Microbiologic Findings in a Cohort of Patients with Erythema Migrans
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Definitions
2.3. Microbiological Analyses
2.3.1. Serological Evaluation
2.3.2. Borrelia Cultivation and Typing
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolcott, K.A.; Margos, G.; Fingerle, V.; Becker, N.S. Host association of Borrelia burgdorferi sensu lato: A review. Ticks Tick-Borne Dis. 2021, 12, 101766. [Google Scholar] [CrossRef] [PubMed]
- Radolf, J.D.; Strle, K.; Lemieux, J.E.; Strle, F. Lyme Disease in Humans. Curr. Issues Mol. Biol. 2021, 42, 333–384. [Google Scholar] [CrossRef]
- Stanek, G.; Strle, F. Lyme borreliosis-from tick bite to diagnosis and treatment. FEMS Microbiol. Rev. 2018, 42, 233–258. [Google Scholar] [CrossRef]
- van den Wijngaard, C.C.; Hofhuis, A.; Simoes, M.; Rood, E.; van Pelt, W.; Zeller, H.; Van Bortel, W. Surveillance perspective on Lyme borreliosis across the European Union and European Economic Area. Eurosurveillance 2017, 22, 30569. [Google Scholar] [CrossRef] [PubMed]
- Stanek, G.; Fingerle, V.; Hunfeld, K.P.; Jaulhac, B.; Kaiser, R.; Krause, A.; Kristoferitsch, W.; O’Connell, S.; Ornstein, K.; Strle, F.; et al. Lyme borreliosis: Clinical case definitions for diagnosis and management in Europe. Clin. Microbiol. Infect. 2011, 17, 69–79. [Google Scholar] [CrossRef]
- Cerar, T.; Ruzic-Sabljic, E.; Cimperman, J.; Strle, F. Comparison of immunofluorescence assay (IFA) and LIAISON in patients with different clinical manifestations of Lyme borreliosis. Wien. Klin. Wochenschr. 2006, 118, 686–690. [Google Scholar] [CrossRef]
- Maraspin, V.; Bogovic, P.; Rojko, T.; Ogrinc, K.; Ruzic-Sabljic, E.; Strle, F. Early Lyme Borreliosis in Patients Treated with Tumour Necrosis Factor-Alfa Inhibitors. J. Clin. Med. 2019, 8, 1857. [Google Scholar] [CrossRef]
- Busch, U.; Teufel, C.H.; Boehmer, R.; Wilske, B.; Preac-Mursic, V. Molecular characterization of Borrelia burgdorferi sensu lato strains by pulsed-field gel electrophoresis. Electrophoresis 1995, 16, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Ruzic-Sabljic, E.; Zore, A.; Strle, F. Characterization of Borrelia burgdorferi sensu lato isolates by pulsed-field gel electrophoresis after MluI restriction of genomic DNA. Res. Microbiol. 2008, 159, 441–448. [Google Scholar] [CrossRef]
