Applications of Bacillus subtilis Protein Display for Medicine, Catalysis, Environmental Remediation, and Protein Engineering
Abstract
1. Introduction
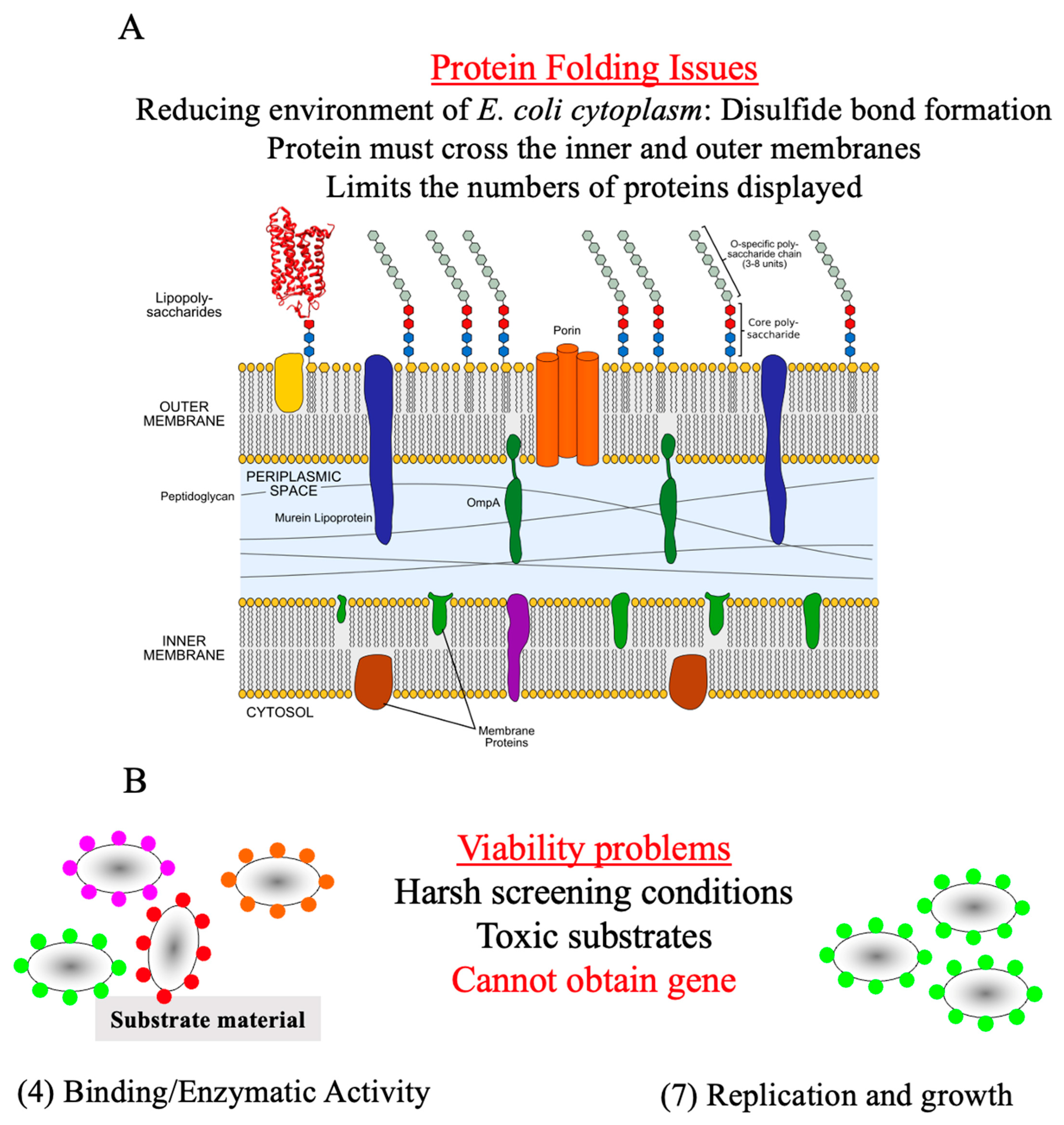
1.1. Protein Display
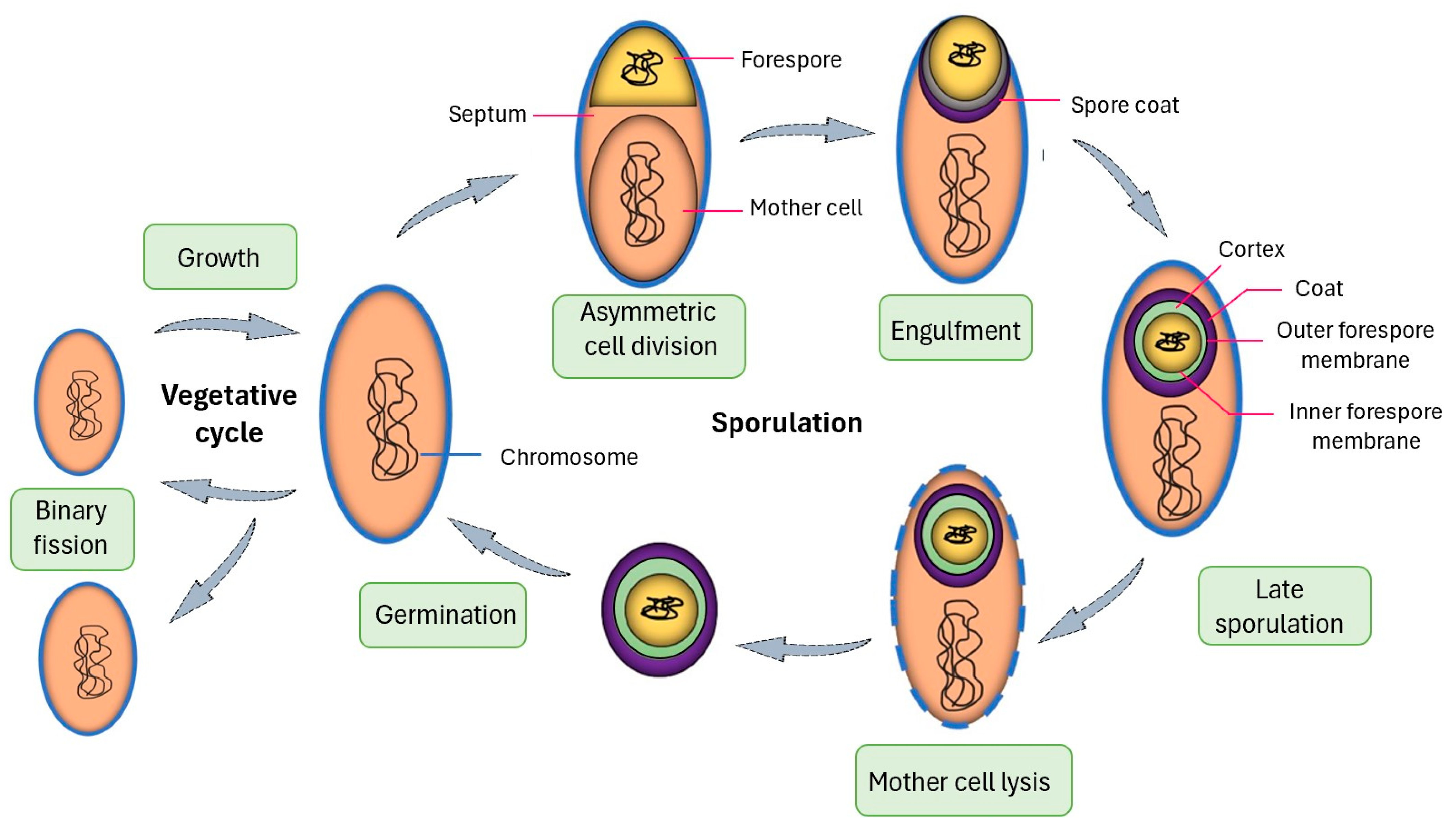
1.2. Advantages of Spore Display
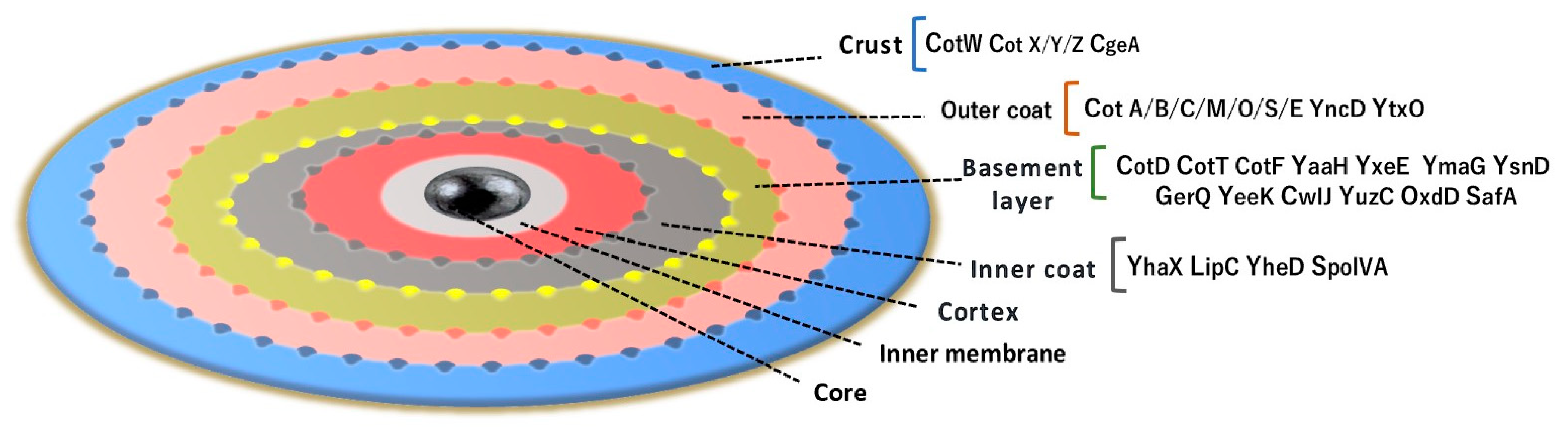
1.3. B. subtilis Anchor Proteins for Protein Display
2. Applications
2.1. Vaccine Development/Drug Delivery
2.2. Biocatalysis
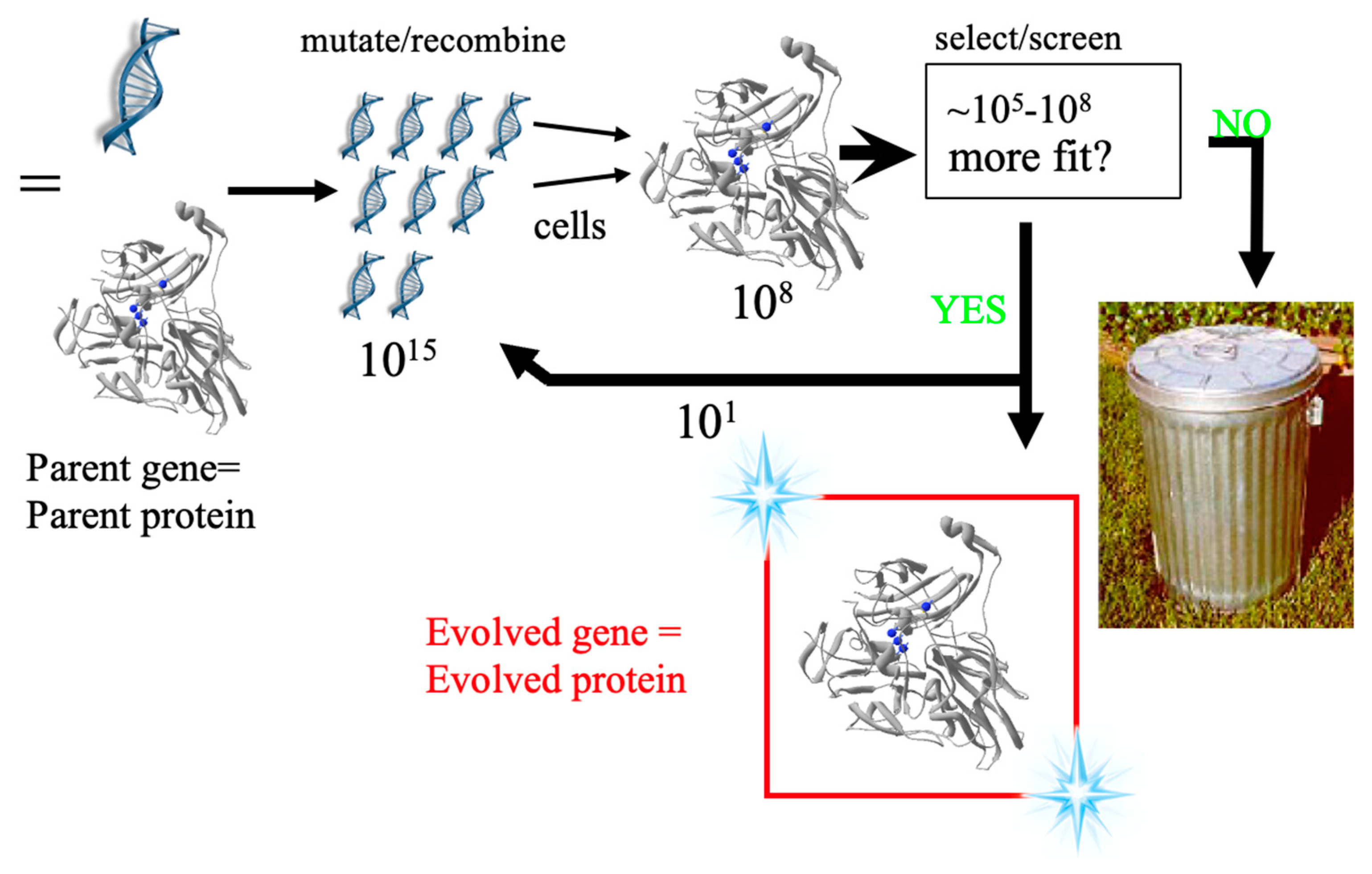
2.3. Protein Engineering and Optimization
2.4. Environmental Applications
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arnold, F.H. Advances in Protein Chemistry. Evolutionary Protein Design; Academic Press: New York, NY, USA, 2001; Volume 55, p. 438. [Google Scholar]
- Amstutz, P.; Forrer, P.; Zahnd, C.; Pluckthun, A. In vitro display technologies: Novel developments and applications. Curr. Opin. Biotechnol. 2001, 12, 400–405. [Google Scholar] [CrossRef]
- Forrer, P.; Jung, S.; Pluckthun, A. Beyond binding: Using phage display to select for structure, folding and enzymatic activity in proteins. Curr. Opin. Struct. Biol. 1999, 9, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Schmoldt, H.U.; Adams, T.M.; Wilhelm, S.; Kolmar, H. Ultra-high-throughput screening based on cell-surface display and fluorescence-activated cell sorting for the identification of novel biocatalysts. Curr. Opin. Biotechnol. 2004, 15, 323–329. [Google Scholar] [CrossRef]
- Lee, S.Y.; Choi, J.H.; Xu, Z. Microbial cell-surface display. Trends Biotechnol. 2003, 21, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Wittrup, K.D. Protein engineering by cell-surface display. Curr. Opin. Biotechnol. 2001, 12, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Bessette, P.H.; Aslund, F.; Beckwith, J.; Georgiou, G. Efficient folding of proteins with multiple disulfide bonds in the Escherichia coli cytoplasm. Proc. Natl. Acad. Sci. USA 1999, 96, 13703–13708. [Google Scholar] [CrossRef]
- Driks, A. Bacillus subtilis spore coat. Microbiol. Mol. Biol. Rev. 1999, 63, 1–20. [Google Scholar] [CrossRef]
- Stragier, P.; Losick, R. Molecular genetics of sporulation in Bacillus subtilis. Annu. Rev. Genet. 1996, 30, 297-41. [Google Scholar] [CrossRef]
- Nicholson, W.L.; Munakata, N.; Horneck, G.; Melosh, H.J.; Setlow, P. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 2000, 64, 548–572. [Google Scholar] [CrossRef]
- Kennedy, M.J.; Reader, S.L.; Swierczynski, L.M. Preservation records of micro-organisms: Evidence of the tenacity of life. Microbiology (Reading) 1994, 140 Pt 10, 2513–2529. [Google Scholar] [CrossRef]
