Abstract
Phytoremediation is a sustainable technique that employs plants to reinforce polluted environments such as agroecosystems. In recent years, new strategies involving the plant microbiome as an adjuvant in remediation processes have been reported. By leveraging this microbial assistance to remediate soils contaminated with heavy metals such As, Pb, Cd, Hg, and Cr, plants can sequester, degrade, or stabilize contaminants more efficiently. Remarkably, some plant species are known for their hyper-accumulative traits in synergy with their microbial partners and can successfully mitigate heavy metal pollutants. This sustainable biotechnology based on plant–microbe associations not only aids in environmental cleanup but also enhances biodiversity, improves soil structure, and promotes plant growth and health, making it a promising solution for addressing agro-pollution challenges worldwide. The current review article emphasizes the potential of synergistic plant–microbe interactions in developing practical and sustainable solutions for heavy metal remediation in agricultural systems, which are essential for food security.
1. Introduction
The growing world population, accompanied by industrial development, mining, and urban sprawl, is accompanied by increased pollution of aquatic, aerial, and terrestrial ecosystems [1]. Soil pollution in agroecosystems is an intensifying global issue because it has significant implications for food security, environmental protection, and agricultural efficiency, depending on the human population [2]. Unsustainable farming practices and unnecessary agrochemical use have raised concerns regarding soil pollution since the Green Revolution [3]. Current statistical analysis has revealed that a substantial proportion of agronomic lands worldwide have been affected by heavy metal pollution, including cadmium (Cd), copper (Cu), chromium (Cr), mercury (Hg), lead (Pb), zinc (Zn), and nickel (Ni), mainly tainting arable soils [4]. According to Chen et al., metalloid arsenic (As) is frequently included in this category because of its analogous chemical properties and environmental behavior [5].
Soil pollution in agricultural areas is unevenly distributed globally, and some regions of the world are aggravated by this issue, mostly owing to their extensive industrialization. Research has shown that in countries such as China and India, a significant portion of the arable land is compromised by heavy metal pollution [6,7]. This problem not only affects crop yield and quality, but also poses profound hazards to the health of producers and consumers. For example, prolonged exposure to arsenic dust can have adverse effects on human health, together with the development of dermal lesions, peripheral neuropathy, and skin cancer. In addition, metals such as lead (Pb), mercury (Hg), and cadmium (Cd) can penetrate the human body through multiple routes, including the inhalation of dust or vapor, the consumption of contaminated food, or direct skin contact [8]. Upon entering the body, they can accumulate in tissues and organs, triggering a broad spectrum of adverse effects, such as neurological damage; cognitive impairment; kidney, liver, and cardiovascular injury; damage to DNA and protein levels; and increased risk of cancer [9]. Consequently, heavy metal exposure is a pressing public health issue that requires careful administration and preventive measures to protect human health [10].
Phytoremediation is a key environmental protection practice that uses soil plants to remediate harmful elements in the environment [10]. Phytoremediation is cost-effective, affordable, and less harmful to the environment in its immediate surroundings than other conventional practices [11]. Hyperaccumulator plant species are highly tolerant of heavy metals. Some examples include Nymphaea tymphaea, Bornmuellera tymphaea, Pteris vittate, Sedum alfredii, and Thlaspi caerulescens [1,2,6,11,12]; however, there are still challenges to overcome, such as slow growth rates and low biomass production [12]. Plant genome engineering can sometimes address this issue in plant species lacking these characteristics or with low heavy metal tolerance.
Microorganisms constantly surround plants and can create robust, long, and beneficial associations that increase their suitability for growth in adverse environments, such as soils contaminated by heavy metals [13]. Some bacteria (e.g., Burkholderia spp., Pseudomonas spp., Bacillus spp., Rhizobium spp., and Frankia spp.) and fungi (e.g., Glomus mosseae and Trichoderma spp.) [2] are also extremely tolerant to heavy metals so that they can endure in rhizospheric environments [14]. In the rhizosphere, the plant excretes root exudates, with nutrients utilized by the soil microbiota to grow and reproduce. Some phyla, such as Firmicutes, Proteobacteria, Bacteroidetes, and Acidobacteria, have highly tolerant species with resistance mechanisms to heavy metal toxicity and are common inhabitants of the rhizosphere [15]. Some of these bacterial groups also colonize and penetrate plant roots, launching themselves as endophytes. From the endosphere, they assist plants in increasing their tolerance and remediation of heavy metals in the soil [16]. Similarly, some fungi, including arbuscular mycorrhizal fungi (AMF), may stimulate phytoremediation through phytoextraction and phytostabilization. Thus, AMF can reduce the stress induced by heavy metals in their plant hosts and curb the processes of metal translocation within the plant [17].
In this study, we explored soil remediation mechanisms carried out by plants and the auxiliary role of their accompanying microbial partners in phytoremediation. We also focused on bacterial and fungal groups that synergize with plants to regain agricultural soils, which are indispensable for food safety and human well-being.
2. Strategies for Phytoremediation: The Current State-of-the-Art
Phytoremediation is a green and environmentally friendly practice that implements plants to eradicate detrimental elements from the environment, predominantly the soil, water, and air. This method affects the natural aptitudes of plants to absorb, gather, degrade, and assuage contaminants, making it a promising solution for addressing pollution in several ecosystems, including agronomic soils polluted with heavy metals [18]. Numerous phytoremediation technologies, such as phytoextraction, phytostabilization, phytodegradation, phytovolatilization, rhizofiltration, and rhizodegradation, are used to exterminate organic and inorganic chemicals from polluted places (each having a diverse means of action) that depend on the cleanup level, the pollutant type, plant types, and the state of the site [19]. Figure 1 shows the different assisted phytoremediation strategies used in this study.
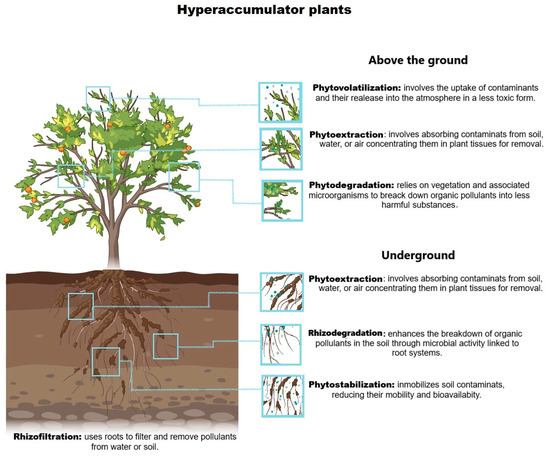
Figure 1.
The mechanism of action for microbial-assisted phytoremediation in soils contaminated with heavy metals. This figure depicts various phytoremediation technologies used to address environmental contamination [11,20,21].
Co-planting and plant growth-promoting rhizobacterial (PGPR) treatment have an encouraging effect on the phytoextraction process. Intercropping and endophytic plant growth-promoting bacterial inoculation (Burkholderia phytofirmans PsJNT) augmented the phytoextraction of Zn, Pb, and Cd directed to Brassica juncea “Małopolska” grown in a monoculture or co-planted with Zea mays L. “Codimon” and Medicago sativa L. “Sanditi” [20]. The association of Bornmuellera nymphaea—Noccaea nymphaea (NB) and Bornmuellera tymphaea–Alyssum murale (AB) inoculated with PGPR increased Ni in roots by 105.8 and 66.4%, respectively, in AB and NB covers, and 39.9 and 79.6% in the shoots. Hence, the collective application of the hyperaccumulator plants N. nymphaea andB.tymphaea administered with one of the two PGPR strains (strain NB24), derived from the rhizosphere of this mixed cover, seemed to be a thought-provoking option for efficient Ni phytoextraction to be verified in the field [21]. Metal accumulation (NiCl2, Pb(CH3COO)2, CuSO4, NaAsO2, and MnCl2) has been reported to be improved by Miscanthus × giganteus and rhizobacterial association along with plant growth in flotation tailings due to PGP properties (indole-3-acetic acid and siderophore production, 1-aminocyclopropane-1-carboxylic acid deaminase activity, and phosphate solubilization) [22].
The impact of phytostabilization in the management of HM-contaminated soils using Koelreuteria paniculata as a test plant considerably amended soil conditions, particularly by enhancing pH levels, fertility, and water retention. The treatment group exhibited a noticeable decline in the bioavailability and migration of heavy metals, such as Zn, Pb, and Mn, with a decrease in runoff loss of 15.7%, 8.4%, and 10.2%, respectively [23]. The potential of rice in connection with the PGPR consortium improved the comparative richness of bacteria related to di (2-ethylhexyl) phthalate degradation (Sphingomonas, Xanthobacteraceae), heavy metal immobilization (Massilia), and soil nutrient cycling (Nitrospira, Vicinamibacterales), which increased plant growth and heavy metal removal from soil, as well as Di (2-ethylhexyl) phthalate [24].
Phytovolatility has made a notable contribution to the detoxification of heavy metals. It remains a key mechanism in arsenic (As) decontamination during the bioremediation process involving Arundo donax L. and the amicrobial assistance of epigenetic changes by a PGPR consortium. PGPR consists of two strains of Stenotrophomonas maltophilia and one Agrobacterium sp. strain. The authors analyzed the methylation-sensitive amplified polymorphisms (MSAPs) of A. donax leaves and detected potential changes by interaction with As and the bacterial consortium. The presence of PGPB did not modify the percentage of volatilized arsenic or induce significant plant growth biomass, but the work demonstrated that phytovolatilization is a robust mechanism for reducing arsenic concentrations in contaminated environments [25].
The rhizofiltration proficiency of Juncus acutus L. was evaluated in a constructed wetland (CW) scheme that was certainly premeditated to treat groundwater polluted with hexavalent chromium (Cr (VI)). J. acutus efficiently eliminated up to 140 μg/L of Cr (VI) from contaminated groundwater. In addition, this study highlighted the role of endophytic bacteria associated with J. acutus in enhancing the plant’s resistance to Cr (VI) toxicity and its total filtration efficacy. Pseudomonas sp. strain R16 exhibited an extraordinary ability to reduce Cr (VI) to the less toxic trivalent form (Cr (III)) under aerophilic conditions. The decrease of 100 mg/L Cr(VI) by Pseudomonas sp. strain R16 after 150 h of incubation underscores the synergistic rapport between the plant and its endophytic bacteria in detoxifying Cr(VI) in contaminated environments [26].
In summary, the amalgamation of co-planting approaches, PGPR injection, and the practice of hyperaccumulators and resistant plant species has shown significant potential in enhancing such processes for the effective remediation of heavy metal-contaminated soils, highlighting an auspicious and synergistic method for environmental decontamination and sustainable land management.
3. Metal-Resistant Microbes
The isolation and application of microbial populations for the remediation of heavy metal ions from the environment have been a focus of particular interest owing to their capacity to effectively highlight pollution challenges. Microbial populations, predominantly those encompassing bacteria and fungi, have acquired various mechanisms to depollute and detain heavy metals, making them suitable candidates for bioremediation [27]. A list of bacterial species that support plants in soil remediation includes Pseudomonas, Bacillus, Variovorax, Klebsiella, Sinorhizobium, Enterobacter, Rhodococcus, Flavobacterium, Ensifer, and Kocuria, to mention but a few [1,2,10,12,13,19]. On the other hand, fungi from the genera Trichoderma, Beauveria, and various arbuscular mycorrhizal fungi (AMF) such as Rhizophagus and Glomus have established their contributions in serving their plant hosts in different soil-cleaning processes, with those that are part of agroecosystems [28].