- Leeflang, M.M.; Ang, C.W.; Berkhout, J.; Bijlmer, H.A.; Van Bortel, W.; Brandenburg, A.H.; Van Burgel, N.D.; Van Dam, A.P.; Dessau, R.B.; Fingerle, V.; et al. The diagnostic accuracy of serological tests for Lyme borreliosis in Europe: A systematic review and meta-analysis. BMC Infect. Dis. 2016, 16, 140. [Google Scholar] [CrossRef]
- Guerin, M.; Shawky, M.; Zedan, A.; Octave, S.; Avalle, B.; Maffucci, I.; Padiolleau-Lefevre, S. Lyme borreliosis diagnosis: State of the art of improvements and innovations. BMC Microbiol. 2023, 23, 204. [Google Scholar] [CrossRef] [PubMed]
- Aguero-Rosenfeld, M.E.; Wang, G.; Schwartz, I.; Wormser, G.P. Diagnosis of lyme borreliosis. Clin. Microbiol. Rev. 2005, 18, 484–509. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, M.; Traweger, A.; Lusa, L.; Stupica, D.; Maraspin, V.; Barrett, P.N.; Strle, F.; Livey, I. Quantitative detection of Borrelia burgdorferi sensu lato in erythema migrans skin lesions using internally controlled duplex real time PCR. PLoS ONE 2013, 8, e63968. [Google Scholar] [CrossRef] [PubMed]
- Wormser, G.P.; Bittker, S.; Cooper, D.; Nowakowski, J.; Nadelman, R.B.; Pavia, C. Yield of large-volume blood cultures in patients with early Lyme disease. J. Infect. Dis. 2001, 184, 1070–1072. [Google Scholar] [CrossRef]
- Stupica, D.; Lusa, L.; Maraspin, V.; Bogovic, P.; Vidmar, D.; O’Rourke, M.; Traweger, A.; Livey, I.; Strle, F. Correlation of Culture Positivity, PCR Positivity, and Burden of Borrelia burgdorferi Sensu Lato in Skin Samples of Erythema Migrans Patients with Clinical Findings. PLoS ONE 2015, 10, e0136600. [Google Scholar] [CrossRef]
- Strle, F.; Lusa, L.; Ruzic-Sabljic, E.; Maraspin, V.; Lotric Furlan, S.; Cimperman, J.; Ogrinc, K.; Rojko, T.; Videcnik Zorman, J.; Stupica, D. Clinical characteristics associated with Borrelia burgdorferi sensu lato skin culture results in patients with erythema migrans. PLoS ONE 2013, 8, e82132. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, P.; Schroder, M.T.; Niiranen, K.; Nevanlinna, A.; Panelius, J.; Ranki, A. The many faces of solitary and multiple erythema migrans. Acta Derm. Venereol. 2013, 93, 693–700. [Google Scholar] [CrossRef]
- Egberts, F.; Moller, M.; Proksch, E.; Schwarz, T. Multiple erythema migrans—Manifestation of systemic cutaneous borreliosis. J. Dtsch. Dermatol. Ges. 2008, 6, 350–353. [Google Scholar] [CrossRef]
- Glatz, M.; Resinger, A.; Semmelweis, K.; Ambros-Rudolph, C.M.; Mullegger, R.R. Clinical spectrum of skin manifestations of Lyme borreliosis in 204 children in Austria. Acta Derm. Venereol. 2015, 95, 565–571. [Google Scholar] [CrossRef]