- Fessner, W.-D. (Ed.) Biocatalysis: From Discovery to Applications; Topics in Current Chemistry; Springer-Verlag: Berlin/Heidelberg, Germany, 2000; Volume 200, p. 254. [Google Scholar]
- Isticato, R.; Cangiano, G.; Tran, H.T.; Ciabattini, A.; Medaglini, D.; Oggioni, M.R.; De Felice, M.; Pozzi, G.; Ricca, E. Surface display of recombinant proteins on Bacillus subtilis spores. J. Bacteriol. 2001, 183, 6294–6301. [Google Scholar] [CrossRef] [PubMed]
- Isticato, R. Bacterial Spore-Based Delivery System: 20 Years of a Versatile Approach for Innovative Vaccines. Biomolecules 2023, 13, 947. [Google Scholar] [CrossRef] [PubMed]
- Duc, L.H.; Hong, H.A.; Fairweather, N.; Ricca, E.; Cutting, S.M. Bacterial spores as vaccine vehicles. Infect. Immun. 2003, 71, 2810–2818. [Google Scholar] [CrossRef] [PubMed]
- Vetráková, A.; Chovanová, R.K.; Rechtoríková, R.; Krajčíková, D.; Barák, I. Bacillus subtilis spores displaying RBD domain of SARS-CoV-2 spike protein. Comput. Struct. Biotechnol. J. 2023, 21, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.C.L.; Li, P.; Tsang, M.S.M.; Sung, J.C.C.; Kwong, K.W.Y.; Zheng, T.; Hon, S.S.M.; Lau, C.P.; Cheng, W.; Chen, F.; et al. Creating a Vaccine-like Supplement against Respiratory Infection Using Recombinant Bacillus subtilis Spores Expressing SARS-CoV-2 Spike Protein with Natural Products. Molecules 2023, 28, 4996. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Guluarte, J.O.; Abbott, D.W.; Inglis, G.D.; Guan, L.L.; Alexander, T.W. Development of a spore-based mucosal vaccine against the bovine respiratory pathogen Mannheimia haemolytica. Sci. Rep. 2023, 13, 12981. [Google Scholar] [CrossRef]
- Yin, L.; Meng, Z.; Zhang, Y.; Hu, K.; Chen, W.; Han, K.; Wu, B.Y.; You, R.; Li, C.H.; Jin, Y.; et al. Bacillus spore-based oral carriers loading curcumin for the therapy of colon cancer. J. Control. Release 2018, 271, 31–44. [Google Scholar] [CrossRef]
- Wang, H.; Yang, R.; Hua, X.; Zhao, W.; Zhang, W. Functional display of active β-galactosidase on Bacillus subtilis spores using crust proteins as carriers. Food Sci. Biotechnol. 2015, 24, 1755–1759. [Google Scholar] [CrossRef]
- Seok, J.K.; Jung, H.C.; Pan, J.G. Transgalactosylation in a water-solvent biphasic reaction system with β-galactosidase displayed on the surfaces of Bacillus subtilis spores. Appl. Environ. Microbiol. 2007, 73, 2251–2256. [Google Scholar]
