Monitoring of Animal Feed Contamination by Mycotoxins: Results of Five Years of Official Control by an Accredited Italian Laboratory
Abstract
1. Introduction
2. Materials and Methods
3. Results
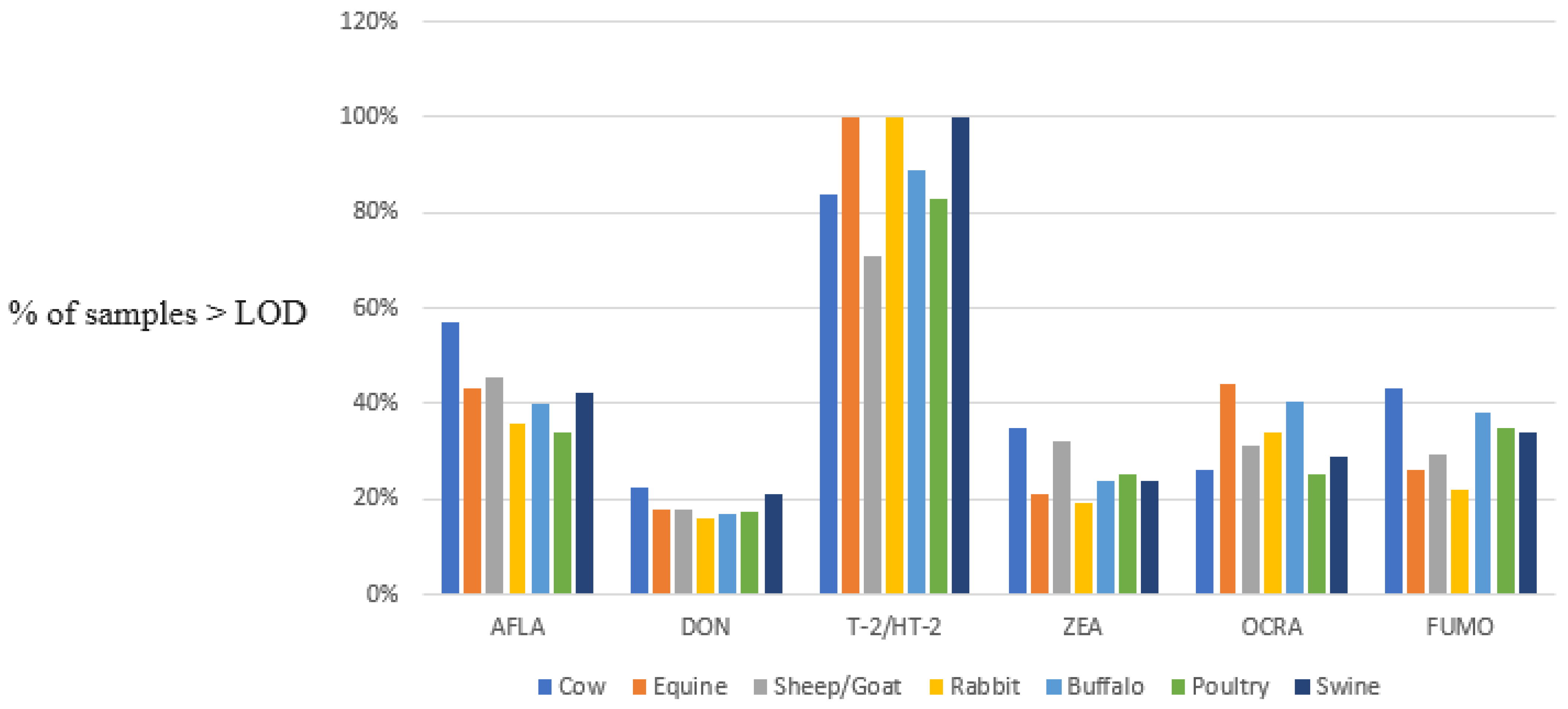
Animal Feed Contamination by Mycotoxins
4. Discussion
4.1. Contamination by Mycotoxins in Feed for Different Species
4.2. Comparison to the Literature
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wilson, D.M.; Payne, G.A. Factors affecting Aspergillus Flavus group infection and Aflatoxin contamination of crops. In The Toxicology of Aflatoxins; Eaton, D.L., Groopman, J.D., Eds.; Academic Press: Cambridge, MA, USA, 1994; pp. 309–325. [Google Scholar] [CrossRef]
- D’Agnello, P.; Vita, V.; Franchino, C.; Urbano, L.; Curiale, A.; Debegnach, F.; Iammarino, M.; Marchesani, G.; Chiaravalle, A.E.; De Pace, R. ELISA and UPLC/FLD as Screening and Confirmatory Techniques for T-2/HT-2 Mycotoxin Determination in Cereals. Appl. Sci. 2021, 11, 1688. [Google Scholar] [CrossRef]
- Vita, V.; Franchino, C.; Iammarino, M.; De Pace, R. Aflatoxins contamination in nuts for direct human consumption: Analytical findings from three years of official control in Italy. Int. J. Food Sci. Technol. 2022, 57, 7496–7504. [Google Scholar] [CrossRef]
- Nardiello, D.; Lo Magro, S.; Iammarino, M.; Palermo, C.; Muscarella, M.; Centonze, D. Recent Advances in the Post-Column Derivatization for the Determination of Mycotoxins in Food Products and Feed Materials by Liquid Chromatography and Fluorescence Detection. Curr. Anal. Chem. 2014, 10, 355–365. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Chemical Agents and Related Occupations—A Review of Human Carcinogens, Volume 100F; IARC: Lyon, France, 2012. [Google Scholar]
- Briden, W. Micotoxin contamination of the feed supply chain: Implications for animal productivity and feed security. Anim. Feed Sci. Technol. 2012, 173, 134–158. [Google Scholar] [CrossRef]
- Dhakal, A.; Sbar, E. Aflatoxin Toxicity; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Tian, E.; Tian, Y.; Zhang, D.; Cai, P.; Lin, H.; Ying, H.; Hu, Q.-N.; Wu, A. Elimination of Fusarium mycotoxin deoxynivalenol (DON) via microbial and enzymatic strategies: Current status and future perspectives. Trends Food Sci. Technol. 2022, 124, 96–107. [Google Scholar] [CrossRef]