3.1. PGPB Contributions
The explanation of plant-advantageous bacteria, largely metal-resistant strains, and the contemplation of their mechanisms to augment plant growth and metal tolerance is pivotal for undertaking phytoremediation-enhancing tactics. Table 1 presents the number of studies directed in this context using PGPR and PGPEs (plant growth-promoting endophytes). Plant health in metal-polluted soils is upgraded in two ways by these bacteria: first, through the direct production of plant growth-beneficial products together with the solubilization/transformation of mineral nutrients (e.g., phosphate, nitrogen, and potassium), the synthesis of phytohormones, siderophores, and definite enzymes; and second, they indirectly support plants by controlling plant pathogens or by bringing a systemic resistance of plants against pathogens. Furthermore, they alter the metal buildup volume in plants by eliminating metal-immobilizing extracellular polymeric substances and metal-mobilizing organic acids and biosurfactants [29].
In a recent study, ten bacterial strains from plant rhizospheres in mining deposits, recognized as Enterobacter, Serratia, Klebsiella, and Escherichia species, have shown significant potential for phytoremediation. Strain Serratia sp. K120 showed a positive correlation between ACC deaminase action and indole-3 acetic acid (IAA) production across numerous heavy metal treatments, including Pb, As, and Cu. This strain boosted plant growth under exposure to these metals and displayed resilience to the adverse effects of Ni, Cd, and Mn, making it a favorable candidate for use in phytoremediation systems aimed at remediating soils contaminated with heavy metals [30].
An inspection of Pseudometallophytes (Betula celtiberica, Cytisus scoparius, and Festuca rubra) in a Pb/Zn mine revealed the influence of metal-tolerant rhizobacteria on phytoremediation. Regardless of their low diversity, these bacteria, principally Actinobacteria, were more rampant in rhizosphere soils and presented tolerance to higher levels of Cd and Zn. Although not all isolates had PGP traits, many of them implicitly improved the growth of Festuca pratensis and Salix viminalis, signifying their potential for phytostabilization and phytoextraction efforts [31]. Eleven Cd-resistant bacterial strains, including Variovorax paradoxus, Rhodococcus sp. and Flavobacterium sp., were obtained from the root area of Brassica juncea planted in Cd-contaminated soils. These variants showed tolerance to various metals (Cd, Zn, Cu, Ni, and Co) and promoted root extension in B. juncea seedlings. Most strains, exclusive of Flavobacterium sp. strain 5P-3, formed ACC deaminase and were associated with enhanced root growth. These bacteria display potential as inoculants to advance B. juncea growth in Cd-polluted soils, supporting their use in phytoremediation strategies [32].
The diazotrophic rhizobacteria Azospirillum baldaniorum Sp245 (formerly A. brasilense) and Azospirillum brasilense Sp7 are two species capable of colonizing different habitats. The former (Sp245) is considered a facultative endophyte, as it can live either as an endophyte or saprophyte, while the Sp7 strain can only colonize the surface of roots. In a study by Kamnev and colleagues [33], they observed that these Azospirillum strains respond differently to the presence of heavy metals. The wild-type Sp245 strain showed a less marked buildup of stress-induced compounds compared to the non-endophytic type strain Sp7 when exposed to metals such as Co2+, Cu2+, and Zn2+. Moreover, both strains revealed reduced indole-3-acetic acid (IAA) production in the presence of Cu2+ or Cd2+, which hypothetically affects their efficiency as plant growth promoters in soils contaminated with heavy metals. This result shows that although there are naturally non-resistant strains to heavy metals such as A. baldaniorum, there are others that can tolerate broader ranges and could be candidates for inoculation in plants, thereby stimulating their fitness and growth, and in turn, indirectly improving remediation processes. The application of heavy metal (HM)-resistant PGPR variants Ralstonia eutropha (B1) and Chrysiobacterium humi (B2) sharply reduced Zn and Cd bioaccumulation in Helianthus annuus, with C. humi (B2) reducing Zn accumulation by up to 67%, Zn bioconcentration factor (BCF) by 20%, Zn uptake by 64%, and Cd uptake and BCF by 27%, whereas it enhanced bacterial diversity in the rhizosphere, thus improving plant stabilization in contaminated soils [34]. These findings emphasize the vital role of bacteria in the progress of phytoremediation efforts, predominantly in heavily polluted environments.

Table 1.
Relevant studies demonstrating the role of plant growth-promoting rhizobacteria and endophytic bacteria in the phytoremediation of metal-contaminated soils, including those used for agriculture.
Table 1.
Relevant studies demonstrating the role of plant growth-promoting rhizobacteria and endophytic bacteria in the phytoremediation of metal-contaminated soils, including those used for agriculture.
| Type | Species/Strain | Plant Used | Mechanism of Action | Impact | References |
|---|---|---|---|---|---|
| Plant Growth-Promoting Rhizobacteria | Sinorhizobium meliloti | Medicago lupulina L. | IAA, ACC deaminase, and siderophores. | Amended biomass, elevated copper uptake, and decreased copper stress. | [35] |
| Variovorax paradoxus | Bornmuellera tymphaea (Hausskn.) Hausskn. Noccaea tymphaea (Hausskn.) F.K.Mey. Alyssum murale L. | IAA, ACC deaminase, siderophores, and P solubilization. | Improved biomass and greater nickel uptake. | [21] | |
| Chryseobacterium humi Pseudomonas reactans Pseudomonas fluorescens | Zea mays L. | ACC deaminase, P solubilization, and siderophores. | Augmented root and shoot growth, higher biomass, and enhanced cadmium uptake. | [36] | |
| Ensifer adhaerens | Indole-3-acetic acid (IAA), siderophores, and ACC deaminase. | Heightened arsenic uptake. | [37] | ||
| Bacillus licheniformis Micrococcus luteus | Vitis vinifera cv. Malbec | Nitrogen assimilation, P solubilization, and Siderophore. | Diminished toxicity from arsenic. | [38] | |
| Kocuria sp. CRB15 | Brassica nigra L. | Indole-3-acetic acid (IAA) and solubilization of phosphorus. | Enhanced growth of roots and shoots. | [39] | |
| Sinorhizobium Saheli | Leucaena leucocephala (Lam.) de Wit | Fixation of nitrogen, solubilization of phosphorus, and synthesis of IAA. | Increased root and shoot growth, elevated biomass, decreased cadmium uptake, and reduced manganese uptake. | [40] | |
| Pseudomonas sp. | Medicago sativa L. | N fixation, P solubilization, IAA biosynthesis, and siderophore generation | Improved root and shoot development, greater biomass, boosted chlorophyll levels, decreased oxidative stress, and elevated Cr accumulation in roots. | [41] | |
| Bacillus sp. EhS7 Acinetobacter sp. RA1 Bacillus sp. RA2 | Perennial ryegrass Tall fescue | IAA biosynthesis and P solubilization. | Greater biomass, less oxidative stress, and reduced uptake of metals. | [42] | |
| Plant growth-promoting endophytic bacteria | Bacillus sp. E2S2 Bacillus sp. E1S2 | Sedum plumbizincicola X.H.Guo and S.B.Zhou ex L.H.Wu | Indole-3-acetic acid (IAA) synthesis, ACC deaminase enzyme activity, phosphorus solubilization, and siderophore production. | Boosted root and shoot growth, augmented biomass, increased cadmium uptake, and higher zinc accumulation. | [37] |
| Serratia sp. AI001 Klebsiella sp. AI002 | Solanum nigrum L. | IAA production. | Increase in biomass, elevation of chlorophyll content, and enhancement of Cd translocation. | [43] |
3.2. Fungal Interventions
During mycoremediation, the degradative power of fungi is used to eliminate or deactivate disparaging chemicals present in soil and water [44]. Dark septate endophyte (DSE), Exophiala pisciphila, triggered a noticeable tolerance to Cd, with a considerable decline in the toxic effects of Cd and a noteworthy increase in maize expansion by activating antioxidant systems, changing metal chemical forms into inactive Cd, and repartitioning subcellular Cd into the cell wall [45]. Exposure of P. libanensis autonomously or along with C. claroideum upgraded plant growth and reformed the physiological status (e.g., electrolyte leakage, chlorophyll, proline, and malondialdehyde contents) in addition to Ni and sodium (Na+) buildup potential (e.g., uptake and translocation factor of Ni and Na+) of H. annuus under Ni and salinity stress either alone or in combination [46]. The introduction of AMF, specifically Funneliformis mosseae (Fm) and Rhizophagus intraradices (Ri), to upland rice grown in soil containing 0, 2, or 10 mg Cd kg−1 not only reduced cadmium accumulation in rice but also tempered reactive oxygen species (ROS) scavenging activities [47]. The symbiont of S. calendulacea with FM provides a theoretical basis and application direction for the remediation of Cd-contaminated soil [48]. AMF were found to be very helpful in removing mercury from rice. Rice plants grown in symbiosis with AMF had much lower mercury levels, with a reduction of between 52.82% and 96.42% compared to rice plants without AMF [49].
A beneficial fungus called Glomus versiforme (Gv) facilitated the growth of upland rice in soil with added Cd. Rice plants with Gv grew taller, absorbed more phosphorus, and had healthier photosynthesis [50]. Planting a cadmium-absorbing plant, Solanum nigrum, in conjunction with rice in contaminated soil, with the help of beneficial fungi, reduced Cd in rice while encouraging plant growth [51].
Trichoderma is a genus of fungi central to agroecosystems because of its multifunctional properties that foster plant health and soil quality. These fungi are recognized for their ability to act as biocontrol agents against various plant pathogens, plummeting the need for chemical pesticides and promoting sustainable agricultural practices [52]. Trichoderma spp. boost plant growth by fabricating growth-promoting substances, solubilizing phosphates, and improving nutrient uptake, which results in increased crop yields. Additionally, Trichoderma subsidizes soil remediation by breaking down organic matter and decomposing soil pollutants, thereby improving soil structure and fertility. Their presence also promotes beneficial microbial communities, creating a more resilient and balanced soil ecosystem [53]. Trichoderma is essential in agroecosystems, as it provides environmental benefits and supports sustainable agriculture by enhancing plant growth and health.
Trichoderma can also support its plant hosts in cleansing agricultural soils, as verified in a study by Kumar and Dwivedi, who appraised the chromium-reducing potential of T. lixii strain CR700. In addition, strain CR700 can tolerate high concentrations of metals such as Cr (1000 mg/L), As (2000 mg/L), Ni (1500 mg/L), Zn (1200 mg/L), Cu (1200 mg/L), Pb (100 mg/L), and Cd (100 mg/L) [54]. Eventually, the authors proved that the phytotoxicity test using the supernatant from T. lixii-treated 100 mg/L Cr (VI) on Vigna radiata and Cicer arietinum showed effective detoxification and remediation of Cr (VI).