- Tavassoli, S.; Hinc, K.; Iwanicki, A.; Obuchowski, M.; Ahmadian, G. Investigation of spore coat display of Bacillus subtilis β-galactosidase for developing of whole cell biocatalyst. Arch. Microbiol. 2013, 195, 197–202. [Google Scholar] [CrossRef]
- He, W.; Jiang, B.; Mu, W.; Zhang, T. Production of d-Allulose with d-Psicose 3-Epimerase Expressed and Displayed on the Surface of Bacillus subtilis Spores. J. Agric. Food Chem. 2016, 64, 7201–7207. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tian, R.; Ni, Z.; Zhang, Q.; Zhang, T.; Chen, Z.; Chen, K.; Yang, S. Surface display of the thermophilic lipase Tm1350 on the spore of Bacillus subtilis by the CotB anchor protein. Extremophiles 2015, 19, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, T.; Jia, J.; Vastermark, A.; Tian, R.; Ni, Z.; Chen, Z.; Chen, K.; Yang, S. Expression and display of a novel thermostable esterase from Clostridium thermocellum on the surface of Bacillus subtilis using the CotB anchor protein. J. Ind. Microbiol. Biotechnol. 2015, 42, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Feng, F.; Chen, L.; Yao, Q.; Chen, K. Surface display of Acetobacter pasteurianus AdhA on Bacillus subtilis spores to enhance ethanol tolerance for liquor industrial potential. Eur. Food Res. Technol. 2014, 238, 285–293. [Google Scholar] [CrossRef]
- Gao, C.; Xu, X.; Zhang, X.; Che, B.; Ma, C.; Qiu, J.; Tao, F.; Xu, P. Chemoenzymatic synthesis of N-acetyl-D-neuraminic acid from N-acetyl-D-glucosamine by using the spore surface-displayed N-acetyl-D-neuraminic acid aldolase. Appl. Environ. Microbiol. 2011, 77, 7080–7083. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, Z.; Ni, Z.; Tian, R.; Zhang, T.; Jia, J.; Chen, K.; Yang, S. Display of Thermotoga maritima MSB8 nitrilase on the spore surface of Bacillus subtilis using out coat protein CotG as the fusion partner. J. Mol. Catal. B Enzym. 2016, 123, 73–80. [Google Scholar] [CrossRef]
- Gupta, N.; Lee, F.S.; Farinas, E.T. Laboratory evolution of laccase for substrate specificity. J. Mol. Catal. B Enzym. 2010, 62, 230–234. [Google Scholar] [CrossRef]
- Gupta, N.; Farinas, E.T. Directed evolution of CotA laccase for increased substrate specificity using Bacillus subtilis spores. Protein Eng. Des. Sel. 2010, 23, 679–682. [Google Scholar] [CrossRef]
- Gupta, N.; Farinas, E.T. Narrowing laccase substrate specificity using active site saturation mutagenesis. Comb. Chem. High Throughput Screen. 2009, 12, 269–274. [Google Scholar] [CrossRef]
