. Microbiome Alterations Using NGS of 16S rRNA Gene
3.5. Stool SCFAs and Lactate
3.6. Limitations, Future Perspectives and Summary of Findings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toren, E.; Burnette, K.S.; Banerjee, R.R.; Hunter, C.S.; Tse, H.M. Partners in Crime: Beta-Cells and Autoimmune Responses Complicit in Type 1 Diabetes Pathogenesis. Front. Immunol. 2021, 12, 756548. [Google Scholar] [CrossRef] [PubMed]
- Del Chierico, F.; Rapini, N.; Deodati, A.; Matteoli, M.C.; Cianfarani, S.; Putignani, L. Pathophysiology of Type 1 Diabetes and Gut Microbiota Role. Int. J. Mol. Sci. 2022, 23, 14650. [Google Scholar] [CrossRef] [PubMed]
- Gregory, G.A.; Robinson, T.I.G.; Linklater, S.E.; Wang, F.; Colagiuri, S.; de Beaufort, C.; Donaghue, K.C.; Magliano, D.J.; Maniam, J.; Orchard, T.J.; et al. Global Incidence, Prevalence, and Mortality of Type 1 Diabetes in 2021 with Projection to 2040: A Modelling Study. Lancet Diabetes Endocrinol. 2022, 10, 741–760. [Google Scholar] [CrossRef] [PubMed]
- Ogrotis, I.; Koufakis, T.; Kotsa, K. Changes in the Global Epidemiology of Type 1 Diabetes in an Evolving Landscape of Environmental Factors: Causes, Challenges, and Opportunities. Medicina 2023, 59, 668. [Google Scholar] [CrossRef]
- Zhou, H.; Sun, L.; Zhang, S.; Zhao, X.; Gang, X.; Wang, G. Evaluating the Causal Role of Gut Microbiota in Type 1 Diabetes and Its Possible Pathogenic Mechanisms. Front. Endocrinol. 2020, 11, 125. [Google Scholar] [CrossRef]
- Mishra, S.; Wang, S.; Nagpal, R.; Miller, B.; Singh, R.; Taraphder, S.; Yadav, H. Probiotics and Prebiotics for the Amelioration of Type 1 Diabetes: Present and Future Perspectives. Microorganisms 2019, 7, 67. [Google Scholar] [CrossRef]
- Gradisteanu Pircalabioru, G.; Corcionivoschi, N.; Gundogdu, O.; Chifiriuc, M.-C.; Marutescu, L.G.; Ispas, B.; Savu, O. Dysbiosis in the Development of Type I Diabetes and Associated Complications: From Mechanisms to Targeted Gut Microbes Manipulation Therapies. Int. J. Mol. Sci. 2021, 22, 2763. [Google Scholar] [CrossRef]
- Gomes, A.C.; Bueno, A.A.; de Souza, R.G.M.; Mota, J.F. Gut Microbiota, Probiotics and Diabetes. Nutr. J. 2014, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Verduci, E.; Mameli, C.; Amatruda, M.; Petitti, A.; Vizzuso, S.; El Assadi, F.; Zuccotti, G.; Alabduljabbar, S.; Terranegra, A. Early Nutrition and Risk of Type 1 Diabetes: The Role of Gut Microbiota. Front. Nutr. 2020, 7, 612377. [Google Scholar] [CrossRef]
- Nelios, G.; Prapa, I.; Nikolaou, A.; Mitropoulou, G.; Yanni, A.E.; Kostomitsopoulos, N.; Kourkoutas, Y. Cereals and Fruits as Effective Delivery Vehicles of Lacticaseibacillus rhamnosus through Gastrointestinal Transit. Appl. Sci. 2023, 13, 8643. [Google Scholar] [CrossRef]
- Prapa, I.; Nikolaou, A.; Panas, P.; Tassou, C.; Kourkoutas, Y. Developing Stable Freeze-Dried Functional Ingredients Containing Wild-Type Presumptive Probiotic Strains for Food Systems. Appl. Sci. 2023, 13, 630. [Google Scholar] [CrossRef]