Beauveria bassiana is an important entomopathogenic fungus that has been extensively documented for its role in biological pest control. As naturally occurring pathogens of various insect species, it is a feasible substitute for chemical pesticides in managing agricultural pests, thus promoting ecologically sound farming practices. B. bassiana contaminates and kills insects through direct contact by infiltrating their cuticle, growing internally, and releasing toxins, ultimately leading to host death. Its broad host adaptability includes several major agricultural pests, such as aphids, beetles, and caterpillars, making it a multipurpose tool in integrated pest management (IPM) programs [55]. In addition, B. bassiana is environmentally friendly as it explicitly targets insects without harming beneficial organisms, humans, or the environment. Its capacity to persist in the environment and reprocess over successive generations of insects has further developed its utility as a long-term pest control plan. Overall, Beauveria bassiana is helpful in helping ecological balance and reducing reliance on synthetic pesticides in agricultural ecosystems [56].
Some cases showing the potential of Beauveria fungi include the work of Gola et al., who stated that a strain of Beauveria bassiana unveiled a high capacity for eliminating multiple metals from polluted wastewater, attaining an 84% removal rate, which is significantly higher than the 61–75% removal rate of individual metals [56]. The proclivity of the fungus to different metals also changed when exposed to a multimodal environment, representing its adaptability to complex adulteration scenarios. Atomic force microscopy (AFM) revealed changes in the surface roughness of B. bassiana due to metal noxiousness, suggesting alterations in its cell structure and function. These findings highlight the potential of B. bassiana as a promising strain for efficient multimodal metal removal in wastewater treatment. Even though this work is not directly linked to agroecosystems, this strain can be practical in water treatment for irrigation in agriculture, a topic that has been little explored in recent research but represents significant potential for future investigation.
In a recent evaluation, Kumar and Dwivedi studied the role of fungi in heavy metal removal and the factors influencing their effectiveness [28]. Thus, industrial development and coal and metal mining pollute water bodies with heavy metals, creating severe ecological hazards. The authors advocate that traditional treatment methods are costly and produce hazardous waste, making affordable and eco-friendly solutions essential. Therefore, bioremediation using fungi offers a sustainable approach, as fungi effectively adsorb and accumulate heavy metals through various mechanisms like bioaccumulation and bioabsorption, which were also discussed. Finally, the productivity of fungal bioremediation depends on ecological aspects such as time, pH, temperature, HM concentration, and fungal biomass. Studies on the involvement of fungi in phytoremediation induced by heavy metals are presented in Table 2.

Table 2.
Recent works on the exploration of fungal roles in phytoremediation of heavy metals in different agrosystems.
Table 2.
Recent works on the exploration of fungal roles in phytoremediation of heavy metals in different agrosystems.
| Fungal Species | Host Plant | Heavy Metals | Consequences | Reference |
|---|---|---|---|---|
| Rhizophagus irregularis | Cannabis sativa L. | Cd remediation | Enhanced Cd compartmentation | [57] |
| Laccaria, L. bicolor and L. japonica | Pinus densiflora Siebold and Zucc. | Cadmium (Cd) or copper (Cu) | Blocked the migration and accumulation of cadmium | [58] |
| Ectomycorrhizal (ECM) fungi | Pinus densiflora Siebold and Zucc. | Cu | Increased seedling performance | [59] |
| Paxillus involutus | P. × canescens | Pb | Increased plant growth and may increase Pb phytostabilization potential | [60] |
| Pisolithus albus | Acacia spirorbis Labill. and Eucalyptus globulus Labill. | Co, Cr, Fe, Mn and Ni | Enhanced plant growth and mineral nutrition while limiting metal uptake | [61] |
| Chaetomium globosum | Zea mays L. | Copper | Increased seedling dry weight, osmotic solute content, and antioxidant enzyme activity | [62] |
| Trichoderma harzianum Rifai 1295-22 | Salix fragilis | Cadmium manganese nickel and zinc | Promoted growth | [63] |
| Glomus intraradices | Linum usitatissimum | Nickel | Alleviated Ni toxicity as indicated by improved plant growth | [64] |
| Trametes versicolor and Trichoderma harzianum, Glomus deserticola and G. claroideum | Eucalyptus globulus Labill. | Arsenic (As) | Increased the shoot and root dry weight, and chlorophyll content | [52] |
| Trichoderma sp. PDR1-7 | Pinus sylvestris L. | lead | Removed heavy metals from mine-tailing soil extract media | [65] |
| Penicillium aculeatum PDR-4 and Trichoderma sp. PDR-16 | Sorghum, Sudangrass | As, Cu, Pb and Zn | Caused growth and As, Pb, and Zn uptake | [66] |
| Arbuscular mycorrhizal fungi | Bread wheat | Cd | Improved nitrogen and phosphorus nutrition, and the immobilization of Cd | [67] |
| Cadophora, Leptodontidium, Phialophora and Phialocephala | N/A | Cd, Pb and Zn | Promoted plant growth, metabolite production, and metal tolerance | [68] |
| Aspergillus fumigatus, Rhizopus sp., Penicillium radicum and Fusarium proliferatum | Lactuca sativa L. | Cr-VI to Cr-III | Detoxified up to 95% of Cr extracellularly | [69] |
| Trichoderma atroviride F6 | Brassica juncea L. Coss. var. foliosa Bailey | Cd, Ni | Caused an 110%, 40%, and 170% increase in fresh weight | [53] |
| Glomus geosporum | Aster tripolium L. | Cd and Cu | Enhanced Cd and Cu root uptake and accumulation | [70] |
| Arbuscular mycorrhizal fungi | Erato polymnioides | Hg | Increased Hg accumulation | [71] |
| Claroideoglomus etunicatum | Zea mays L. | La and Cd | Caused metal uptake and transport | [72] |
| Arbuscular mycorrhizal fungi (AMF) | Solanum melongena L. | Pb, Cd, and As as Pb (NO3)2, CdCl2·5H2O, and As3S2 | Improved growth, biomass, and the antioxidative defense response | [73] |
| Arbuscular mycorrhiza (AM) | Trifolium pratense L. | Zn | Increased Zn uptake and root accumulation, enhanced plant growth and P nutrition, and alleviated Zn toxicity | [74] |
| Arbuscular mycorrhizal fungi | Populus cathayana | Pb | Increased P uptake, antioxidant enzyme activity, and Pb accumulation | [75] |
| Glomus intraradices (AH01) | Oryza sativa L. | Arsenite | Decreased arsenite uptake and immobilized arsenite in rice roots, preventing translocation to shoots | [76] |
| Glomus intraradices (AH01) | Oryza sativa L. | Arsenate | Increased OsPT11 expression, enhanced P concentration and biomass, decreased arsenate concentration and uptake in rice, and raised the P/As molar ratio | [77] |
| AMF Funneliformis mosseae (Fm) or Rhizophagus intraradices (Ri) | Oryza sativa L. | Cd | Reduced rice Cd uptake by altering Cd transporter expression | [78] |
| Fusarium sp. CBRF44, Penicillium sp. CBRF65, and Alternaria sp. CBSF68 | Brassica napus | Pb and Cd | Increased biomass and metal extraction | [79] |
| Aspergillus fumigatus, Aspergillus niger, Fusarium equiseti, Fusarium chlamydosporum, Paecilomyces lilacinus, Trametes versicolor, Penicillium cataractum, Perenniporia subtephropora, Daldinia starbaeckii, Antrodia serialis, Cerrena aurantiopora, Phanerochaete concrescens and Polyporales species. | Prosopis juliflora Sw. | As,Cr,Cu,Fe,Mn,Ni, Pb,Zn | Enhanced biomass, increased root and shoot lengths, elevated carotenoids and chlorophyll, increased levels of L-phenylalanine and L-leucine, increased heavy metal accumulation, upregulated antioxidant genes, improved growth and metal tolerance, and served as an effective bioremediation strategy | [44] |
| Trichoderma harzianum | Amaranthus hypochondriacus L. | Cd and Zn | Promoted phytoremediation of Cd and Zn and enhanced the prevalence of heavy metal resistant genes (MRGs) and antibiotic resistance genes (ARGs), MRGs were influenced by available Zn and Cd, and MRGs were linked to specific bacterial hosts | [80] |
4. Plant–Microbe Synergism in Soil Recovery
The alliance of microorganisms and phytoremediation has acknowledged increasing consideration and is a well thought out, promising remediation technology for improving the adsorption and modification of heavy metals [81]. Plant growth-promoting bacteria (PGPB)-assisted phytoremediation has been used to enhance the phytoremediation of HM-contaminated soils [82]. Plant growth-promoting bacteria (PGPB) enhance plant growth through various mechanisms, both directly and indirectly. This mode of action includes nitrogen fixation, where bacteria convert atmospheric nitrogen into a plant-usable form, and phosphate solubilization, which makes phosphorus more accessible for plant uptake. Furthermore, PGPB emit siderophores that chelate and transport iron to the plant, phytohormones such as auxins and gibberellins that control growth and development, and ACC deaminase, an enzyme that alleviates plant stress by breaking down the ethylene precursor ACC, thus promoting healthy plant growth [83]. The indirect elevation of plant development by PGPB usually defends plants against various pathogens or recovers their resistance to environmental stresses such as drought, salt, heavy metals, and organic contaminants [84]. Among these growth-promoting microbes, some strains have been found to be tolerant to high concentrations of HMs [85]. Apart from modifying plant cell metabolism to drive growth, these bacteria can provide additional benefits to host plants, such as mitigating the harmful effects of heavy metals (HMs) and permitting plants to withstand high HM concentrations. With respect to this, heavy metal-tolerant plant growth-promoting bacteria (HMT-PGPB) have a substantial probability for phytoremediation by increasing plant survival and growth in HM-contaminated soils [86]. Rhizospheric and internal plant microbes can stimulate biofilm production around the root zones of host plants. Biofilm buildup, which comprises the accumulation of microbial cells in a self-produced extracellular matrix, augments the resilience of these microbes in intimate environments containing toxic pollutants. This defensive biofilm layer not only helps microbes adhere to root surfaces but also forms a microenvironment that protects them from stressors, such as heavy metals and other contaminants. Additionally, biofilms can enable nutrient exchange between microbes and plants, enhance pollutant degradation, and advance the overall health and growth of host plants under adverse conditions. Kocuria flava and Bacillus vietnamensis, when applied as an inoculum, as well as Kocuria flava and Bacillus vietnamensis, not only promoted the growth of rice seedlings but also decreased As uptake and accumulation in plants [87]. Pseudomonas sp. H13 and Brevundomonas sp. H16 initiate the production of extracellular polysaccharides and inorganic labile sulfides and strengthen biofilm formation, thereby significantly improving the removal efficiency of Cu2+, Zn2+, Cd2+, and Pb2+ [88]. With the integration of metal-tolerant bacteria isolated from polluted soil with plant growth promotion (PGP) potential, Brassica juncea and Lupinus albus phytoaccumulation increased up to 85% for As and up to 45% for Hg [89]. Biofilm communities are thus proficient in the sorption and metabolism of organic pollutants and heavy metals through a well-controlled expression pattern of genes governed by quorum sensing [90]. The main policy for improving the phytoremediation of contaminated soils is to enhance the expression of specific genes using CRISPR technology, thereby boosting the production of metal-binding proteins such as metallothioneins (MT) and phytochelatins (PC), metal transporter proteins (from the MATE, CDF, HMA, ZIP, and YSL families) [91], plant growth hormones (such as auxins, cytokinins, and gibbrellic acids) [92], and root exudates [93]. CRISPR is a powerful gene-editing tool that allows for precise modifications of an organism’s DNA, making its application in areas such as soil remediation and cleanup highly valued. Some examples include the targeted activation or repression of genes responsible for metal uptake, transport, and sequestration.