- Jia, H.; Lee, F.S.; Farinas, E.T. Bacillus subtilis spore display of laccase for evolution under extreme conditions of high concentrations of organic solvent. ACS Combi. Sci. 2014, 16, 665–669. [Google Scholar] [CrossRef]
- Sheng, S.; Jia, H.; Topiol, S.; Farinas, E.T. Engineering CotA Laccase for Acidic pH Stability Using Bacillus subtilis Spore Display. J. Microbiol. Biotechnol. 2017, 27, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Sheng, S.; Farinas, E.T. Laccase and Its Mutant Displayed on the Bacillus subtilis Spore Coat for Oxidation of Phenolic Compounds in Organic Solvents. Catalysts 2021, 11, 606. [Google Scholar] [CrossRef]
- Hosseini-Abari, A.; Kim, B.G.; Lee, S.H.; Emtiazi, G.; Kim, W.; Kim, J.H. Surface display of bacterial tyrosinase on spores of Bacillus subtilis using CotE as an anchor protein. J. Basic. Microbiol. 2016, 56, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Song, T.; Jiang, H.; Pei, C.; Huang, Q.; Xi, H. Bacillus subtilis Spore Surface Display of Haloalkane Dehalogenase DhaA. Curr. Microbiol. 2019, 76, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Wang, J.; Zhang, Z.; Shi, S.; Li, D.; Shen, W.; Shen, E.; Zhou, J. Catalytic transformation of HODAs using an efficient meta-cleavage product hydrolase-spore surface display system. J. Mol. Catal. B Enzym. 2014, 102, 204–210. [Google Scholar] [CrossRef]
- Hinc, K.; Ghandili, S.; Karbalaee, G.; Shali, A.; Noghabi, K.A.; Ricca, E.; Ahmadian, G. Efficient binding of nickel ions to recombinant Bacillus subtilis spores. Res. Microbiol. 2010, 161, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Li, S.; Camilleri, E.; Korza, G.; Yankova, M.; King, S.M.; Setlow, P. Accumulation and release of rare earth ions by spores of Bacillus species and the location of these ions in spores. Appl. Environ. Microbiol. 2019, 85, e00956-19. [Google Scholar] [CrossRef]
- Valenzuela-García, L.I.; Alarcón-Herrera, M.T.; Ayala-García, V.M.; Barraza-Salas, M.; Salas-Pacheco, J.M.; Díaz-Valles, J.F.; Pedraza-Reyes, M. Design of a Whole-Cell Biosensor Based on Bacillus subtilis Spores and the Green Fluorescent Protein to Monitor Arsenic. Microbiol. Spectr. 2023, 11, e00432-23. [Google Scholar] [CrossRef]
- McKenney, P.T.; Driks, A.; Eichenberger, P. The Bacillus subtilis endospore: Assembly and functions of the multilayered coat. Nat. Rev. Microbiol. 2013, 11, 33–44. [Google Scholar] [CrossRef]
- Kunst, F.; Ogasawara, N.; Moszer, I.; Albertini, A.M.; Alloni, G.; Azevedo, V.; Bertero, M.G.; Bessieres, P.; Bolotin, A.; Borchert, S.; et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 1997, 390, 249–256. [Google Scholar] [CrossRef]