- Panagopoulou, E.A.; Chiou, A.; Nikolidaki, E.K.; Christea, M.; Karathanos, V.T. Corinthian Raisins (Vitis vinifera L., Var. Apyrena) Antioxidant and Sugar Content as Affected by the Drying Process: A 3-year Study. J. Sci. Food Agric. 2019, 99, 915–922. [Google Scholar] [CrossRef]
- Kompoura, V.; Prapa, I.; Vasilakopoulou, P.B.; Mitropoulou, G.; Nelios, G.; Balafas, E.; Kostomitsopoulos, N.; Chiou, A.; Karathanos, V.T.; Bezirtzoglou, E.; et al. Corinthian Currants Supplementation Restores Serum Polar Phenolic Compounds, Reduces IL-1beta, and Exerts Beneficial Effects on Gut Microbiota in the Streptozotocin-Induced Type-1 Diabetic Rat. Metabolites 2023, 13, 415. [Google Scholar] [CrossRef]
- Pavlatou, C.; Nikolaou, A.; Prapa, I.; Tegopoulos, K.; Plesssas, S.; Grigoriou, M.E.; Bezirtzoglou, E.; Kourkoutas, Y. Effect of Immobilized Pediococcus acidilactici ORE5 Cells on Pistachio Nuts on the Functional Regulation of the Novel Katiki Domokou-Type Cheese Microbiome. Appl. Sci. 2023, 13, 8047. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Yanni, A.E.; Mitropoulou, G.; Prapa, I.; Agrogiannis, G.; Kostomitsopoulos, N.; Bezirtzoglou, E.; Kourkoutas, Y.; Karathanos, V.T. Functional Modulation of Gut Microbiota in Diabetic Rats Following Dietary Intervention with Pistachio Nuts (Pistacia vera L.). Metabol. Open 2020, 7, 100040. [Google Scholar] [CrossRef]
- Yayla, M.; Binnetoglu, D. Experimental Approaches to Diabetes Mellitus. Eurasian J. Med. 2023, 54, S145–S153. [Google Scholar] [CrossRef]
- Lagkouvardos, I.; Fischer, S.; Kumar, N.; Clavel, T. Rhea: A Transparent and Modular R Pipeline for Microbial Profiling Based on 16S rRNA Gene Amplicons. PeerJ 2017, 5, e2836. [Google Scholar] [CrossRef] [PubMed]
- Prapa, I.; Yanni, A.E.; Nikolaou, A.; Kostomitsopoulos, N.; Kalogeropoulos, N.; Bezirtzoglou, E.; Karathanos, V.T.; Kourkoutas, Y. Dietary Pistachio (Pistacia vera L.) Beneficially Alters Fatty Acid Profiles in Streptozotocin-Induced Diabetic Rat. Appl. Sci. 2022, 12, 4606. [Google Scholar] [CrossRef]
- Huda-Faujan, N.; Abdulamir, A.S.; Fatimah, A.B.; Anas, O.M.; Shuhaimi, M.; Yazid, A.M.; Loong, Y.Y. The Impact of the Level of the Intestinal Short Chain Fatty Acids in Inflammatory Bowel Disease Patients versus Healthy Subjects. Open Biochem. J. 2010, 4, 53–58. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Kourkoutas, Y. Effect of Cooling Rate, Freeze-Drying, and Storage on Survival of Free and Immobilized Lactobacillus casei ATCC 393. LWT - Food Sci. Technol. 2016, 69, 468–473. [Google Scholar] [CrossRef]