Numerous studies since the early 2000s have discovered that specific plant and bacterial genes, when combined with the target plant genomes, might enhance phytoremediation abilities [94]. For example, expression of the NAS1 gene (responsible for encoding the enzyme nicotianamine synthase-1) in tobacco and Arabidopsis plants resulted in enhanced metal tolerance for Cd and Zn [95]. Likewise, some genes, including czcD, are important for bacteria to purify Cd2+ [96]. Some of the metal resistance genes are listed in Table 3.

Table 3.
Exploration of metal-resistant genes from bacteria.
Table 3.
Exploration of metal-resistant genes from bacteria.
| Species Name | Resistant Genes | HM | References |
|---|---|---|---|
| Citrobacter, Desulfocurvus and Stappia | czcA, czcB, czcC | Cadmium | [97] |
| Caenispirillum, Halomonas, Stappia, Thauera | ctpA, copZ, copR and copB | Cadmium | [98] |
| Serratia marcescens CCMA 1010 | zntR gene | Pb2+ | [99] |
| Pseudomonas, Escherichia coli and Staphylococcus aureus | merR, merD | Hg2+ | [100] |
| Sporosarcina ginsengisoli | copK | As(III) | [101] |
| Georgenia sp. SUBG003 | czcD | Cobalt/zinc/cadmium resistance protein | [102] |
| Cupriavidus metallidurans | pbrR and its derivatives, pbrR2 and pbrD | Lead | [103] |
| Escherichia coli | Metallothionein (MT) | Cd | [104] |
| Thiobacillus, Hydrogenophaga and Flavihumibacter | aioA, arsC, arrA and soxB genes | Arsenic | [105] |
| Microbacterium paraoxydans | arsR, arsB, arsC, acr1, acr2 and acr3 | Arsenic | [106] |
| Pseudomonas putida ARS1 | aio, arr, and arsM | Arsenic | [107] |
| Desulfurella and Clostridium | asrA and arsB | Arsenic | [108] |
| Acinetobacter sp. (ADHR1) | chrR | Cr (VI) | [109] |
| Alphaproteobacteria Xanthobacter autotrophicus Py2 | mer1 and mer2 | Mercury (Hg) | [110] |
| Bacillus megaterium | merA and merB | Mercury (Hg) | [111] |
| Pseudomonas putida | mer73 | Mercury (Hg) | [112] |
| E. coli K12 | nikA, nikE, nikC, rcnA and nikB, | Ni2+ | [113] |
| Alcaligenes xylosoxydans, Ralstonia metallidurans and Helicobacter mustelae | nccA, cnrA and cznA | Ni2+ | [113] |
| Arthrobacter rhombi AY509239, Clavibacter xyli AY509235, Microbacterium arabinogalactanolyticum AY509226, Rhizobium mongolense AY509209 and Variovorax paradoxus AY512828 | czc, chr, mer and ncc | Arsenate, cadmium, chromium, zinc, mercury, lead, cobalt, copper, and nickel | [114] |
| Pseudomonas aeruginosa JP-11 | cad operon, and czc operon | Cadmium | [115] |
| Staphylococcus aureus | cadB | Cadmium | [116] |
| Staphylococcus lugdunensis | cadX | Cadmium | [117] |
| Ochrobactrum tritici | chrBACF | Chromium | [118] |
| Arthrobacter sp. | chrJ, chrK, and chrL | Chromium | [119] |
| Escherichia coli | cueO | Copper | [120] |
| Pseudomonas fluorescens | copRSCD operon | Copper | [121] |
| Bacillus subtilis | ycnJ | Copper | [122] |
| Helicobacter pylori | hpcopA and hpcopP | Copper | [123] |
| C. metallidurans | pbrU | Lead | [124] |
| Pseudomonas aeruginosa strain WI-1 | bmtA | Lead | [125] |
| Acidithiobacillus ferrooxidans | merC | Mercury | [126] |
| Cupriavidus (Ralstonia) metallidurans | cnrCBA | Nickle | [127] |
| Achromobacter xylosoxidans 31A | nre | Nickle | [128] |
| Escherichia coli | yohM | Nickle | [129] |
| Escherichia coli | NiCoT efflux gene (rcnA) | Nickle and Cobalt | [130] |
| Escherichia coli | ppk | Mercury | [131] |
Heavy metal transport can be influenced by the interaction between host plants and diverse AM fungal isolates, and the contact of heavy metals with other metals [132]. Arbuscular mycorrhizal fungi (AMF) are obligate symbionts of an extensive array of plants. Their contribution as adjuncts in phytoremediation must be alongside their plant hosts, unlike fungi such as Trichoderma, which can independently colonize the soil and be cultivated and/or applied in soil remediation interventions without an associated host.
AM fungi may enhance stress tolerance in polluted soils by trapping heavy metals in their extraradical hyphae and plant root systems [133]. Arbuscular mycorrhizal (AM) fungi are crucial for enhancing the accumulation of glomalin-related soil proteins, organic matter, and organic carbon. This is achieved through several mechanisms. AM fungi contribute to stabilizing and forming soil aggregates by producing glomalin, a glycoprotein that binds soil particles together, improving soil structure, and enhancing organic matter content. This stabilization process can alter the particle size distribution, leading to more structured and less erodible soil. Additionally, by forming symbiotic relationships with plant roots, AM fungi enhance plant growth and nutrient uptake, increasing the input of organic matter into the soil as root exudates and decaying plant material. AM fungi can also mitigate metal toxicity in areas contaminated with heavy metals by sequestering metals in their hyphal networks and reducing their bioavailability, thereby further influencing soil health and structure [134]. A cyclin, specifically SiPHO80, within the protein family, may be crucial for maintaining inorganic phosphate balance and managing tolerance to heavy metal stress in Serendipita indica, an osmotolerant AM fungal species [135]. Therefore, S. indica has been suggested as a potential biofertilizer. We can also benefit from their role in heavy-metal phytoextraction. AMF-assisted remediation of metal contamination involves several modes of action, such as increasing photosynthetic capability, facilitating nutrient absorption, accelerating biochemical and enzymatic activities, restricting heavy metal uptake, altering soil pH, and immobilizing or compartmentalizing heavy metals in plant organs and fungal structures, thus enhancing plant performance [136]. AMF (e.g., Glomus, Rhizophagus) boost plant growth and resilience to heavy metal stress for effective phytoremediation [137].
5. Engineering the Meta-Organism
The meta-organism is the plant and its associated microbiome; therefore, it has been proposed that engineering could be a viable strategy to make phytoremediation processes more efficient. This proposal was made by Thijs et al. [138] a few years ago, where, based on different studies, they described four main strategies for engineering a meta-organism. Phytoremediation, traditionally focused on selecting plants with fast growth, high biomass, and hyperaccumulation capabilities, often overlooks the influence of plants on their associated microbiomes, which is crucial for the success of phytoremediation [1,6]. The selection of plant species significantly affects the structure and function of microbial communities, making it essential to modify plant-associated microbial communities. Therefore, integrating microbiome functions and promoting specific microbial assemblies can improve both phytoremediation and biomass yields. Practical strategies include evaluating the effects of plants on microbiomes and implementing interventions to enhance the microbiomes and phytoremediation outcomes. This approach is termed “Microbiome-based plant selection” [2].
Another strategy proposed by the authors was the manipulation of root exudates. Known previously as “top-down” engineering by Orozco-Mosqueda and colleagues, this strategy involves manipulating the plant genome to produce exudates that increase or stimulate the abundance of beneficial microbial groups [139]. Plant molecules, such as strigolactones, flavonoids, and cutin monomers, act as signals detected by rhizosphere microorganisms. Similarly, the rhizobiome influences the plant transcriptome and metabolism, thereby impacting its overall fitness. This interplay involves mutual effects, where both plants and their rhizobiomes interact through the secretion and detection of signals in the rhizosphere. Despite significant efforts to understand the chemicals coordinating symbiotic interactions in the rhizosphere, redirecting rhizosphere microbial communities remains challenging owing to the dynamic nature and variability of rhizodeposits, which are influenced by plant species, physiological stage, neighboring plants, soil characteristics, soil contaminants, and microbial community context [11]. Future research should further explore this topic, as it holds promise for manipulating rhizomicrobiomes through plant root exudates.
The third strategy involves modification or perturbation of the driving forces. Competition can favor the selection of beneficial plant growth promoters (PGPs) and degrading microorganisms in the rhizosphere. Metagenomic data have shown that many microbial genes required for phytoremediation are already present in the environment. However, this is sometimes insufficient for achieving high biodegradation activity. Contaminant degradation results from various competitive interactions, including interference competition and resource exploitation, as well as cooperative interactions, such as coexistence, mutualism, and symbiosis, which affect partners spatially and temporally. Identifying and understanding these interactions between the host plant and its microbiome are crucial for optimizing the organism [138]. Improving our understanding of these relationships presents challenges, including designing studies to determine whether interactions are direct or indirect, and addressing issues related to statistical assessment and testing of the dynamics, feedback, and uncertainties in host–microbiome relationships.
Finally, engineering rhizomicrobial catabolic capabilities have been proposed [138]. This approach addresses cases in which the abundance of degrading traits in the surrounding soil is low. Increasing the frequency of beneficial plant growth-promoting and degrading microorganisms can be achieved through enrichment or inoculation, thereby enhancing net immigration from the environment through competitive interactions [2]. Additionally, preselecting highly colonizing strains with competitive capabilities in multiple environments would increase the confidence in achieving a bioinoculant with better field results. For example, under saline conditions in agricultural soils, certain mycobacterial agents in a bioinoculant should have strategies to survive such stress, such as biofilm production, membrane component modification, and osmoprotectant production.
6. Assessment of Perspectives
This study has emphasized the dynamic role of soil microorganisms, predominantly bacteria and fungi, in supplementing the proficiency of phytoremediation for agronomic soils polluted with heavy metals. Although plants have intrinsic capabilities to absorb, degrade, and stabilize pollutants during phytoextraction, phytodegradation, and phytostabilization, their microbial cohorts play a secondary role in enhancing these methods. These microbes, dwelling in the rhizosphere and endosphere, increase plant tolerance to heavy metals, activate and immobilize contaminants, and enable nutrient uptake, thereby promoting plant growth and overall phytoremediation efficiency [140].
Numerous research directions have emerged in this regard. A crucial priority is to attain a comprehensive understanding of the diverse microbial ecosystems connected with specific plant species and their roles in heavy metal remediation. Accomplishing this demand requires advanced molecular techniques for describing these microbial populations, such as metagenomics, metatranscriptomics, and metaproteomics. These omics sciences are fundamental to unveiling novel mechanisms of plant–microbe interactions and genes upregulated in the presence of heavy metals, particularly in agricultural soils, etc. [141]. Additionally, recognizing and isolating new microbial strains with improved metal resistance and plant growth-promoting capabilities, especially those specializing in heavy metals and plant species, is vital. Reconnoitering the potential of extremophiles adapted to environments with high heavy metal contamination is also essential to continue investigating and taking advantage of these groups of prokaryotes, particularly those that exhibit beneficial interactions with plant crops [142]. Furthermore, genetic engineering/genome modification approaches can be leveraged to boost the phytoremediation effectiveness of plants and their symbiotic microorganisms. This involves incorporating genes that enhance metal tolerance, increasing the production of chelating agents, or modifying microbial metabolic pathways to improve metal transformation efficiency. These are promising options, with their interactions and molecular mechanisms only beginning to be understood for enhancing phytoremediation in agricultural soils, particularly through the use of soil-adapted and endemic microorganisms.