- Liao, Y.; Peng, Z.; Chen, L.; Nüssler, A.K.; Liu, L.; Yang, W. Deoxinyvalenol, gut microbiota and immunotoxicity: A potential approach? Food Chem. Toxicol. 2018, 112, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Mostrom, M.S.; Evans, T.J. Zearalenone. In Veterinary Toxicology, 3rd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 1055–1063. [Google Scholar]
- Bulgaru, C.V.; Marin, D.E.; Pistol, G.C.; Taranu, I. Zearalenone and the immune response. Toxins 2021, 13, 248. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wen, J.; Tang, Y.; Shi, J.; Mu, G.; Yan, R.; Cai, J.; Long, M. Research progress on fumonisin B1 contamination and toxicity: A review. Molecules 2021, 26, 5238. [Google Scholar] [CrossRef]
- Van der Merwe, K.J.; Steyn, P.S.; Fourie, L.; Scott, D.B.; Theron, J.J. Ochratoxin A, a toxic metabolite produced by Aspergillus ochraceus wilh. Nature 1965, 205, 1112–1113. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Liu, F.; Wang, Q.; Selvaraj, J.N.; Xing, F.; Zhao, Y.; Liu, Y. Ochratoxin A Producing Fungi, Biosynthetic Pathway and Regulatory Mechanisms. Toxins 2016, 8, 83. [Google Scholar] [CrossRef]
- Tao, Y.; Xie, S.; Xu, F.; Liu, A.; Wang, Y.; Chen, D.; Pan, Y.; Huang, L.; Peng, D.; Wang, X.; et al. Ochratoxin A: Toxicity, oxidative stress and metabolism. Food Chem. Toxicol. 2018, 112, 320–331. [Google Scholar] [CrossRef]
- Meneely, J.; Greer, B.; Kolawole, O.; Elliott, C. T-2 and HT-2 Toxins: Toxicity, Occurrence and Analysis: A Review. Toxins 2023, 15, 481. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Qin, Z.; Kuca, K.; You, L.; Zhao, Y.; Liu, A.; Musilek, K.; Chrienova, Z.; Nepovimova, E.; Oleksak, P.; et al. An update on T-2 toxin and its modified forms: Metabolism, immunotoxicity mechanism, and human exposure assessment. Arch. Toxicol. 2020, 94, 3645–3669. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EU) No 574/2011 of 16 June 2011 amending Annex I to Directive 2002/32/EC of the European Parliament and of the Council as regards maximum levels for nitrite, melamine, Ambrosia spp. and carry-over of certain coccidiostats and histomonostats and consolidating Annexes I and II thereto. Off. J. Eur. Union 2011, L159, 7–24. [Google Scholar]