- Moszer, I. The complete genome of Bacillus subtilis: From sequence annotation to data management and analysis. FEBS Lett. 1998, 430, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Cutting, S.M. Bacillus probiotics. Food Microbiol. 2011, 28, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.A.; Huang, J.M.; Khaneja, R.; Hiep, L.V.; Urdaci, M.C.; Cutting, S.M. The safety of Bacillus subtilis and Bacillus indicus as food probiotics. J. Appl. Microbiol. 2008, 105, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wan, Q.; Krajcikova, D.; Tang, J.; Tzokov, S.B.; Barak, I.; Bullough, P.A. Diverse supramolecular structures formed by self-assembling proteins of the Bacillus subtilis spore coat. Mol. Microbiol. 2015, 97, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Bartels, J.; Lopez Castellanos, S.; Radeck, J.; Mascher, T. Sporobeads: The Utilization of the Bacillus subtilis Endospore Crust as a Protein Display Platform. ACS Synth. Biol. 2018, 7, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Zilhao, R.; Serrano, M.; Isticato, R.; Ricca, E.; Moran, C.P., Jr.; Henriques, A.O. Interactions among CotB, CotG, and CotH during assembly of the Bacillus subtilis spore coat. J. Bacteriol. 2004, 186, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Isticato, R.; Esposito, G.; Zilhao, R.; Nolasco, S.; Cangiano, G.; De Felice, M.; Henriques, A.O.; Ricca, E. Assembly of multiple CotC forms into the Bacillus subtilis spore coat. J. Bacteriol. 2004, 186, 1129–1135. [Google Scholar] [CrossRef]
- Donovan, W.; Zheng, L.; Sandman, K.; Losick, R. Genes encoding spore coat polypeptides from Bacillus subtilis. J. Mol. Biol. 1987, 196, 1–10. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, C.S.; Kim, B.G. Spore-displayed streptavidin: A live diagnostic tool in biotechnology. Biochem. Biophys. Res. Commun. 2005, 331, 210–214. [Google Scholar] [CrossRef]
- Sacco, M.; Ricca, E.; Losick, R.; Cutting, S. An additional GerE-controlled gene encoding an abundant spore coat protein from Bacillus subtilis. J. Bacteriol. 1995, 177, 372–377. [Google Scholar] [CrossRef]
- Kim, J.; Schumann, W. Display of proteins on Bacillus subtilis endospores. Cell. Mol. Life Sci. 2009, 66, 3127–3136. [Google Scholar] [CrossRef] [PubMed]
- Ciabattini, A.; Parigi, R.; Isticato, R.; Oggioni, M.R.; Pozzi, G. Oral priming of mice by recombinant spores of Bacillus subtilis. Vaccine 2004, 22, 4139–4143. [Google Scholar] [CrossRef] [PubMed]