7. Conclusions
Further research is important to improve the application of microbial consortia in phytoremediation, considering variables such as soil type, pollutant concentrations, and plant species. Inspecting the interactions between microbial strains and their community impact on pollutant degradation can reveal synergistic effects that augment remediation efficiency. Understanding the genetic and metabolic responses of microbes and plants to contaminants can lead to the development of more targeted and resilient phytoremediation strategies. By exploring these factors, we can optimize the use of microbial consortia to address persistent heavy-metal contamination and improve soil health and fertility [143,144]. In turn, this will support advancements in food security, promote sustainable agricultural practices, and contribute to overall environmental restoration.
Author Contributions
Z.K.: Writing—original draft, Visualization, Methodology. M.d.C.O.-M.: Writing—original draft, Methodology, Investigation. G.S.: Writing—review and editing, Supervision, Methodology, Conceptualization, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the ICTI-Michoacán and CIC-UMSNH.
Acknowledgments
We acknowledge Aurora Flores for their assistance with this figure.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Asad, S.A.; Farooq, M.; Afzal, A.; West, H. Integrated phytobial heavy metal remediation strategies for a sustainable clean environment—A review. Chemosphere 2019, 217, 925–941. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Muhammad, H.; Lv, X.; Wei, T.; Ren, X.; Jia, H.; Atif, S.; Hua, L. Prospects and applications of plant growth promoting rhizobacteria to mitigate soil metal contamination: A review. Chemosphere 2020, 246, 125823. [Google Scholar] [CrossRef]
- Grobelak, A.; Napora, A.; Kacprzak, M. Using plant growth-promoting rhizobacteria (PGPR) to improve plant growth. Ecol. Eng. 2015, 84, 22–28. [Google Scholar] [CrossRef]
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Shi, H.; Tao, J.; Chen, L.; Liu, Y.; Lei, G.; Liu, X.; Smol, J.P. Industrial arsenic contamination causes catastrophic changes in freshwater ecosystems. Sci. Rep. 2015, 5, 17419. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A.; Kaur, P.; Singh Sidhu, G.P.; Bali, A.S.; Bhardwaj, R.; Thukral, A.K.; Cerda, A. Pollution assessment of heavy metals in soils of India and ecological risk assessment: A state-of-the-art. Chemosphere 2019, 216, 449–462. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Cocârţă, D.M.; Neamţu, S.; Reşetar Deac, A.M. Carcinogenic risk evaluation for human health risk assessment from soils contaminated with heavy metals. Int. J. Environ. Sci. Technol. 2016, 13, 2025–2036. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; He, X.; Li, B.; Du, S. Plant growth-promoting rhizobacteria: A good companion for heavy metal phytoremediation. Chemosphere 2023, 338, 139475. [Google Scholar] [CrossRef]
- Manoj, S.R.; Karthik, C.; Kadirvelu, K.; Arulselvi, P.I.; Shanmugasundaram, T.; Bruno, B.; Rajkumar, M. Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: A review. J. Environ. Manag. 2020, 254, 109779. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Guan, D.-X.; Cao, Y.; Wang, C.; Liu, C.; Ma, L.Q. Arsenic-Hyperaccumulator Pteris vittata Effectively Uses Sparingly-Soluble Phosphate Rock: Rhizosphere Solubilization, Nutrient Improvement, and Arsenic Accumulation. Environ. Sci. Technol. 2024, 58, 7870–7879. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Zaidi, A.; Wani, P.A.; Oves, M. Role of plant growth promoting rhizobacteria in the remediation of metal contaminated soils. Environ. Chem. Lett. 2009, 7, 1–19. [Google Scholar] [CrossRef]
- Oyewole, O.A.; Zobeashia, S.S.L.-T.; Oladoja, E.O.; Raji, R.O.; Odiniya, E.E.; Musa, A.M. Biosorption of heavy metal polluted soil using bacteria and fungi isolated from soil. SN Appl. Sci. 2019, 1, 857. [Google Scholar] [CrossRef]
- Zuo, Y.; Li, Y.; Chen, H.; Ran, G.; Liu, X. Effects of multi-heavy metal composite pollution on microorganisms around a lead-zinc mine in typical karst areas, southwest China. Ecotoxicol. Environ. Saf. 2023, 262, 115190. [Google Scholar] [CrossRef]
- Qin, H.; Wang, Z.; Sha, W.; Song, S.; Qin, F.; Zhang, W. Role of Plant-Growth-Promoting Rhizobacteria in Plant Machinery for Soil Heavy Metal Detoxification. Microorganisms 2024, 12, 700. [Google Scholar] [CrossRef]
- Göhre, V.; Paszkowski, U. Contribution of the arbuscular mycorrhizal symbiosis to heavy metal phytoremediation. Planta 2006, 223, 1115–1122. [Google Scholar] [CrossRef]
- Mahajan, P.; Kaushal, J. Role of Phytoremediation in Reducing Cadmium Toxicity in Soil and Water. J. Toxicol. 2018, 2018, 4864365. [Google Scholar] [CrossRef]
- Jacob, J.M.; Karthik, C.; Saratale, R.G.; Kumar, S.S.; Prabakar, D.; Kadirvelu, K.; Pugazhendhi, A. Biological approaches to tackle heavy metal pollution: A survey of literature. J. Environ. Manag. 2018, 217, 56–70. [Google Scholar] [CrossRef]
- Konkolewska, A.; Piechalak, A.; Ciszewska, L.; Antos-Krzemińska, N.; Skrzypczak, T.; Hanć, A.; Sitko, K.; Małkowski, E.; Barałkiewicz, D.; Małecka, A. Combined use of companion planting and PGPR for the assisted phytoextraction of trace metals (Zn, Pb, Cd). Environ. Sci. Pollut. Res. 2020, 27, 13809–13825. [Google Scholar] [CrossRef]
- Durand, A.; Piutti, S.; Rue, M.; Morel, J.L.; Echevarria, G.; Benizri, E. Improving nickel phytoextraction by co-cropping hyperaccumulator plants inoculated by plant growth promoting rhizobacteria. Plant Soil 2016, 399, 179–192. [Google Scholar] [CrossRef]
- Rakić, T.; Pešić, M.; Kostić, N.; Andrejić, G.; Fira, D.; Dželetović, Ž.; Stanković, S.; Lozo, J. Rhizobacteria associated with Miscanthus x giganteus improve metal accumulation and plant growth in the flotation tailings. Plant Soil 2021, 462, 349–363. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Su, R.; Chen, Y. Phytostabilization of Heavy Metals and Fungal Community Response in Manganese Slag under the Mediation of Soil Amendments and Plants. Toxics 2024, 12, 333. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Wang, W.; Chen, X.; Zheng, X.; Fu, W.; Wang, G.; Ji, J.; Guan, C. Phytoremediation of DEHP and heavy metals co-contaminated soil by rice assisted with a PGPR consortium: Insights into the regulation of ion homeostasis, improvement of photosynthesis and enrichment of beneficial bacteria in rhizosphere soil. Environ. Pollut. 2022, 314, 120303. [Google Scholar] [CrossRef]
- Guarino, F.; Miranda, A.; Castiglione, S.; Cicatelli, A. Arsenic phytovolatilization and epigenetic modifications in Arundo donax L. assisted by a PGPR consortium. Chemosphere 2020, 251, 126310. [Google Scholar] [CrossRef]
- Dimitroula, H.; Syranidou, E.; Manousaki, E.; Nikolaidis, N.P.; Karatzas, G.P.; Kalogerakis, N. Mitigation measures for chromium-VI contaminated groundwater—The role of endophytic bacteria in rhizofiltration. J. Hazard. Mater. 2015, 281, 114–120. [Google Scholar] [CrossRef]
- Tirry, N.; Tahri Joutey, N.; Sayel, H.; Kouchou, A.; Bahafid, W.; Asri, M.; El Ghachtouli, N. Screening of plant growth promoting traits in heavy metals resistant bacteria: Prospects in phytoremediation. J. Genet. Eng. Biotechnol. 2018, 16, 613–619. [Google Scholar] [CrossRef]
- Kumar, V.; Dwivedi, S.K. Mycoremediation of heavy metals: Processes, mechanisms, and affecting factors. Environ. Sci. Pollut. Res. 2021, 28, 10375–10412. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Zhang, C.; Freitas, H. Beneficial role of bacterial endophytes in heavy metal phytoremediation. J. Environ. Manag. 2016, 174, 14–25. [Google Scholar] [CrossRef]
- Carlos, M.-H.J.; Stefani, P.-V.Y.; Janette, A.-M.; Melani, M.-S.S.; Gabriela, P.-O. Assessing the effects of heavy metals in ACC deaminase and IAA production on plant growth-promoting bacteria. Microbiol. Res. 2016, 188–189, 53–61. [Google Scholar] [CrossRef]
- Becerra-Castro, C.; Monterroso, C.; Prieto-Fernández, A.; Rodríguez-Lamas, L.; Loureiro-Viñas, M.; Acea, M.J.; Kidd, P.S. Pseudometallophytes colonising Pb/Zn mine tailings: A description of the plant–microorganism–rhizosphere soil system and isolation of metal-tolerant bacteria. J. Hazard. Mater. 2012, 217–218, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Belimov, A.A.; Hontzeas, N.; Safronova, V.I.; Demchinskaya, S.V.; Piluzza, G.; Bullitta, S.; Glick, B.R. Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.). Soil Biol. Biochem. 2005, 37, 241–250. [Google Scholar] [CrossRef]
- Kamnev, A.A.; Tugarova, A.V.; Antonyuk, L.P.; Tarantilis, P.A.; Polissiou, M.G.; Gardiner, P.H.E. Effects of heavy metals on plant-associated rhizobacteria: Comparison of endophytic and non-endophytic strains of Azospirillum brasilense. J. Trace Elem. Med. Biol. 2005, 19, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.P.G.C.; Moreira, H.; Franco, A.R.; Rangel, A.O.S.S.; Castro, P.M.L. Inoculating Helianthus annuus (sunflower) grown in zinc and cadmium contaminated soils with plant growth promoting bacteria—Effects on phytoremediation strategies. Chemosphere 2013, 92, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Mohamad, O.A.; Deng, Z.; Liu, X.; Glick, B.R.; Wei, G. Rhizobial symbiosis effect on the growth, metal uptake, and antioxidant responses of Medicago lupulina under copper stress. Environ. Sci. Pollut. Res. 2015, 22, 12479–12489. [Google Scholar] [CrossRef]