- Nikolaou, A.; Sgouros, G.; Mitropoulou, G.; Santarmaki, V.; Kourkoutas, Y. Freeze-Dried Immobilized Kefir Culture in Low Alcohol Winemaking. Foods 2020, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic: Expert Consensus Document. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Mitropoulou, G.; Nedovic, V.; Goyal, A.; Kourkoutas, Y. Immobilization Technologies in Probiotic Food Production. J. Nutr. Metab. 2013, 2013, 716861. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus casei CCFM419 attenuates type 2 diabetes via a gut microbiota dependent mechanism. Food Funct. 2017, 8, 3814. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, W.L.; Chen, G.M.; Qian, M.; Han, J.Z.; Lv, X.C.; Chen, L.J.; Rao, P.F.; Ai, L.Z.; Ni, L. Pediococcus acidilactici FZU106 alleviates high-fat diet-induced lipid metabolism disorder in association with the modulation of intestinal microbiota in hyperlipidemic rats. Curr. Res. Food Sci. 2022, 5, 775–788. [Google Scholar] [CrossRef]
- Mathias Akinlade, O.; Victor Owoyele, B.; Olufemi Soladoye, A. Streptozotocin-Induced Type 1 and 2 Diabetes in Rodents: A Model for Studying Diabetic Cardiac Autonomic Neuropathy. Afr. Health Sci. 2021, 21, 719–727. [Google Scholar] [CrossRef]
- Singer, J.; Allen, J.; Morris, J.; Gold, A. Management of Eating Disorders in Patients with Type 1 (Insulin Dependent) Diabetes. In Multidisciplinary Management of Eating Disorders; Morris, J., McKinlay, A., Eds.; Springer: Cham, Switzerland, 2018; pp. 189–207. [Google Scholar] [CrossRef]
- Pongrac Barlovic, D.; Harjutsalo, V.; Groop, P.H. Exercise and nutrition in type 1 diabetes: Insights from the FinnDiane cohort. Front. Endocrinol. 2022, 13, 1064185. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, R.C.M.F.; Brandão, A.B.P.; De Abreu, I.C.M.E.; Ferreira, F.G.; Santos, L.B.; Moreira, L.N.; Taddei, C.R.; Aimbire, F.; Cunha, T.S. Saccharomyces boulardii Tht 500101 Changes Gut Microbiota and Ameliorates Hyperglycaemia, Dyslipidaemia, and Liver Inflammation in Streptozotocin-Diabetic Mice. Benef. Microbes 2019, 10, 901–912. [Google Scholar] [CrossRef]
- Henschel, A.M.; Cabrera, S.M.; Kaldunski, M.L.; Jia, S.; Geoffrey, R.; Roethle, M.F.; Lam, V.; Chen, Y.-G.; Wang, X.; Salzman, N.H.; et al. Modulation of the Diet and Gastrointestinal Microbiota Normalizes Systemic Inflammation and β-Cell Chemokine Expression Associated with Autoimmune Diabetes Susceptibility. PLoS ONE 2018, 13, e0190351. [Google Scholar] [CrossRef]
- Calabrese, C.M.; Valentini, A.; Calabrese, G. Gut Microbiota and Type 1 Diabetes Mellitus: The Effect of Mediterranean Diet. Front. Nutr. 2021, 7, 612773. [Google Scholar] [CrossRef]
- Holt, R.I.G.; DeVries, J.H.; Hess-Fischl, A.; Hirsch, I.B.; Kirkman, M.S.; Klupa, T.; Ludwig, B.; Nørgaard, K.; Pettus, J.; Renard, E.; et al. The Management of Type 1 Diabetes in Adults. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2021, 64, 2609–2652. [Google Scholar] [CrossRef] [PubMed]