- Mauriello, E.M.; Duc, L.H.; Isticato, R.; Cangiano, G.; Hong, H.A.; De Felice, M.; Ricca, E.; Cutting, S.M. Display of heterologous antigens on the Bacillus subtilis spore coat using CotC as a fusion partner. Vaccine 2004, 22, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.D.; Fulton, R.W.; Lehenbauer, T.W.; Step, D.L.; Confer, A.W. The epidemiology of bovine respiratory disease: What is the evidence for predisposing factors? Can. Vet. J. 2010, 51, 1095–1102. [Google Scholar] [PubMed]
- Li, W.; Li, J.; Dai, X.; Liu, M.; Khalique, A.; Wang, Z.; Zeng, Y.; Zhang, D.; Ni, X.; Zeng, D.; et al. Surface Display of porcine circovirus type 2 antigen protein cap on the spores of Bacillus subtilis 168: An effective mucosal vaccine candidate. Front. Immunol. 2022, 13, 1007202. [Google Scholar] [CrossRef]
- Lê, K.A.; Robin, F.; Roger, O. Sugar replacers: From technological challenges to consequences on health. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 310–315. [Google Scholar] [CrossRef]
- Hossain, A.; Yamaguchi, F.; Matsuo, T.; Tsukamoto, I.; Toyoda, Y.; Ogawa, M.; Nagata, Y.; Tokuda, M. Rare sugar d-allulose: Potential role and therapeutic monitoring in maintaining obesity and type 2 diabetes mellitus. Pharmacol. Ther. 2015, 155, 49–59. [Google Scholar] [CrossRef]
- Gaonkar, S.K.; Alvares, J.J.; Furtado, I.J. Recent advances in the production, properties and applications of haloextremozymes protease and lipase from haloarchaea. World J. Microbiol. Biotechnol. 2023, 39, 322. [Google Scholar] [CrossRef]
- Peng, Y.; Su, A.; Huang, W.; Lan, S.; Yang, T.; Tan, Q. Research progress on microbial thermophilic lipase. Food. Ferment. Ind. 2021, 47, 289–294. [Google Scholar]
- Maldonado, R.R.; Lopes, D.B.; Aguiar-Oliveira, E.; Kamimura, E.S.; Macedo, G.A. A review on Geotrichum lipases: Production, purification, immobilization and applications. Chem. Biochem. Eng. Q. 2016, 30, 439–454. [Google Scholar] [CrossRef]
- Brígida, A.I.S.; Amaral, P.F.F.; Coelho, M.A.Z.; Gonçalves, L.R.B. Lipase from Yarrowia lipolytica: Production, characterization and application as an industrial biocatalyst. J. Mol. Catal. B Enzym. 2014, 101, 148–158. [Google Scholar] [CrossRef]
- Pace, H.C.; Brenner, C. The nitrilase superfamily: Classification, structure and function. Genome Biol. 2001, 2, reviews0001.1–reviews0001.9. [Google Scholar] [CrossRef] [PubMed]
- Solomon, E.I.; Sundaram, U.M.; Machonkin, T.E. Multicopper oxidases and oxygenases. Chem. Rev. 1996, 96, 2563–2605. [Google Scholar] [CrossRef] [PubMed]