- Moreira, H.; Pereira, S.I.A.; Marques, A.P.G.C.; Rangel, A.O.S.S.; Castro, P.M.L. Selection of metal resistant plant growth promoting rhizobacteria for the growth and metal accumulation of energy maize in a mine soil—Effect of the inoculum size. Geoderma 2016, 278, 1–11. [Google Scholar] [CrossRef]
- Ma, Y.; Oliveira, R.S.; Nai, F.; Rajkumar, M.; Luo, Y.; Rocha, I.; Freitas, H. The hyperaccumulator Sedum plumbizincicola harbors metal-resistant endophytic bacteria that improve its phytoextraction capacity in multi-metal contaminated soil. J. Environ. Manag. 2015, 156, 62–69. [Google Scholar] [CrossRef]
- Funes Pinter, I.; Salomon, M.V.; Berli, F.; Bottini, R.; Piccoli, P. Characterization of the As(III) tolerance conferred by plant growth promoting rhizobacteria to in vitro-grown grapevine. Appl. Soil Ecol. 2017, 109, 60–68. [Google Scholar] [CrossRef]
- Hansda, A.; Kumar, V.; Anshumali. Cu-resistant Kocuria sp. CRB15: A potential PGPR isolated from the dry tailing of Rakha copper mine. 3 Biotech 2017, 7, 132. [Google Scholar] [CrossRef]
- Kang, X.; Yu, X.; Zhang, Y.; Cui, Y.; Tu, W.; Wang, Q.; Li, Y.; Hu, L.; Gu, Y.; Zhao, K.; et al. Inoculation of Sinorhizobium saheli YH1 Leads to Reduced Metal Uptake for Leucaena leucocephala Grown in Mine Tailings and Metal-Polluted Soils. Front. Microbiol. 2018, 9, 1853. [Google Scholar] [CrossRef]
- Tirry, N.; Kouchou, A.; El Omari, B.; Ferioun, M.; El Ghachtouli, N. Improved chromium tolerance of Medicago sativa by plant growth-promoting rhizobacteria (PGPR). J. Genet. Eng. Biotechnol. 2021, 19, 149. [Google Scholar] [CrossRef] [PubMed]
- Ke, T.; Guo, G.; Liu, J.; Zhang, C.; Tao, Y.; Wang, P.; Xu, Y.; Chen, L. Improvement of the Cu and Cd phytostabilization efficiency of perennial ryegrass through the inoculation of three metal-resistant PGPR strains. Environ. Pollut. 2021, 271, 116314. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Mateen, A.; Ahmad, M.A.; Munir, I.; Iqbal, A.; Alghamdi, K.M.S.; Al-Solami, H.M.; Siddiqui, M.F. Heavy metal ATPase genes (HMAs) expression induced by endophytic bacteria, “AI001, and AI002” mediate cadmium translocation and phytoremediation. Environ. Pollut. 2022, 293, 118508. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Hamid, F.S.; Pariatamby, A.; Ossai, I.C.; Ahmed, A.; Barasarathi, J.; Auta, H.S. Influence of bioaugmented fungi on tolerance, growth and phytoremediation ability of Prosopis juliflora Sw. DC in heavy metal–polluted landfill soil. Environ. Sci. Pollut. Res. 2024, 31, 28671–28694. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-L.; Li, T.; Liu, G.-Y.; Smith, J.M.; Zhao, Z.-W. Unraveling the role of dark septate endophyte (DSE) colonizing maize (Zea mays) under cadmium stress: Physiological, cytological and genic aspects. Sci. Rep. 2016, 6, 22028. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Oliveira, R.S.; Zhang, C.; Freitas, H. Potential of plant beneficial bacteria and arbuscular mycorrhizal fungi in phytoremediation of metal-contaminated saline soils. J. Hazard. Mater. 2019, 379, 120813. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.W.; Wu, L.; Luo, N.; Huang, W.X.; Mo, C.H.; Li, Y.W.; Xiang, L.; Zhao, H.M.; Cai, Q.Y.; et al. Effects of arbuscular mycorrhizal fungi on redox homeostasis of rice under Cd stress. Plant Soil 2020, 455, 121–138. [Google Scholar] [CrossRef]
- Lu, R.-R.; Hu, Z.-H.; Zhang, Q.-L.; Li, Y.-Q.; Lin, M.; Wang, X.-L.; Wu, X.-N.; Yang, J.-T.; Zhang, L.-Q.; Jing, Y.-X.; et al. The effect of Funneliformis mosseae on the plant growth, Cd translocation and accumulation in the new Cd-hyperaccumulator Sphagneticola calendulacea. Ecotoxicol. Environ. Saf. 2020, 203, 110988. [Google Scholar] [CrossRef]
- Li, X.; Zhou, M.; Shi, F.; Meng, B.; Liu, J.; Mi, Y.; Dong, C.; Su, H.; Liu, X.; Wang, F.; et al. Influence of arbuscular mycorrhizal fungi on mercury accumulation in rice (Oryza sativa L.): From enriched isotope tracing perspective. Ecotoxicol. Environ. Saf. 2023, 255, 114776. [Google Scholar] [CrossRef]
- Zhu, Q.; Xu, P.; Lei, L.; Jing, Y. Transcriptome analysis reveals decreased accumulation and toxicity of Cd in upland rice inoculated with arbuscular mycorrhizal fungi. Appl. Soil Ecol. 2022, 177, 104501. [Google Scholar] [CrossRef]
- Yang, X.; Qin, J.; Li, J.; Lai, Z.; Li, H. Upland rice intercropping with Solanum nigrum inoculated with arbuscular mycorrhizal fungi reduces grain Cd while promoting phytoremediation of Cd-contaminated soil. J. Hazard. Mater. 2021, 406, 124325. [Google Scholar] [CrossRef] [PubMed]
- Arriagada, C.; Aranda, E.; Sampedro, I.; Garcia-Romera, I.; Ocampo, J.A. Contribution of the saprobic fungi Trametes versicolor and Trichoderma harzianum and the arbuscular mycorrhizal fungi Glomus deserticola and G. claroideum to arsenic tolerance of Eucalyptus globulus. Bioresour. Technol. 2009, 100, 6250–6257. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Jiang, M.; Zeng, Z.; Du, A.; Tan, H.; Liu, Y. Trichoderma atroviride F6 improves phytoextraction efficiency of mustard (Brassica juncea (L.) Coss. var. foliosa Bailey) in Cd, Ni contaminated soils. Chemosphere 2008, 71, 1769–1773. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Dwivedi, S.K. Hexavalent chromium stress response, reduction capability and bioremediation potential of Trichoderma sp. isolated from electroplating wastewater. Ecotoxicol. Environ. Saf. 2019, 185, 109734. [Google Scholar] [CrossRef]
- Gola, D.; Tyagi, P.K.; Chauhan, N.; Malik, A.; Srivastava, S.K. Beauveria bassiana assisted remediation of chromium and indanthane blue. J. Environ. Chem. Eng. 2021, 9, 105552. [Google Scholar] [CrossRef]
- Gola, D.; Dey, P.; Bhattacharya, A.; Mishra, A.; Malik, A.; Namburath, M.; Ahammad, S.Z. Multiple heavy metal removal using an entomopathogenic fungi Beauveria bassiana. Bioresour. Technol. 2016, 218, 388–396. [Google Scholar] [CrossRef]
- Sun, S.; Fan, X.; Feng, Y.; Wang, X.; Gao, H.; Song, F. Arbuscular mycorrhizal fungi influence the uptake of cadmium in industrial hemp (Cannabis sativa L.). Chemosphere 2023, 330, 138728. [Google Scholar] [CrossRef]
- Quan, L.; Shi, L.; Zhang, S.; Yao, Q.; Yang, Q.; Zhu, Y.; Liu, Y.; Lian, C.; Chen, Y.; Shen, Z.; et al. Ectomycorrhizal fungi, two species of Laccaria, differentially block the migration and accumulation of cadmium and copper in Pinus densiflora. Chemosphere 2023, 334, 138857. [Google Scholar] [CrossRef]
- Chen, Y.; Nara, K.; Wen, Z.; Shi, L.; Xia, Y.; Shen, Z.; Lian, C. Growth and photosynthetic responses of ectomycorrhizal pine seedlings exposed to elevated Cu in soils. Mycorrhiza 2015, 25, 561–571. [Google Scholar] [CrossRef]
- Szuba, A.; Karliński, L.; Krzesłowska, M.; Hazubska-Przybył, T. Inoculation with a Pb-tolerant strain of Paxillus involutus improves growth and Pb tolerance of Populus × canescens under in vitro conditions. Plant Soil 2017, 412, 253–266. [Google Scholar] [CrossRef]
- Jourand, P.; Hannibal, L.; Majorel, C.; Mengant, S.; Ducousso, M.; Lebrun, M. Ectomycorrhizal Pisolithus albus inoculation of Acacia spirorbis and Eucalyptus globulus grown in ultramafic topsoil enhances plant growth and mineral nutrition while limits metal uptake. J. Plant Physiol. 2014, 171, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Abou Alhamed, M.F.; Shebany, Y.M. Endophytic Chaetomium globosum enhances maize seedling copper stress tolerance. Plant Biol. 2012, 14, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.; De-Leij, F.A.A.M.; Lynch, J.M. Trichoderma harzianum Rifai 1295-22 Mediates Growth Promotion of Crack Willow (Salix fragilis) Saplings in Both Clean and Metal-Contaminated Soil. Microb. Ecol. 2007, 54, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Amna; Masood, S.; Syed, J.H.; Munis, M.F.H.; Chaudhary, H.J. Phyto-extraction of Nickel by Linum usitatissimum in Association with Glomus intraradices. Int. J. Phytoremediation 2015, 17, 981–987. [Google Scholar] [CrossRef]
- Babu, A.G.; Shea, P.J.; Oh, B.-T. Trichoderma sp. PDR1-7 promotes Pinus sylvestris reforestation of lead-contaminated mine tailing sites. Sci. Total Environ. 2014, 476–477, 561–567. [Google Scholar] [CrossRef]
- Babu, A.G.; Shim, J.; Shea, P.J.; Oh, B.-T. Penicillium aculeatum PDR-4 and Trichoderma sp. PDR-16 promote phytoremediation of mine tailing soil and bioenergy production with sorghum-sudangrass. Ecol. Eng. 2014, 69, 186–191. [Google Scholar] [CrossRef]
- Baghaie, A.H.; Aghili, F.; Jafarinia, R. Soil-indigenous arbuscular mycorrhizal fungi and zeolite addition to soil synergistically increase grain yield and reduce cadmium uptake of bread wheat (through improved nitrogen and phosphorus nutrition and immobilization of Cd in roots). Environ. Sci. Pollut. Res. 2019, 26, 30794–30807. [Google Scholar] [CrossRef]
- Berthelot, C.; Leyval, C.; Foulon, J.; Chalot, M.; Blaudez, D. Plant growth promotion, metabolite production and metal tolerance of dark septate endophytes isolated from metal-polluted poplar phytomanagement sites. FEMS Microbiol. Ecol. 2016, 92, fiw144. [Google Scholar] [CrossRef]
- Bibi, S.; Hussain, A.; Hamayun, M.; Rahman, H.; Iqbal, A.; Shah, M.; Irshad, M.; Qasim, M.; Islam, B. Bioremediation of hexavalent chromium by endophytic fungi; safe and improved production of Lactuca sativa L. Chemosphere 2018, 211, 653–663. [Google Scholar] [CrossRef]
- Carvalho, L.M.; Caçador, I.; Martins-Loução, M.A. Arbuscular mycorrhizal fungi enhance root cadmium and copper accumulation in the roots of the salt marsh plant Aster tripolium L. Plant Soil 2006, 285, 161–169. [Google Scholar] [CrossRef]