- Wang-Fischer, Y.; Garyantes, T. Improving the Reliability and Utility of Streptozotocin-Induced Rat Diabetic Model. J. Diabetes Res. 2018, 2018, 8054073. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.N.A.; Cruz, N.T.S.; Martins, A.A.; Silva, R.C.M.; Almeida, H.C.; Costa, H.E.S.; Santos, K.M.O.; Vieira, B.R.; Sousa, F.B.; Junior, F.S.; et al. Probiotic Lactobacillus rhamnosus EM1107 Prevents Hyperglycemia, Alveolar Bone Loss, and Inflammation in a Rat Model of Diabetes and Periodontitis. J. Periodontol. 2023, 94, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Bibbò, S.; Dore, M.P.; Pes, G.M.; Delitala, G.; Delitala, A.P. Is There a Role for Gut Microbiota in Type 1 Diabetes Pathogenesis? Ann. Med. 2017, 49, 11–22. [Google Scholar] [CrossRef]
- Siljander, H.; Honkanen, J.; Knip, M. Microbiome and Type 1 Diabetes. EBioMedicine 2019, 46, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Bielka, W.; Przezak, A.; Pawlik, A. The Role of the Gut Microbiota in the Pathogenesis of Diabetes. Int. J. Mol. Sci. 2022, 23, 480. [Google Scholar] [CrossRef]
- Yang, S.-C. Effect of Synbiotics on Intestinal Microflora and Digestive Enzyme Activities in Rats. World J. Gastroenterol. 2005, 11, 7413. [Google Scholar] [CrossRef]
- Farida, E.; Nuraida, L.; Giriwono, P.E.; Jenie, B.S.L. Lactobacillus rhamnosus Reduces Blood Glucose Level through Downregulation of Gluconeogenesis Gene Expression in Streptozotocin-Induced Diabetic Rats. Int. J. Food Sci. 2020, 2020, 6108575. [Google Scholar] [CrossRef]
- Wei, S.-H.; Chen, Y.-P.; Chen, M.-J. Selecting Probiotics with the Abilities of Enhancing GLP-1 to Mitigate the Progression of Type 1 Diabetes In Vitro and In Vivo. J. Funct. Foods 2015, 18, 473–486. [Google Scholar] [CrossRef]
- Abhari, K.; Shekarforoush, S.S.; Sajedianfard, J.; Hosseinzadeh, S.; Nazifi, S. The Effects of Probiotic, Prebiotic and Synbiotic Diets Containing Bacillus coagulans and Inulin on Rat Intestinal Microbiota. Iran. J. Vet. Res. 2015, 16, 267–273. [Google Scholar] [PubMed]
- Patterson, E.; Marques, T.M.; O’Sullivan, O.; Fitzgerald, P.; Fitzgerald, G.F.; Cotter, P.D.; Dinan, T.G.; Cryan, J.F.; Stanton, C.; Ross, R.P. Streptozotocin-Induced Type-1-Diabetes Disease Onset in Sprague–Dawley Rats Is Associated with an Altered Intestinal Microbiota Composition and Decreased Diversity. Microbiology 2015, 161, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.U.; Shabbir, M.Z.; Steiner, J.M.; Ahmad, S.; Kamran, Z.; Anwar, H.; Hussain, G.; Shaukat, A.; Ullah, M.I.; Suchodolski, J.S. Molecular Analysis of the Gut Microbiome of Diabetic Rats Supplemented with Prebiotic, Probiotic, and Synbiotic Foods. Int. J. Diabetes Dev. Ctries 2017, 37, 419–425. [Google Scholar] [CrossRef]