- Mayolo-Deloisa, K.; González-González, M.; Rito-Palomares, M. Laccases in Food Industry: Bioprocessing, Potential Industrial and Biotechnological Applications. Front. Bioeng. Biotechnol. 2020, 8, 00222. [Google Scholar] [CrossRef] [PubMed]
- Khatami, S.H.; Vakili, O.; Movahedpour, A.; Ghesmati, Z.; Ghasemi, H.; Taheri-Anganeh, M. Laccase: Various types and applications. Biotechnol. Appl. Biochem. 2022, 69, 2658–2672. [Google Scholar] [CrossRef]
- Hamid, R.; Khan, M.A.; Ahmad, M.; Ahmad, M.M.; Abdin, M.Z.; Musarrat, J.; Javed, S. Chitinases: An update. J. Pharm. Bioallied Sci. 2013, 5, 21–29. [Google Scholar]
- Rostami, A.; Hinc, K.; Goshadrou, F.; Shali, A.; Bayat, M.; Hassanzadeh, M.; Amanlou, M.; Eslahi, N.; Ahmadian, G. Display of B. pumilus chitinase on the surface of B. subtilis spore as a potential biopesticide. Pestic. Biochem. Physiol. 2017, 140, 17–23. [Google Scholar] [CrossRef]
- Hsieh, H.Y.; Lin, C.H.; Hsu, S.Y.; Stewart, G.C. A Bacillus spore-based display system for bioremediation of atrazine. Appl. Environ. Microbiol. 2020, 86, e01230-20. [Google Scholar] [CrossRef]
- Qu, Y.; Shi, S.; Ma, Q.; Kong, C.; Zhou, H.; Zhang, X.; Zhou, J. Multistep conversion of para-Substituted Phenols by Phenol Hydroxylase and 2,3-Dihydroxybiphenyl 1,2-Dioxygenase. Appl. Biochem. Biotechnol. 2013, 169, 2064–2075. [Google Scholar] [CrossRef]
- Seah, S.Y.K.; Labbé, G.; Nerdinger, S.; Johnson, M.R.; Snieckus, V.; Eltis, L.D. Identification of a serine hydrolase as a key determinant in the microbial degradation of polychlorinated biphenyls. J. Biol. Chem. 2000, 275, 15701–15708. [Google Scholar] [CrossRef][Green Version]
- Haber, L.T.; Bates, H.K.; Allen, B.C.; Vincent, M.J.; Oller, A.R. Derivation of an oral toxicity reference value for nickel. Regul. Toxicol. Pharmacol. 2017, 87, S1–S18. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Udensi, U.K.; Pacurari, M.; Stevens, J.J.; Patlolla, A.K.; Noubissi, F.; Kumar, S. State of the science review of the health effects of inorganic arsenic: Perspectives for future research. Environ. Toxicol. 2019, 34, 188–202. [Google Scholar] [CrossRef] [PubMed]

| Application | Anchor | Fusion | Function | References |
|---|---|---|---|---|
| Vaccine; adjuvant; drug delivery | CotB | C-terminal tetanus fragment (TTFC) | Intranasal dosage produced a mucosal (IgA) and systemic (IgG) reaction in murine mod | [13,14,15] |
| CotZ, CotY | SARS-CoV-2 Receptor binding domain | Oral vaccine against SARS-CoV-2 virus | [16,17] | |
| Spore | Neutralizing epitopes from Mannheimia haemolytica | Adjuvant for bovine respiratory disease | [18] | |