- Ruzic-Sabljic, E.; Strle, F. Comparison of growth of Borrelia afzelii, B. garinii, and B. burgdorferi sensu stricto in MKP and BSK-II medium. Int. J. Med. Microbiol. 2004, 294, 407–412. [Google Scholar] [CrossRef]
- Maraspin, V.; Ogrinc, K.; Rojko, T.; Bogovic, P.; Ruzic-Sabljic, E.; Kastrin, A.; Wormser, G.P.; Strle, F. Characteristics of spirochetemic patients with a solitary erythema migrans skin lesion in Europe. PLoS ONE 2021, 16, e0250198. [Google Scholar] [CrossRef] [PubMed]
- Ogrinc, K.; Kastrin, A.; Lotric-Furlan, S.; Bogovic, P.; Rojko, T.; Maraspin, V.; Ruzic-Sabljic, E.; Strle, K.; Strle, F. Colocalization of radicular pain and erythema migrans in patients with Bannwarth’s syndrome suggests a direct spread of borrelia into the central nervous system. Clin. Infect. Dis. 2021, 75, 81–87. [Google Scholar] [CrossRef] [PubMed]
| Female Sex | 141 (48.3) |
| Age (years) | 51 (40–60) |
| Underlying chronic illness | 108 (37.0) |
| Previous LB | 57 (19.5) |
| Tick bite * | 144 (49.3) |
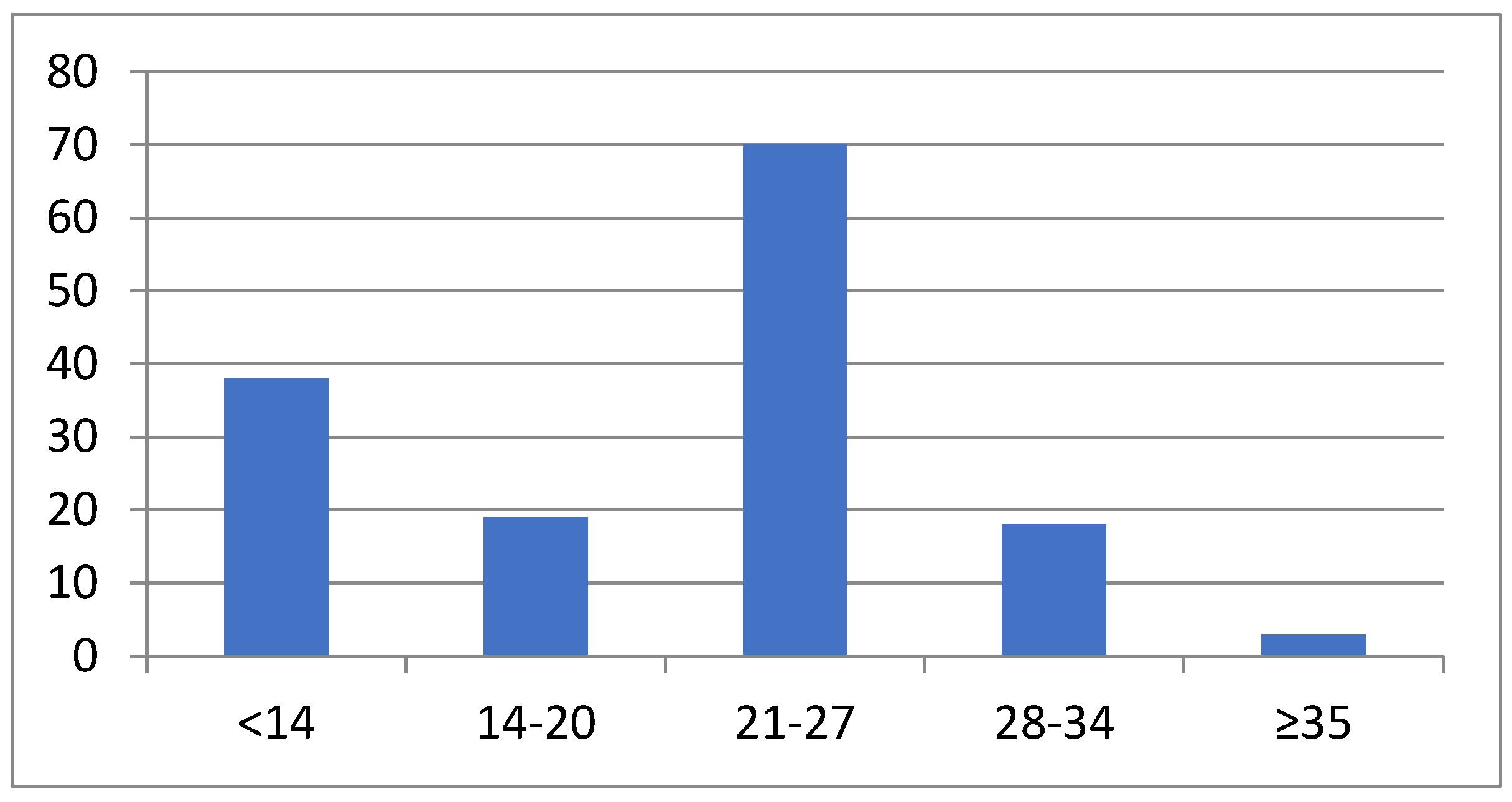
| Duration of EM at diagnosis | 12 (4–29) |
| Diameter of EM at diagnosis | 16 (10–24) |
| Multiple EM | 40 (13.7) |
| Symptoms at the site of EM | 138 (47.3) |
| Constitutional symptoms | 99 (33.9) |
| Borrelia Antibodies in Serum | |
| IgM-positive | 95 (32.5) |
| borderline | 23 (7.9) |
| negative | 174 (59.6) |
| IgM levels: all patients | 13.2 (7.3–32.4) |
| seropositive patients | 54.3 (32.4–156.0) |
| IgG-positive | 169 (57.9) |
| borderline | 25 (8.6) |
| negative | 98 (33.6) |
| IgG levels: all patients * | 23.3 (7.4–75.0) |
| seropositive patients ** | 56.3 (28.0–147.6) |
| IgM- and/or IgG-positive | 195 (66.8) |
| Isolation of Borrelia burgdorferi sensu lato from skin | |
| Isolation | 182 (62.3%) |
| Time from skin biopsy to positive culture (days) | 24 (13–26) range 11–58 |
| Borrelia species *** | |
| B. afzelii | 142 (95.9) |
| B. garinii | 4 (2.7) |
| B. burgdorferi s.s. | 2 (1.4) |
| IgM- and/or IgG- and/or Borrelia culture-positive | 261 (89.4) |
| Parameter | All Patients | IgM-pos | IgM-neg * | p | IgG-pos | IgG-neg * | p | Skin Culture-pos | Skin Culture-neg | p |
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 51 (40–60) | 44 (34–57) | 53 (42–62) | <0.001 | 52 (40–61) | 50 (38–59) | 0.184 | 52 (40–61) | 50 (38–60) | 0.408 |