- Leiva-Gea, I.; Sánchez-Alcoholado, L.; Martín-Tejedor, B.; Castellano-Castillo, D.; Moreno-Indias, I.; Urda-Cardona, A.; Tinahones, F.J.; Fernández-García, J.C.; Queipo-Ortuño, M.I. Gut Microbiota Differs in Composition and Functionality between Children with Type 1 Diabetes and MODY2 and Healthy Control Subjects: A Case-Control Study. Diabetes Care 2018, 41, 2385–2395. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, P.; Metos, J.; Anandh Babu, P.V. Impact of Type 1 Diabetes on the Composition and Functional Potential of Gut Microbiome in Children and Adolescents: Possible Mechanisms, Current Knowledge, and Challenges. Gut Microbes 2021, 13, 1926841. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, Y.-W.; Shao, L.; Sun, S.-H.; Wu, J.; Song, Q.-H.; Zou, H.-S.; Ling, Z.-X. Gut Microbiota Dysbiosis in Chinese Children with Type 1 Diabetes Mellitus: An Observational Study. World J. Gastroenterol. 2021, 27, 2394–2414. [Google Scholar] [CrossRef]
- Mikami, A.; Ogita, T.; Namai, F.; Shigemori, S.; Sato, T.; Shimosato, T. Oral Administration of Flavonifractor plautii Attenuates Inflammatory Responses in Obese Adipose Tissue. Mol. Biol. Rep. 2020, 47, 6717–6725. [Google Scholar] [CrossRef]
- Borgo, F.; Garbossa, S.; Riva, A.; Severgnini, M.; Luigiano, C.; Benetti, A.; Pontiroli, A.E.; Morace, G.; Borghi, E. Body Mass Index and Sex Affect Diverse Microbial Niches within the Gut. Front. Microbiol. 2018, 9, 213. [Google Scholar] [CrossRef]
- Alauzet, C.; Lozniewski, A.; Cailliez-Grimal, C. Flavonifractor. In Bergey’s Manual of Systematics of Archaea and Bacteria; Wiley: Hoboken, NJ, USA, 2023; pp. 1–11. [Google Scholar]
- Feng, P.; Yang, J.; Zhao, S.; Ling, Z.; Han, R.; Wu, Y.; Salama, E.-S.; Kakade, A.; Khan, A.; Jin, W.; et al. Human Supplementation with Pediococcus acidilactici GR-1 Decreases Heavy Metals Levels through Modifying the Gut Microbiota and Metabolome. NPJ Biofilms Microbiomes 2022, 8, 63. [Google Scholar] [CrossRef]
- Ding, F.; Zhou, N.; Luo, Y.; Wang, T.; Li, W.; Qiao, F.; Du, Z.; Zhang, M. Probiotic Pediococcus pentosaceus Restored Gossypol-Induced Intestinal Barrier Injury by Increasing Propionate Content in Nile Tilapia. J. Anim. Sci. Biotechnol. 2024, 15, 54. [Google Scholar] [CrossRef]
- van Muijlwijk, G.H.; Rice, T.A.; Flavell, R.A.; Palm, N.W.; de Zoete, M.R. Allobaculum mucilyticum sp. nov. and Allobaculum fili sp. nov., Isolated from the Human Intestinal Tract. Int. J. Syst. Evol. Microbiol. 2023, 73, 005635. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.V.; Leung, J.M.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D.; et al. Altering the Intestinal Microbiota during a Critical Developmental Window Has Lasting Metabolic Consequences. Cell 2014, 158, 705–721. [Google Scholar] [CrossRef]
- Thevaranjan, N.; Puchta, A.; Schulz, C.; Naidoo, A.; Szamosi, J.C.; Verschoor, C.P.; Loukov, D.; Schenck, L.P.; Jury, J.; Foley, K.P.; et al. Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host Microbe 2017, 21, 455–466.E4. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Qin, P.; Wang, J. High-Fat Diet Alters the Intestinal Microbiota in Streptozotocin-Induced Type 2 Diabetic Mice. Microorganisms 2019, 7, 176. [Google Scholar] [CrossRef]