| Spore | Covalent linkage of curcumin and folate | Drug delivery for colon cancer | [19] | |
| Biocatalysis | CotG | β-galactosidase | Transgalactosylation in biphasic reaction mixtures | [20,21] |
| CotC, CotY, CotZ | β-galactosidase | β-gal reaction; evaluate as potential enzymatic anchors | [22] | |
| CotZ | D-psicose 3-epimerase | Allulose synthesis | [23] | |
| CotB | Lipase | Demonstrates advantage of enzyme displayed enzyme comparted to non-immobilized biocatalysis | [24] | |
| CotB | Esterase | Demonstrates advantage of enzyme displayed enzyme comparted to non-immobilized biocatalysis | [25] | |
| CotC | Alcohol dehydrogenase | Ethanol tolerance for flavor production in liquor | [26] | |
| CotG | N-acetyl-D-neuraminic acid aldolase | Synthesis of N-acetyl-D-neuraminic from N-acetyl-D-glucosamine | [27] | |
| CotG | Nitrilase | Hydrolysis of nitriles to ammonia and carboxylic acid | [28] | |
| CotC | Genomic substitution of wild-type CotC (laccase) | [29,30,31,32,33,34] | ||
| Environmental applications | CotE | Tyrosinase | Phenol polluted environments | [35] |
| CotG | Haloalkane dehalogenase | Degradation of sulfur mustard | [36] | |
| CotG | Meta-cleavage product (MCP) hydrolase (MfphA and BphD) | 2-hydroxy-6-oxohexa-2,4-dienoic acids transformation | [37] | |
| CotB | His18 | Nickel binding | [38] | |
| Spore | Not applicable | Rare earth element binding | [39] | |
| Spore | Not applicable | Arsenic sensor | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoodi, A.; Farinas, E.T. Applications of Bacillus subtilis Protein Display for Medicine, Catalysis, Environmental Remediation, and Protein Engineering. Microorganisms 2024, 12, 97. https://doi.org/10.3390/microorganisms12010097
Mahmoodi A, Farinas ET. Applications of Bacillus subtilis Protein Display for Medicine, Catalysis, Environmental Remediation, and Protein Engineering. Microorganisms. 2024; 12(1):97. https://doi.org/10.3390/microorganisms12010097
Chicago/Turabian StyleMahmoodi, Asieh, and Edgardo T. Farinas. 2024. "Applications of Bacillus subtilis Protein Display for Medicine, Catalysis, Environmental Remediation, and Protein Engineering" Microorganisms 12, no. 1: 97. https://doi.org/10.3390/microorganisms12010097
APA StyleMahmoodi, A., & Farinas, E. T. (2024). Applications of Bacillus subtilis Protein Display for Medicine, Catalysis, Environmental Remediation, and Protein Engineering. Microorganisms, 12(1), 97. https://doi.org/10.3390/microorganisms12010097