| Duration of EM at diagnosis | 12 (4–29) | 11 (5–28) | 12 (4–30) | 0.900 | 14 (6–30) | 9 (4–21) | 0.001 | 9 (4–23) | 14 (7–31) | 0.095 |
| Diameter of EM at diagnosis | 16 (10–24) | 18 (10–26) | 15 (10–23) | 0.606 | 19 (13–26) | 12 (8–18) | 0.003 | 15 (10–24) | 17 (12–24) | 0.853 |
| Number of Patients No = 292 | IgM-pos N=95 | IgM-neg * N=187 | p Value | IgG-pos N=169 | IgG-neg * N=123 | p Value | Isolation-pos N=182 | Isolation-neg No=110 | p Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Female sex | 141 (48.3%) | 45 (47.4%) | 96 (51.3%) | 0.6142 | 73 (51.8%) | 68 (55.3%) | 0.0545 | 85 (46.7%) | 56 (50.9%) | 0.5646 |
| Underlying illness | 108 (37.0%) | 28 (25.9%) | 80 (42.8%) | 0.0410 | 61 (56.6%) | 47 (38.2%) | 0.8048 | 62 (57.4%) | 42 (38.2%) | 0.4767 |
| Previous LB | 57 (19.5%) | 16 (28.1%) | 41 (21.9%) | 0.0772 | 42 (24.8%) | 15 (12.2%) | 0.0109 | 40 (22.0%) | 17 (15.5%) | 0.2266 |
| Tick bite ** | 144 (49.3%) | 41 (43.2%) | 103 (55.1%) | 0.3966 | 78 (46.2%) | 66 (53.7%) | 0.2510 | 93 (51.1%) | 51 (46.4%) | 0.5070 |
| Multiple EM | 40 (13.7%) | 24 (25.3%) | 16 (8.6%) | 0.0003 | 28 (16.6%) | 12 (9.8%) | 0.1338 | 22 (12.1%) | 18 (16.4%) | 0.3931 |
| Symptoms at the site of EM | 138 (47.3%) | 41 (43.2%) | 97 (51.9%) | 0.2086 | 66 (39.1%) | 72 (58.5%) | 0.0015 | 87 (47.8%) | 51 (46.4%) | 0.9064 |
| Constitutional symptoms | 99 (33.9%) | 33 (34.7%) | 66 (35.3%) | 0.9686 | 59 (34.9%) | 40 (32.5%) | 0.7634 | 64 (35.2%) | 35 (31.8%) | 0.6471 |
| IgM pos | 95 (32.5%) | / | / | / | 61 (36.1%) | 34 (27.6%) | 0.1628 | 40 (22.5%) | 55 (50%) | <0.0001 |
| IgG pos | 169 (57.9%) | 61 (64.2%) | 108 (57.8%) | 0.3590 | / | / | / | 105 (57.7%) | 64 (58.2%) | 0.9679 |
| Skin culture pos | 182 (62.3%) | 60 (63.2%) | 122 (65.2%) | 0.8307 | 105 (62.1%) | 77 (62.6%) | 0.9679 | / | / | / |
| B | Wald | df | Sig. | |
|---|---|---|---|---|
| Intercept | 2.256 | 9.290 | 1 | 0.002 |
| Sex | 0.154 | 0.253 | 1 | 0.615 |
| Age | −0.040 | 11.549 | 1 | 0.001 |
| Underlying chronic illness | 0.037 | 0.012 | 1 | 0.914 |
| Previous LB | 0.109 | 0.077 | 1 | 0.781 |
| Tick bite * | −0.123 | 0.160 | 1 | 0.689 |
| Multiple EM | −1.183 | 7.212 | 1 | 0.007 |
| Symptoms at the site of EM | 0.098 | 0.311 | 1 | 0.752 |
| Constitutional symptoms | 0.002 | 0.329 | 1 | 0.995 |
| Duration of EM at diagnosis | 0.003 | 0.158 | 1 | 0.691 |
| Diameter of EM at diagnosis | 0.002 | 0.034 | 1 | 0.853 |
| IgG-pos | −0.536 | 2.608 | 1 | 0.106 |
| Skin culture-pos | −0.21 | 0.453 | 1 | 0.501 |