- European Commission. Commission Recommendation of 27 March 2013 on the presence of T-2 and HT-2 toxin in cereals and cereal products. Off. J. Eur. Union 2013, L91, 12–15. [Google Scholar]
- European Commission. Commission Recommendation of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2006, L229, 7–9. [Google Scholar]
- Binder, E.M.; Tan, L.M.; Chin, L.J.; Handl, J.; Richard, J. Wordwide occurance of micotoxins in commodities, feeds and feed ingredients. Anim. Feed Sci. Technol. 2007, 137, 265–282. [Google Scholar] [CrossRef]
- Medina, A.; Akbar, A.; Baazeem, A.; Rodriguez, A.; Magan, N. Climate change, food security and micotoxins: Do we know enough? Fungal Biol. Rev. 2017, 31, 143–154. [Google Scholar] [CrossRef]
- Pleadin, J.; Zadravec, M.; Lešić, T.; Vulić, A.; Vahčić, N.; Kudumija, N.; Frece, J.; Markov, K. Chapter II Ochratoxin A contamination of traditional dry-cured meat products. In Ochratoxin A and Aflatoxin B1: New Research; Nova Science Publishers: Hauppauge, NY, USA, 2020; pp. 31–66. [Google Scholar]
- European Commission. Commission Regulation (EC) No 152/2009 of 27 January 2009 laying down the methods of sampling and analysis for the official control of feed. Off. J. Eur. Union 2009, L54, 1–130. [Google Scholar]
- ISO/IEC 17025; Testing and Calibration Laboratories. ISO: Geneva, Switzerland, 2018.
- European Commission. Commission Implementing Regulation (EU) 2021/808 of 22 March 2021 on the performance of analytical methods for residues of pharmacologically active substances used in food-producing animals and on the interpretation of results as well as on the methods to be used for sampling and repealing Decisions 2002/657/EC and 98/179/EC. Off. J. Eur. Union 2021, L180, 84–109. [Google Scholar]
- Iammarino, M. Simplified Guidelines for Chromatographic Methods Validation; LAP Lambert Academic Publishing: Riga, Latvia, 2019. [Google Scholar]
- Menichini, E.; Viviano, G.; Working Group Istituto Superiore di Sanità. Treatment of Data below the Detection Limit in the Calculation of Analytical Results—Rapporti ISTISAN 04/15; Istituto Superiore di Sanità: Rome Italy, 2004; Available online: https://www.iss.it/documents/20126/955767/0415.1106219644.pdf/51c15924-7b63-07ca-cce4-9a37d772190d?t=1575578775857 (accessed on 30 October 2023).