- Thomaz, F.S.; Altemani, F.; Panchal, S.K.; Worrall, S.; Dekker Nitert, M. The Influence of Wasabi on the Gut Microbiota of High-Carbohydrate, High-Fat Diet-Induced Hypertensive Wistar Rats. J. Hum. Hypertens. 2021, 35, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, E.; Yin, B.; Fang, D.; Chen, P.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Effects of Lactobacillus casei CCFM419 on Insulin Resistance and Gut Microbiota in Type 2 Diabetic Mice. Benef. Microbes 2017, 8, 421–432. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA). Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of Pasteurised Akkermansia muciniphila as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, 6780. [Google Scholar] [CrossRef]
- Li, J.; Yang, G.; Zhang, Q.; Liu, Z.; Jiang, X.; Xin, Y. Function of Akkermansia muciniphila in Type 2 Diabetes and Related Diseases. Front. Microbiol. 2023, 14, 1172400. [Google Scholar] [CrossRef]
- Hänninen, A.; Toivonen, R.; Pöysti, S.; Belzer, C.; Plovier, H.; Ouwerkerk, J.P.; Emani, R.; Cani, P.D.; De Vos, W.M. Akkermansia Muciniphila Induces Gut Microbiota Remodelling and Controls Islet Autoimmunity in NOD Mice. Gut 2018, 67, 1445–1453. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, Q.; Liu, M.; Zhang, X.; He, F.; Wang, G. Akkermansia muciniphila Can Reduce the Damage of Gluco/Lipotoxicity, Oxidative Stress and Inflammation, and Normalize Intestine Microbiota in Streptozotocin-Induced Diabetic Rats. Pathog. Dis. 2018, 76, fty028. [Google Scholar] [CrossRef]
- Dempsey, E.; Corr, S.C. Lactobacillus spp. for Gastrointestinal Health: Current and Future Perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wu, F.; Zhou, Q.; Wei, W.; Yue, J.; Xiao, B.; Luo, Z. Lactobacillus and Intestinal Diseases: Mechanisms of Action and Clinical Applications. Microbiol. Res. 2022, 260, 127019. [Google Scholar] [CrossRef] [PubMed]
- Aoki, R.; Kamikado, K.; Suda, W.; Takii, H.; Mikami, Y.; Suganuma, N.; Hattori, M.; Koga, Y. A Proliferative Probiotic Bifidobacterium Strain in the Gut Ameliorates Progression of Metabolic Disorders via Microbiota Modulation and Acetate Elevation. Sci. Rep. 2017, 7, 43522. [Google Scholar] [CrossRef]
- Li, H.; Liu, F.; Lu, J.; Shi, J.; Guan, J.; Yan, F.; Li, B.; Huo, G. Probiotic Mixture of Lactobacillus plantarum Strains Improves Lipid Metabolism and Gut Microbiota Structure in High Fat Diet-Fed Mice. Front. Microbiol. 2020, 11, 512. [Google Scholar] [CrossRef]
- Dolpady, J.; Sorini, C.; Di Pietro, C.; Cosorich, I.; Ferrarese, R.; Saita, D.; Clementi, M.; Canducci, F.; Falcone, M. Oral Probiotic VSL#3 Prevents Autoimmune Diabetes by Modulating Microbiota and Promoting Indoleamine 2,3-Dioxygenase-Enriched Tolerogenic Intestinal Environment. J. Diabetes Res. 2016, 2016, 7569431. [Google Scholar] [CrossRef] [PubMed]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Tonucci, L.B.; Olbrich dos Santos, K.M.; Licursi de Oliveira, L.; Rocha Ribeiro, S.M.; Duarte Martino, H.S. Clinical Application of Probiotics in Type 2 Diabetes Mellitus: A Randomized, Double-Blind, Placebo-Controlled Study. Clin. Nutr. 2017, 36, 85–92. [Google Scholar] [CrossRef]