- Chamba, I.; Rosado, D.; Kalinhoff, C.; Thangaswamy, S.; Sánchez-Rodríguez, A.; Gazquez, M.J. Erato polymnioides—A novel Hg hyperaccumulator plant in ecuadorian rainforest acid soils with potential of microbe-associated phytoremediation. Chemosphere 2017, 188, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Diao, F.-W.; Wang, Q.-F.; Pan, L.; Dang, Z.-H.; Guo, W. Effects of arbuscular mycorrhizal symbiosis on growth, nutrient and metal uptake by maize seedlings (Zea mays L.) grown in soils spiked with Lanthanum and Cadmium. Environ. Pollut. 2018, 241, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, R.; Favas, P.; Pratas, J.; Varun, M.; Paul, M.S. Assessment of edibility and effect of arbuscular mycorrhizal fungi on Solanum melongena L. grown under heavy metal(loid) contaminated soil. Ecotoxicol. Environ. Saf. 2018, 148, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.D.; Li, X.L.; Tao, H.Q.; Christie, P.; Wong, M.H. The role of arbuscular mycorrhiza in zinc uptake by red clover growing in a calcareous soil spiked with various quantities of zinc. Chemosphere 2003, 50, 839–846. [Google Scholar] [CrossRef]
- Chen, L.; Hu, X.; Yang, W.; Xu, Z.; Zhang, D.; Gao, S. The effects of arbuscular mycorrhizal fungi on sex-specific responses to Pb pollution in Populus cathayana. Ecotoxicol. Environ. Saf. 2015, 113, 460–468. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Chan, W.F.; Wu, C.; Wu, F.; Wu, S.; Wong, M.H. Arsenite transporters expression in rice (Oryza sativa L.) associated with arbuscular mycorrhizal fungi (AMF) colonization under different levels of arsenite stress. Chemosphere 2012, 89, 1248–1254. [Google Scholar] [CrossRef]
- Chen, X.W.; Wu, F.Y.; Li, H.; Chan, W.F.; Wu, C.; Wu, S.C.; Wong, M.H. Phosphate transporters expression in rice (Oryza sativa L.) associated with arbuscular mycorrhizal fungi (AMF) colonization under different levels of arsenate stress. Environ. Exp. Bot. 2013, 87, 92–99. [Google Scholar] [CrossRef]
- Chen, X.W.; Wu, L.; Luo, N.; Mo, C.H.; Wong, M.H.; Li, H. Arbuscular mycorrhizal fungi and the associated bacterial community influence the uptake of cadmium in rice. Geoderma 2019, 337, 749–757. [Google Scholar] [CrossRef]
- Shi, Y.; Xie, H.; Cao, L.; Zhang, R.; Xu, Z.; Wang, Z.; Deng, Z. Effects of Cd- and Pb-resistant endophytic fungi on growth and phytoextraction of Brassica napus in metal-contaminated soils. Environ. Sci. Pollut. Res. 2017, 24, 417–426. [Google Scholar] [CrossRef]
- Song, J.; Chen, Y.; Mi, H.; Xu, R.; Zhang, W.; Wang, C.; Rensing, C.; Wang, Y. Prevalence of antibiotic and metal resistance genes in phytoremediated cadmium and zinc contaminated soil assisted by chitosan and Trichoderma harzianum. Environ. Int. 2024, 183, 108394. [Google Scholar] [CrossRef]
- Glick, B.R. Using soil bacteria to facilitate phytoremediation. Biotechnol. Adv. 2010, 28, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xu, M.; Zhao, Q.; Xia, Y.; Chen, C.; Shen, Z. Complete Genome Sequence of Cd(II)-Resistant Arthrobacter sp. PGP41, a Plant Growth-Promoting Bacterium with Potential in Microbe-Assisted Phytoremediation. Curr. Microbiol. 2018, 75, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Prasad, M.N.V.; Rajkumar, M.; Freitas, H. Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol. Adv. 2011, 29, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R.; Todorovic, B.; Czarny, J.; Cheng, Z.; Duan, J.; McConkey, B. Promotion of plant growth by bacterial ACC deaminase. Crit. Rev. Plant Sci. 2007, 26, 227–242. [Google Scholar] [CrossRef]
- Abou-Aly, H.E.; Youssef, A.M.; Tewfike, T.A.; El-Alkshar, E.A.; El-Meihy, R.M. Reduction of heavy metals bioaccumulation in sorghum and its rhizosphere by heavy metals-tolerant bacterial consortium. Biocatal. Agric. Biotechnol. 2021, 31, 101911. [Google Scholar] [CrossRef]
- Ren, Z.; Cheng, R.; Chen, P.; Xue, Y.; Xu, H.; Yin, Y.; Huang, G.; Zhang, W.; Zhang, L. Plant-associated Microbe System in Treatment of Heavy Metals–contaminated Soil: Mechanisms and Applications. Water Air Soil Pollut. 2023, 234, 39. [Google Scholar] [CrossRef]
- Mallick, I.; Bhattacharyya, C.; Mukherji, S.; Dey, D.; Sarkar, S.C.; Mukhopadhyay, U.K.; Ghosh, A. Effective rhizoinoculation and biofilm formation by arsenic immobilizing halophilic plant growth promoting bacteria (PGPB) isolated from mangrove rhizosphere: A step towards arsenic rhizoremediation. Sci. Total Environ. 2018, 610–611, 1239–1250. [Google Scholar] [CrossRef]
- Xing, Y.; Tan, S.; Liu, S.; Xu, S.; Wan, W.; Huang, Q.; Chen, W. Effective immobilization of heavy metals via reactive barrier by rhizosphere bacteria and their biofilms. Environ. Res. 2022, 207, 112080. [Google Scholar] [CrossRef]
- Franchi, E.; Rolli, E.; Marasco, R.; Agazzi, G.; Borin, S.; Cosmina, P.; Pedron, F.; Rosellini, I.; Barbafieri, M.; Petruzzelli, G. Phytoremediation of a multi contaminated soil: Mercury and arsenic phytoextraction assisted by mobilizing agent and plant growth promoting bacteria. J. Soils Sediments 2017, 17, 1224–1236. [Google Scholar] [CrossRef]
- Mishra, S.; Huang, Y.; Li, J.; Wu, X.; Zhou, Z.; Lei, Q.; Bhatt, P.; Chen, S. Biofilm-mediated bioremediation is a powerful tool for the removal of environmental pollutants. Chemosphere 2022, 294, 133609. [Google Scholar] [CrossRef]
- Pinto, A.P.; de Varennes, A.; Fonseca, R.; Teixeira, D.M. Phytoremediation of Soils Contaminated with Heavy Metals: Techniques and Strategies. In Phytoremediation: Management of Environmental Contaminants; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 1, pp. 133–155. [Google Scholar]
- Chen, Z.; Liu, Q.; Chen, D.; Wu, Y.; Hamid, Y.; Lin, Q.; Zhang, S.; Feng, Y.; He, Z.; Yin, X.; et al. Enhancing the phytoextraction efficiency of heavy metals in acidic and alkaline soils by Sedum alfredii Hance: A study on the synergistic effect of plant growth regulator and plant growth-promoting bacteria. Sci. Total Environ. 2024, 932, 173029. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Liang, Y.; Wang, M.; Hu, R.; Song, Z.; Xu, X.; Zheng, L.; Shen, Z.; Chen, C. Less is more: A new strategy combining nanomaterials and PGPB to promote plant growth and phytoremediation in contaminated soil. J. Hazard. Mater. 2024, 469, 134110. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Q.; Chen, Y.; Li, Y.-Y.; Ding, C.-Y.; Li, B.-L.; Han, H.; Chen, Z.-J. Plant growth-promoting bacteria improve the Cd phytoremediation efficiency of soils contaminated with PE–Cd complex pollution by influencing the rhizosphere microbiome of sorghum. J. Hazard. Mater. 2024, 469, 134085. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.-C.; Lo, J.-C.; Yeh, K.-C. Genes Associated with Heavy Metal Tolerance and Accumulation in Zn/Cd Hyperaccumulator Arabidopsis halleri: A Genomic Survey with cDNA Microarray. Environ. Sci. Technol. 2006, 40, 6792–6798. [Google Scholar] [CrossRef] [PubMed]
- Zainab, N.; Glick, B.R.; Bose, A.; Amna; Ali, J.; Rehman, F.U.; Paker, N.P.; Rengasamy, K.; Kamran, M.A.; Hayat, K.; et al. Deciphering the mechanistic role of Bacillus paramycoides (PM51) and Bacillus tequilensis (PM52) in bio-sorption and phyto-assimilation of Cadmium via Linum usitatissimum L. Seedlings. Plant Physiol. Biochem. 2024, 211, 108652. [Google Scholar] [CrossRef]
- Huang, W.; Chen, Z.; Liu, Y.; Li, D.; Wei, Z. Sulfide-carbonate-mineralized functional bacterial consortium for cadmium removal in flue gas. Chemosphere 2024, 363, 142869. [Google Scholar] [CrossRef]
- Huang, W.; Chen, Z.; Liu, H.; Wang, H.; Wei, Z. Microbial induced carbonate precipitation for cadmium removal in flue gas from sludge incineration. J. Environ. Chem. Eng. 2024, 12, 112573. [Google Scholar] [CrossRef]
- Carlier, J.D.; dos Reis Ferreira, G.M.; Schwan, R.F.; da Silva, C.F.; Costa, M.C. Pb2+ biosorption by Serratia marcescens CCMA 1010 and its relation with zntR gene expression and ZntA efflux pump regulation. Environ. Adv. 2024, 15, 100479. [Google Scholar] [CrossRef]
- Nanda, M.; Kumar, V.; Sharma, D.K. Multimetal tolerance mechanisms in bacteria: The resistance strategies acquired by bacteria that can be exploited to ‘clean-up’ heavy metal contaminants from water. Aquat. Toxicol. 2019, 212, 1–10. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X.; Fu, Q.; Zhang, D. Biomineralization based remediation of As(III) contaminated soil by Sporosarcina ginsengisoli. J. Hazard. Mater. 2012, 201–202, 178–184. [Google Scholar] [CrossRef]
- Oza, T.; Patel, P.; Thaker, V.S. A brief study on heavy metal resistance genes from 10 genomes of Georgenia sp. and in vitro confirmation on Georgenia sp. SUBG003. J. Hazard. Mater. Lett. 2024, 5, 100097. [Google Scholar] [CrossRef]
- Hui, C.-Y.; Ma, B.-C.; Wang, Y.-Q.; Yang, X.-Q.; Cai, J.-M. Designed bacteria based on natural pbr operons for detecting and detoxifying environmental lead: A mini-review. Ecotoxicol. Environ. Saf. 2023, 267, 115662. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Yao, W.; Tang, L. Surface expression of metallothionein enhances bioremediation in Escherichia coli. Desalination Water Treat. 2024, 317, 100070. [Google Scholar] [CrossRef]
- Zhang, M.; Xiong, Y.; Sun, H.; Xiao, T.; Xiao, E.; Sun, X.; Li, B.; Sun, W. Selective pressure of arsenic and antimony co-contamination on microbial community in alkaline sediments. J. Hazard. Mater. 2024, 464, 132948. [Google Scholar] [CrossRef]
- Mandal, D.; Das, S.K.; Adhikari, J.; Chatterjee, D.; Bandyopadhyay, T.K.; Basu, A. Genome sequencing, annotation and application of a strain of Microbacterium paraoxydans—A bacterium with arsenic bioremediation and plant growth promoting potential. Microbe 2024, 4, 100132. [Google Scholar] [CrossRef]