| B | Wald | df | Sig. | |
|---|---|---|---|---|
| Intercept | −0.670 | 0.890 | 1 | 0.345 |
| Sex | −0.558 | 3.679 | 1 | 0.055 |
| Age | 0.029 | 6.508 | 1 | 0.011 |
| Underlying chronic illness | −0.558 | 2.956 | 1 | 0.086 |
| Previous LB | 1.146 | 8.554 | 1 | 0.003 |
| Tick bite * | 0.076 | 0.064 | 1 | 0.800 |
| Multiple EM | −0.725 | 2.285 | 1 | 0.131 |
| Duration of EM at diagnosis | 0.025 | 8.405 | 1 | 0.004 |
| Diameter of EM at diagnosis | 0.028 | 5.773 | 1 | 0.016 |
| Symptoms at the site of EM | −0.680 | 5.344 | 1 | 0.021 |
| Constitutional symptoms | −0.016 | 0.002 | 1 | 0.960 |
| IgM-pos | −0.599 | 3.192 | 1 | 0.074 |
| Skin culture-pos | 0.390 | 1.626 | 1 | 0.202 |
| B | Wald | df | Sig. | |
|---|---|---|---|---|
| Intercept | −0.633 | 0.919 | 1 | 0.338 |
| Sex | −0.181 | 0.426 | 1 | 0.514 |
| Age | 0.018 | 2.820 | 1 | 0.093 |
| Underlying chronic illness | −0.684 | 5.003 | 1 | 0.025 |
| Previous LB | 0.503 | 1.832 | 1 | 0.176 |
| Tick bite * | 0.260 | 0.864 | 1 | 0.353 |
| Multiple EM | 0.330 | 0.582 | 1 | 0.446 |
| Symptoms at the site of EM | −0.105 | 0.136 | 1 | 0.712 |
| Constitutional symptoms | 0.242 | 0.622 | 1 | 0.430 |
| Duration of EM at diagnosis | −0.005 | 0.532 | 1 | 0.466 |
| Diameter of EM at diagnosis | 0.003 | 0.069 | 1 | 0.793 |
| IgM-pos | −0.228 | 0.527 | 1 | 0.468 |
| IgG-pos | 0.423 | 1.968 | 1 | 0.161 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ružić-Sabljić, E.; Maraspin, V.; Bogovič, P.; Rojko, T.; Ogrinc, K.; Jaklič, M.; Strle, F. Microbiologic Findings in a Cohort of Patients with Erythema Migrans. Microorganisms 2024, 12, 185. https://doi.org/10.3390/microorganisms12010185
Ružić-Sabljić E, Maraspin V, Bogovič P, Rojko T, Ogrinc K, Jaklič M, Strle F. Microbiologic Findings in a Cohort of Patients with Erythema Migrans. Microorganisms. 2024; 12(1):185. https://doi.org/10.3390/microorganisms12010185
Chicago/Turabian StyleRužić-Sabljić, Eva, Vera Maraspin, Petra Bogovič, Tereza Rojko, Katarina Ogrinc, Martina Jaklič, and Franc Strle. 2024. "Microbiologic Findings in a Cohort of Patients with Erythema Migrans" Microorganisms 12, no. 1: 185. https://doi.org/10.3390/microorganisms12010185
APA StyleRužić-Sabljić, E., Maraspin, V., Bogovič, P., Rojko, T., Ogrinc, K., Jaklič, M., & Strle, F. (2024). Microbiologic Findings in a Cohort of Patients with Erythema Migrans. Microorganisms, 12(1), 185. https://doi.org/10.3390/microorganisms12010185