- Kosicki, R.; Błajet-Kosicka, A.; Grajewski, J.; Twarużek, M. Multiannual mycotoxin survey in feed materials and feedingstuffs. Anim. Feed Sci. Technol. 2016, 215, 165–180. [Google Scholar] [CrossRef]
- Twarużek, M.; Skrzydlewski, P.; Kosicki, R.; Grajewski, J. Mycotoxins survey in feed materials and feedingstuffs in years 2015–2020. Toxicon 2021, 202, 27–39. [Google Scholar] [CrossRef]
- Ferrari, L.; Fumagalli, F.; Rizzi, N.; Grandi, E.; Vailati, S.; Manoni, M.; Ottoboni, M.; Cheli, F.; Pinotti, L. An Eight-Year Survey on Aflatoxin B1 Indicates High Feed Safety in Animal Feed and Forages in Northern Italy. Toxins 2022, 14, 763. [Google Scholar] [CrossRef]
- Vandicke, J.; De Visschere, K.; Ameye, M.; Croubels, S.; De Saeger, S.; Audenaert, K.; Haesaert, G. Multi-Mycotoxin Contamination of Maize Silages in Flanders, Belgium: Monitoring Mycotoxin Levels from Seed to Feed. Toxins 2021, 13, 202. [Google Scholar] [CrossRef] [PubMed]
- Karlovsky, P.; Suman, M.; Berthiller, F.; De Meester, J.; Eisenbrand, G.; Perrin, I.; Oswald, I.P.; Speijers, G.; Chiodini, A.; Recker, T.; et al. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016, 32, 179–205. [Google Scholar] [CrossRef] [PubMed]
- Čolović, R.; Puvača, N.; Cheli, F.; Avantaggiato, G.; Greco, D.; Đuragić, O.; Kos, J.; Pinotti, L. Decontamination of Mycotoxin-Contaminated Feedstuffs and Compound Feed. Toxins 2019, 11, 617. [Google Scholar] [CrossRef]
- Iammarino, M.; dell’Oro, D.; Bortone, N.; Chiaravalle, A.E. Beta emitter radionuclides (90Sr) contamination in animal feed: Validation and application of a radiochemical method by ultra low level liquid scintillation counting. Ital. J. Food Saf. 2015, 4, 13–17. [Google Scholar] [CrossRef][Green Version]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Savi, G.D.; Cardoso, W.A.; Furtado, B.G.; Bortolotto, T.; Zanoni, E.T.; Scussel, R.; Rezende, L.F.; Machado-de-Ávila, R.A.; Montedo, O.R.K.; Angioletto, E. Antifungal activities against toxigenic Fusarium specie and deoxynivalenol adsorption capacity of ion-exchanged zeolites. J. Environ. Sci. Health Part B 2018, 53, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Bakutis, B.A.B.; Violeta, K.; Paokevieius, A. Use of biological method for detoxification of Mycotoxins. Bot. Lith. 2005, 7, 123–129. [Google Scholar]