- Wang, Z.-W.; Yang, G.; Chen, J.; Zhou, Y.; Núñez Delgado, A.; Cui, H.-L.; Duan, G.-L.; Rosen, B.P.; Zhu, Y.-G. Fundamentals and application in phytoremediation of an efficient arsenate reducing bacterium Pseudomonas putida ARS1. J. Environ. Sci. 2024, 137, 237–244. [Google Scholar] [CrossRef]
- Gou, J.; Xia, J.; Li, Y.; Qiu, Y.; Jiang, F. A novel sulfidogenic process via sulfur reduction to remove arsenate in acid mine drainage: Insights into the performance and microbial mechanisms. Water Res. 2024, 254, 121423. [Google Scholar] [CrossRef]
- Montes-Robledo, A.; Baena-Baldiris, D.; Baldiris-Avila, R. Reduction of Cr(VI) by planktonic cells and biofilm of Acinetobacter sp. (ADHR1) isolated from electroplating wastewater. Environ. Technol. Innov. 2024, 33, 103521. [Google Scholar] [CrossRef]
- Petrus Amanda, K.; Rutner, C.; Liu, S.; Wang, Y.; Wiatrowski Heather, A. Mercury Reduction and Methyl Mercury Degradation by the Soil Bacterium Xanthobacter autotrophicus Py2. Appl. Environ. Microbiol. 2015, 81, 7833–7838. [Google Scholar] [CrossRef]
- Huang, C.-C.; Narita, M.; Yamagata, T.; Phung, L.T.; Endo, G.; Silver, S. Characterization of two regulatory genes of the mercury resistance determinants from TnMERI1 by luciferase-based examination. Gene 2002, 301, 13–20. [Google Scholar] [CrossRef]
- Leonhäuser, J.; Wang, W.; Deckwer, W.-D.; Wagner-Döbler, I. Functioning of the mercury resistance operon at extremely high Hg(II) loads in a chemostat: A proteome analysis. J. Biotechnol. 2007, 132, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zheng, X.; Zhang, D.; Iqbal, W.; Liu, C.; Yang, B.; Zhao, X.; Lu, X.; Mao, Y. Microbial characterization of heavy metal resistant bacterial strains isolated from an electroplating wastewater treatment plant. Ecotoxicol. Environ. Saf. 2019, 181, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Abou-Shanab, R.A.I.; van Berkum, P.; Angle, J.S. Heavy metal resistance and genotypic analysis of metal resistance genes in gram-positive and gram-negative bacteria present in Ni-rich serpentine soil and in the rhizosphere of Alyssum murale. Chemosphere 2007, 68, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, J.; Das, S. Characterization and cadmium-resistant gene expression of biofilm-forming marine bacterium Pseudomonas aeruginosa JP-11. Environ. Sci. Pollut. Res. 2014, 21, 14188–14201. [Google Scholar] [CrossRef]
- Smith, K.; Novick Richard, P. Genetic Studies on Plasmid-Linked Cadmium Resistance in Staphylococcus aureus. J. Bacteriol. 1972, 112, 761–772. [Google Scholar] [CrossRef]
- Chaouni, L.B.-A.; Etienne, J.; Greenland, T.; Vandenesch, F. Nucleic Acid Sequence and Affiliation of pLUG10, a Novel Cadmium Resistance Plasmid from Staphylococcus lugdunensis. Plasmid 1996, 36, 1–8. [Google Scholar] [CrossRef]
- Branco, R.; Chung Ana, P.; Johnston, T.; Gurel, V.; Morais, P.; Zhitkovich, A. The Chromate-Inducible chrBACF Operon from the Transposable Element TnOtChr Confers Resistance to Chromium(VI) and Superoxide. J. Bacteriol. 2008, 190, 6996–7003. [Google Scholar] [CrossRef]
- Henne, K.L.; Nakatsu, C.H.; Thompson, D.K.; Konopka, A.E. High-level chromate resistance in Arthrobactersp. strain FB24 requires previously uncharacterized accessory genes. BMC Microbiol. 2009, 9, 199. [Google Scholar] [CrossRef]
- Singh Satish, K.; Grass, G.; Rensing, C.; Montfort William, R. Cuprous Oxidase Activity of CueO from Escherichia coli. J. Bacteriol. 2004, 186, 7815–7817. [Google Scholar] [CrossRef]
- Hu, Y.-H.; Wang, H.-L.; Zhang, M.; Sun, L. Molecular analysis of the copper-responsive CopRSCD of a pathogenic Pseudomonas fluorescens strain. J. Microbiol. 2009, 47, 277–286. [Google Scholar] [CrossRef]
- Chillappagari, S.; Miethke, M.; Trip, H.; Kuipers Oscar, P.; Marahiel Mohamed, A. Copper Acquisition Is Mediated by YcnJ and Regulated by YcnK and CsoR in Bacillus subtilis. J. Bacteriol. 2009, 191, 2362–2370. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Taylor, D.E. Helicobacter pylori genes hpcopA and hpcopP constitute a cop operon involved in copper export. FEMS Microbiol. Lett. 1996, 145, 181–188. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taghavi, S.; Lesaulnier, C.; Monchy, S.; Wattiez, R.; Mergeay, M.; van der Lelie, D. Lead(II) resistance in Cupriavidus metallidurans CH34: Interplay between plasmid and chromosomally-located functions. Antonie Van Leeuwenhoek 2009, 96, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Naik, M.M.; Pandey, A.; Dubey, S.K. Pseudomonas aeruginosa strain WI-1 from Mandovi estuary possesses metallothionein to alleviate lead toxicity and promotes plant growth. Ecotoxicol. Environ. Saf. 2012, 79, 129–133. [Google Scholar] [CrossRef]
- Sasaki, Y.; Hayakawa, T.; Inoue, C.; Miyazaki, A.; Silver, S.; Kusano, T. Generation of Mercury-Hyperaccumulating Plants through Transgenic Expression of the Bacterial Mercury Membrane Transport Protein MerC. Transgenic Res. 2006, 15, 615–625. [Google Scholar] [CrossRef]
- Grass, G.; Große, C.; Nies Dietrich, H. Regulation of the cnr Cobalt and Nickel Resistance Determinant from Ralstonia sp. Strain CH34. J. Bacteriol. 2000, 182, 1390–1398. [Google Scholar] [CrossRef]
- Grass, G.; Fricke, B.; Nies, D.H. Control of Expression of a Periplasmic Nickel Efflux Pump by Periplasmic Nickel Concentrations. Biometals 2005, 18, 437–448. [Google Scholar] [CrossRef]
- Rodrigue, A.; Effantin, G.; Mandrand-Berthelot, M.-A. Identification of rcnA (yohM), a Nickel and Cobalt Resistance Gene in Escherichia coli. J. Bacteriol. 2005, 187, 2912–2916. [Google Scholar] [CrossRef]
- Duprey, A.; Chansavang, V.; Frémion, F.; Gonthier, C.; Louis, Y.; Lejeune, P.; Springer, F.; Desjardin, V.; Rodrigue, A.; Dorel, C. “NiCo Buster”: Engineering E. coli for fast and efficient capture of cobalt and nickel. J. Biol. Eng. 2014, 8, 19. [Google Scholar] [CrossRef]
- Ruiz, O.N.; Alvarez, D.; Gonzalez-Ruiz, G.; Torres, C. Characterization of mercury bioremediation by transgenic bacteria expressing metallothionein and polyphosphate kinase. BMC Biotechnol. 2011, 11, 82. [Google Scholar] [CrossRef]
- Zhang, S.; Qi, J.; Jiang, H.; Chen, X.; You, Z. Improving vanadium removal from contaminated river water in constructed wetlands: The role of arbuscular mycorrhizal fungi. Environ. Pollut. 2024, 347, 123804. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Kataki, S.; Chatterjee, S.; Gupta, D.K. Potential of nano-phytoremediation of heavy metal contaminated soil: Emphasizing the role of mycorrhizal fungi in the amelioration process. Int. J. Environ. Sci. Technol. 2024, 21, 6405–6428. [Google Scholar] [CrossRef]
- Hao, S.; Tian, Y.; Lin, Z.; Xie, L.; Zhou, X.; Bañuelos, G.S. Effects of arbuscular mycorrhizal fungi on the reduction of arsenic accumulation in plants: A meta-analysis. Front. Plant Sci. 2024, 15, 1327649. [Google Scholar] [CrossRef] [PubMed]
- Loha, A.; Kashyap, A.K.; Sharma, P. A putative cyclin, SiPHO80 from root endophytic fungus Serendipita indica regulates phosphate homeostasis, salinity and heavy metal toxicity tolerance. Biochem. Biophys. Res. Commun. 2018, 507, 414–419. [Google Scholar] [CrossRef]
- Khalid, M.; Ur-Rahman, S.; Hassani, D.; Hayat, K.; Zhou, P.; Hui, N. Advances in fungal-assisted phytoremediation of heavy metals: A review. Pedosphere 2021, 31, 475–495. [Google Scholar] [CrossRef]
- Hnini, M.; Rabeh, K.; Oubohssaine, M. Interactions between beneficial soil microorganisms (PGPR and AMF) and host plants for environmental restoration: A systematic review. Plant Stress 2024, 11, 100391. [Google Scholar] [CrossRef]
- Thijs, S.; Sillen, W.; Rineau, F.; Weyens, N.; Vangronsveld, J. Towards an Enhanced Understanding of Plant–Microbiome Interactions to Improve Phytoremediation: Engineering the Metaorganism. Front. Microbiol. 2016, 7, 341. [Google Scholar] [CrossRef]
- del Carmen Orozco-Mosqueda, M.; Fadiji, A.E.; Babalola, O.O.; Glick, B.R.; Santoyo, G. Rhizobiome engineering: Unveiling complex rhizosphere interactions to enhance plant growth and health. Microbiol. Res. 2022, 263, 127137. [Google Scholar] [CrossRef]
- Abbaszadeh-Dahaji, P.; Omidvari, M.; Ghorbanpour, M. Increasing Phytoremediation Efficiency of Heavy Metal-Contaminated Soil Using PGPR for Sustainable Agriculture. In Plant-Microbe Interaction: An Approach to Sustainable Agriculture; Choudhary, D.K., Varma, A., Tuteja, N., Eds.; Springer Singapore: Singapore, 2016; pp. 187–204. [Google Scholar]
- Al Mamun, A.; Rahman, M.M.; Huq, M.A.; Rahman, M.M.; Rana, M.R.; Rahman, S.T.; Khatun, M.L.; Alam, M.K. Phytoremediation: A transgenic perspective in omics era. Transgenic Res. 2024, 33, 175–194. [Google Scholar] [CrossRef]
- Timmusk, S.; Pall, T.; Raz, S.; Fetsiukh, A.; Nevo, E. The potential for plant growth-promoting bacteria to impact crop productivity in future agricultural systems is linked to understanding the principles of microbial ecology. Front. Microbiol. 2023, 14, 1141862. [Google Scholar] [CrossRef]
- Santoyo, G.; Guzmán-Guzmán, P.; Parra-Cota, F.I.; de los Santos-Villalobos, S.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant Growth Stimulation by Microbial Consortia. Agronomy 2021, 11, 219. [Google Scholar] [CrossRef]
- Hao, X.; Gu, Y.; Zhang, H.; Wang, X.; Liu, X.; Chen, C.; Wang, C.; Zhang, X.; Liu, X.; Shen, X. Synthetic Microbial Community Promotes Bacterial Communities Leading to Soil Multifunctionality in Desertified Land. Microorganisms 2024, 12, 1117. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).