- Moravejolahkami, A.R.; Shakibaei, M.; Fairley, A.M.; Sharma, M. Probiotics, Prebiotics, and Synbiotics in Type 1 Diabetes Mellitus: A Systematic Review and Meta-analysis of Clinical Trials. Diabetes Metab. Res. Rev. 2024, 40, e3655. [Google Scholar] [CrossRef]






| Storage Temperature | Form | Nature | Logcfu | % Survival | |||
|---|---|---|---|---|---|---|---|
| d0 | d15 | d30 | d15 | d30 | |||
| RT | Immobilized | W | 8.62 ± 0.05 | 6.74 ± 0.08 | 2.30 ± 0.01 | 78.19 ± 1.80 | 26.68 ± 0.35 |
| FD | 8.59 ± 0.05 | 7.75 ± 0.02 | 6.38 ± 0.01 | 90.22 ± 0.85 | 74.27 ± 0.62 | ||
| Free | W | 9.61 ± 0.05 | 7.43 ± 0.02 | 5.09 ± 0.01 | 77.31 ± 2.02 | 52.96 ± 0.39 | |
| FD | 9.46 ± 0.03 | 7.62 ± 0.01 | 6.54 ± 0.02 | 80.54 ± 0.61 | 69.13 ± 0.84 | ||
| 4 °C | Immobilized | W | 8.62 ± 0.05 | 8.41 ± 0.02 | 7.55 ± 0.02 | 97.56 ± 0.99 | 87.58 ± 0.88 |
| FD | 8.59 ± 0.05 | 8.51 ± 0.01 | 8.28 ± 0.01 | 99.06 ± 1.01 | 96.39 ± 0.65 | ||
| Free | W | 9.61 ± 0.05 | 8.97 ± 0.01 | 7.41 ± 0.01 | 93.34 ± 0.81 | 77.10 ± 0.55 | |
| FD | 9.46 ± 0.03 | 9.08 ± 0.03 | 8.23 ± 0.01 | 95.98 ± 0.64 | 86.99 ± 0.71 | ||
| Cecum (Tissue Adherent) | Cecal Content | |||||||
|---|---|---|---|---|---|---|---|---|
| Group | H_IPC | H_FP | D_IPC | D_FP | H_IPC | H_FP | D_IPC | D_FP |
| TAC | 5.70 ± 0.06 | 5.42 ± 0.14 | 6.30 ± 0.26 b | 5.88 ± 0.16 | 8.52 ± 0.11 | 6.75 ± 0.03 a | 7.70 ± 0.25 a,b | 7.50 ± 0.03 a,b |
| Enterobacteriaceae | 3.15 ± 0.16 | 2.43 ± 0.17 | 3.86 ± 0.48 b | 5.31 ± 0.10 a,b,c | 5.58 ± 0.28 | 3.43 ± 0.31 | 6.02 ± 0.01 b | 5.29 ± 0.08 b |
| Coliforms | 3.13 ± 0.13 | 2.40 ± 0.17 | 3.53 ± 0.56 | 5.20 ± 0.06 a,b,c | 5.57 ± 0.28 | 3.35 ± 0.15 a | 6.28 ± 0.09 a,b | 5.29 ± 0.08 b,c |
| E. coli | 3.01 ± 0.16 | 2.33 ± 0.03 | 3.69 ± 0.51 b | 5.25 ± 0.10 a,b,c | 5.41 ± 0.32 | 3.47 ± 0.15 | 5.74 ± 0.05 b | 5.12 ± 0.02 b |
| Staphylococcus spp. | 3.02 ± 0.10 | 2.90 ± 0.07 | 4.86 ± 0.12 a,b | 4.68 ± 0.21 a,b | 5.55 ± 0.13 | 4.52 ± 0.08 a | 4.94 ± 0.09 a,b | 5.16 ± 0.01 a,b |
| Εnterococcus spp. | 3.84 ± 0.05 | 3.93 ± 0.29 | 3.87 ± 0.15 | 4.86 ± 0.19 a,b,c | 6.12 ± 0.20 | 4.85 ± 0.11 a | 5.31 ± 0.04 a | 4.64 ± 0.04 a,c |
| Clostridium spp. | 5.26 ± 0.09 | 5.00 ± 0.12 | 5.39 ± 0.12 | 5.61 ± 0.11 b | 6.93 ± 0.30 | 6.53 ± 0.06 | 6.55 ± 0.01 | 6.54 ± 0.01 |
| LAB | 5.78 ± 0.07 | 5.12 ± 0.03 a | 6.21 ± 0.28 b | 5.34 ± 0.09 a | 8.23 ± 0.07 | 6.28 ± 0.10 a | 6.90 ± 0.28 a | 7.87 ± 0.15 b,c |
| Bifidobacterium spp. | 5.30 ± 0.05 | 5.53 ± 0.14 | 6.22 ± 0.05 a,b | 5.67 ± 0.19 c | 7.61 ± 0.18 | 7.48 ± 0.02 | 7.88 ± 0.05 | 6.71 ± 0.13 a,b,c |
| H_IPC | H_FP | D_IPC | D_FP | |||||
|---|---|---|---|---|---|---|---|---|
| Weeks | 0 | 4 | 0 | 4 | 0 | 4 | 0 | 4 |