- Cheng, B.; Wan, C.; Yang, S.; Xu, H.; Wei, H.U.A.; Liu, J.; Tian, W.; Zeng, M. Detoxification of Deoxynivalenol by bacillus strains. J. Food Saf. 2010, 30, 599–614. [Google Scholar] [CrossRef]
- Li, M.; Guan, E.; Bian, K. Detoxification of Deoxynivalenol by 60Co γ-ray irradiation and toxicity analyses of radiolysis products. J. AOAC Int. 2019, 102, 1749–1755. [Google Scholar] [CrossRef]
- Li, Y.; Gao, H.; Wang, R.; Xu, Q. Deoxynivalenol in food and feed: Recent advances in decontamination strategies. Front. Microbiol. 2023, 14, 1141378. [Google Scholar] [CrossRef]
- Adunphatcharaphon, S.; Petchkongkaew, A.; Visessanguan, W. In Vitro Mechanism Assessment of Zearalenone Removal by Plant-Derived Lactobacillus plantarum BCC 47723. Toxins 2021, 13, 286. [Google Scholar] [CrossRef]
- Jard, G.; Liboz, T.; Mathieu, F.; Guyonvarc’h, A.; Lebrihi, A. Adsorption of zearalenone by Aspergillus japonicus conidia: New trends for biological decontamination in animal feed. World Mycotoxin J. 2009, 2, 391–397. [Google Scholar] [CrossRef]
- Zhou, L.-H.; Wang, Y.-L.; Qiu, M.; Shi, Q.; Sun, L.-J.; Liao, J.-M.; Xu, D.-F.; Liu, Y.; Fang, Z.-J.; Gooneratne, R. Analysis of T-2 Toxin Removal Factors in a Lactococcus Fermentation System. J. Food Prot. 2017, 80, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
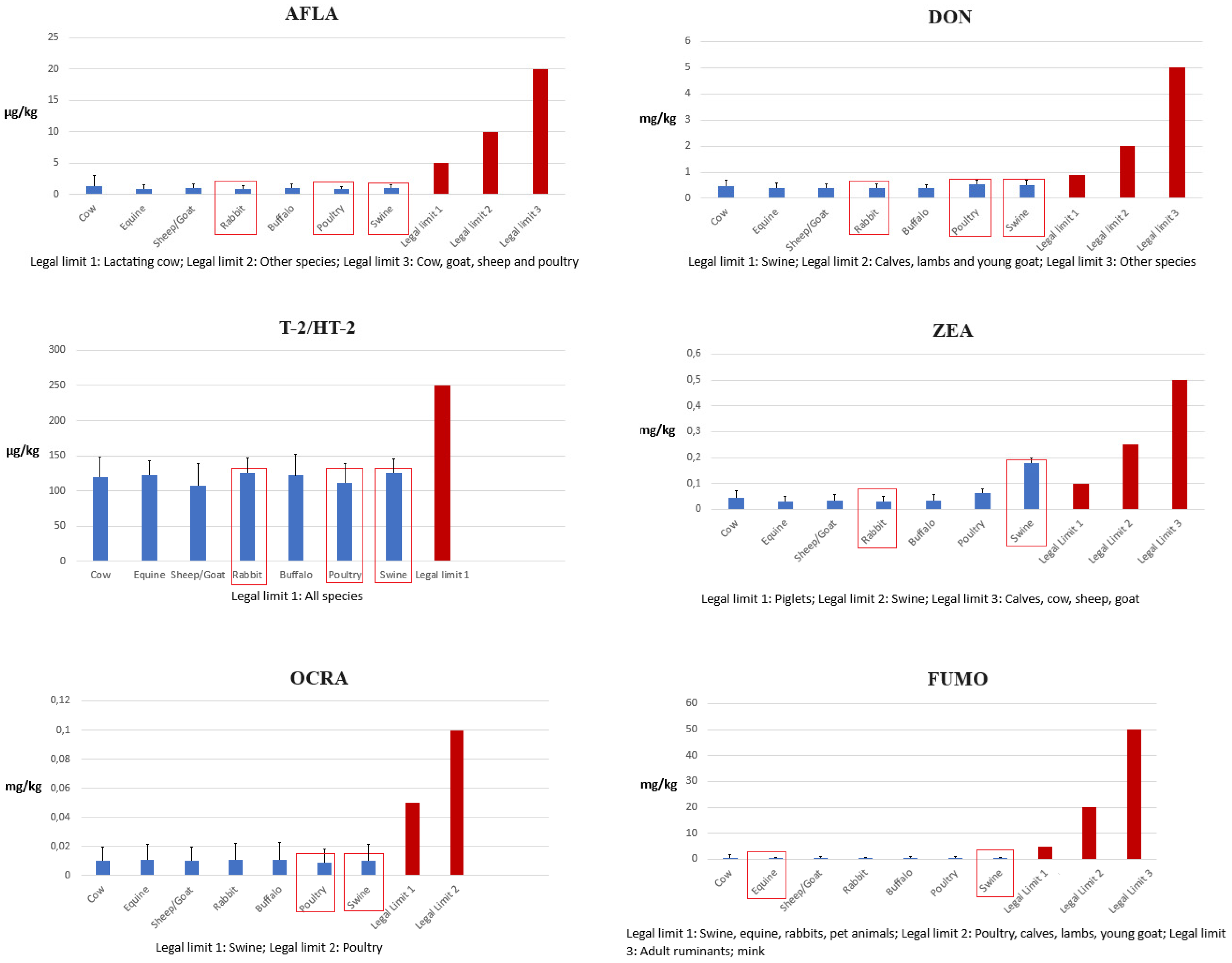
| Mycotoxin | Animal Species | Legal Limit (mg kg−1) |
|---|---|---|
| AF | Cow | 0.005 |
| Other species | 0.010 | |
| Cow, goat, sheep and poultry | 0.020 | |
| DON | Swine | 0.9 |
| Calves, lambs and young goat | 2 | |
| Other species | 5 | |
| ZEA | Piglets | 0.1 |
| Fattening pigs | 0.25 | |
| Calves, cow, sheep and goat | 0.5 | |
| OCRA | Swine | 0.05 |
| Poultry | 0.1 | |
| FUMO | Swine, equine, rabbits and pet animals | 5 |
| Poultry, calves, lambs and young goat | 20 | |
| Adult ruminants and mink | 50 | |
| T-2/HT-2 | All species | 0.25 |
| Mycotoxin | LOD (µg kg−1) | SD * | CV (%) * | Recovery (%) * |
|---|---|---|---|---|
| Aflatoxin B1 (AF) | 1.0 | 0.304 | 9.5 | 98.0% |