| Actinobacteria | 0.06 ± 0.00 | 0.0 ± 0.02 | 0.06 ± 0.02 | 0.05 ± 0.00 | 1.12 ± 0.95 | 8.04 ± 2.02 a,b,c | 2.08 ± 0.79 | 11.73 ± 0.11 a,b,c |
| Bacteroidetes | 22.24 ± 2.64 | 15.14 ± 4.72 | 22.71 ± 0.59 | 24.86 ± 8.61 | 20.12 ± 3.99 | 22.49 ± 1.95 | 16.97 ± 14.47 | 5.61 ± 0.59 |
| Firmicutes | 77.29 ± 2.38 | 83.51 ± 5.87 | 76.86 ± 0.59 | 74.23 ± 7.96 | 71.75 ± 4.23 | 55.90 ± 2.38 | 72.94 ± 9.69 | 76.42 ± 2.62 |
| Tenericutes | 0.30 ± 0.24 | 1.22 ± 1.18 | 0.27 ± 0.04 | 0.80 ± 0.62 | 0.21 ± 0.01 | 0.10 ± 0.02 | 0.16 ± 0.11 | 0.26 ± 0.09 |
| Verrucomicrobia | 0.11 ± 0.02 | 0.06 ± 0.01 | 0.10 ± 0.03 | 0.06 ± 0.02 | 6.80 ± 0.72 | 13.46 ± 2.42 b,c | 7.85 ± 3.88 | 5.98 ± 2.01 |
| Lactic | Acetic | Propionic | Isobutyric | Butyric | Isovaleric | Valeric | |
|---|---|---|---|---|---|---|---|
| H_IPC | 2.796 ± 0.170 | 18.481 ± 0.502 | 1.382 ± 0.047 | 0.059 ± 0.006 | 0.657 ± 0.099 | 0.058 ± 0.007 | 0.106 ± 0.012 |
| H_FP | 4.014 ± 0.438 | 26.552 ± 2.610 | 1.890 ± 0.105 | 0.559 ± 0.381 | 1.654 ± 0.522 | 0.332 ± 0.197 | 0.160 ± 0.022 |
| D_IPC | 6.121 ± 0.783 a | 37.280 ± 2.680 a,b | 1.899 ± 0.148 | 0.147 ± 0.101 | 1.166 ± 0.315 | 0.046 ± 0.018 | 0.131 ± 0.016 |
| D_FP | 4.307 ± 0.688 | 38.094 ± 2.712 a,b | 1.904 ± 0.188 | 0.219 ± 0.130 | 1.005 ± 0.258 | 0.062 ± 0.024 | 0.092 ± 0.019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prapa, I.; Kompoura, V.; Pavlatou, C.; Nelios, G.; Mitropoulou, G.; Kostomitsopoulos, N.; Plessas, S.; Bezirtzoglou, E.; Karathanos, V.T.; Yanni, A.E.; et al. Effects of Free or Immobilized Pediococcus acidilactici ORE5 on Corinthian Currants on Gut Microbiome of Streptozotocin-Induced Diabetic Rats. Microorganisms 2024, 12, 2004. https://doi.org/10.3390/microorganisms12102004
Prapa I, Kompoura V, Pavlatou C, Nelios G, Mitropoulou G, Kostomitsopoulos N, Plessas S, Bezirtzoglou E, Karathanos VT, Yanni AE, et al. Effects of Free or Immobilized Pediococcus acidilactici ORE5 on Corinthian Currants on Gut Microbiome of Streptozotocin-Induced Diabetic Rats. Microorganisms. 2024; 12(10):2004. https://doi.org/10.3390/microorganisms12102004
Chicago/Turabian StylePrapa, Ioanna, Vasiliki Kompoura, Chrysoula Pavlatou, Grigorios Nelios, Gregoria Mitropoulou, Nikolaos Kostomitsopoulos, Stavros Plessas, Eugenia Bezirtzoglou, Vaios T. Karathanos, Amalia E. Yanni, and et al. 2024. "Effects of Free or Immobilized Pediococcus acidilactici ORE5 on Corinthian Currants on Gut Microbiome of Streptozotocin-Induced Diabetic Rats" Microorganisms 12, no. 10: 2004. https://doi.org/10.3390/microorganisms12102004
APA StylePrapa, I., Kompoura, V., Pavlatou, C., Nelios, G., Mitropoulou, G., Kostomitsopoulos, N., Plessas, S., Bezirtzoglou, E., Karathanos, V. T., Yanni, A. E., & Kourkoutas, Y. (2024). Effects of Free or Immobilized Pediococcus acidilactici ORE5 on Corinthian Currants on Gut Microbiome of Streptozotocin-Induced Diabetic Rats. Microorganisms, 12(10), 2004. https://doi.org/10.3390/microorganisms12102004