| Deoxynivalenol (DON) | 555 | 0.327 | 9.0 | 90.5% |
| Fumonisins (FUMO) | 0.2 | 0.527 | 6.5 | 117.0% |
| Ochratoxin A (OCRA) | 12.5 | 0.021 | 5.5 | 106.5% |
| T-2/HT-2 toxins | 75.0 | 12.165 | 6.5 | 102.0% |
| Zearalenone (ZEA) | 8.0 | 0.029 | 8.5 | 97.0% |
| AF (µg kg−1) | DON (mg kg−1) | T-2/HT-2 (µg kg−1) | ZEA (mg kg−1) | OCRA (mg kg−1) | FUMO (mg kg−1) | Total (N° of N.C.) | ||
|---|---|---|---|---|---|---|---|---|
| COW | N° of samples | 380 | 288 | 55 | 288 | 288 | 288 | |
| <LOD | 164 | 223 | 9 | 188 | 214 | 163 | 380 | |
| Min–Max | 0.5–15.5 | 0.28–6.87 | 37.5–214.3 | 0.004–0.730 | 0.006–0.140 | 0.1–9.3 | (6) | |
| Mean | 1.3 | 0.46 | 119.3 | 0.045 | 0.010 | 0.4 | ||
| N° of N.C. | 2 (12.6–15.5) | 1 (6.87) | 0 | 3 (0.57–0.73–1.23) | 0 | 0 | ||
| EQUINE | N° of samples | 51 | 34 | 7 | 34 | 34 | 34 | |
| <LOD | 29 | 28 | 0 | 27 | 19 | 25 | 51 | |
| Min–Max | 0.5–2.2 | 0.277–1.730 | 99.96–179.14 | 0.004–0.510 | 0.006–0.035 | 0.10–0.73 | (0) | |
| Mean | 0.94 | 0.411 | 121.51 | 0.029 | 0.011 | 0.17 | ||
| N° of N.C. | 0 | 0 | 0 | 0 | 0 | 0 | ||
| SHEEP/GOAT | N° of samples | 79 | 71 | 14 | 71 | 71 | 71 | |
| <LOD | 43 | 58 | 4 | 48 | 49 | 50 | 79 | |
| Min–Max | 0.50–7.16 | 0.277–1.730 | 37.50–179.14 | 0.004–0.510 | 0.006–0.035 | 0.1–6.3 | (2) | |
| Mean | 1.04 | 0.404 | 107.45 | 0.033 | 0.010 | 0.4 | ||
| N° of N.C. | 1 (7.16) | 0 | 0 | 1 (0.510) | 0 | 0 | ||
| N° of samples | 39 | 32 | 5 | 32 | 32 | 32 | ||
| RABBIT | <LOD | 25 | 27 | 0 | 26 | 21 | 25 | 39 |
| Min–Max | 0.5–2.2 | 0.277–1.730 | 99.96–179.14 | 0.004–0.510 | 0.006–0.035 | 0.10–0.73 | (0) | |
| Mean | 0.9 | 0.415 | 125.26 | 0.030 | 0.011 | 0.17 | ||
| N° of N.C. | 0 | 0 | 0 | 0 | 0 | 0 | ||
| N° of samples | 48 | 42 | 9 | 42 | 42 | 42 | ||
| BUFFALO | <LOD | 29 | 35 | 1 | 32 | 25 | 26 | 48 |
| Min–Max | 0.5–4.9 | 0.277–1.730 | 37.50–181.64 | 0.004–0.510 | 0.006–0.035 | 0.10–3.08 | (0) | |
| Mean | 1.1 | 0.407 | 122.38 | 0.033 | 0.011 | 0.34 | ||
| N° of N.C. | 0 | 0 | 0 | 0 | 0 | 0 | ||
| N° of samples | 47 | 40 | 6 | 40 | 40 | 40 | ||
| POULTRY | <LOD | 31 | 33 | 1 | 30 | 30 | 26 | 47 |
| Min–Max | 0.5–2.2 | 0.28–4.68 | 37.50–179.14 | 0.004–0.730 | 0.006–0.035 | 0.10–2.99 | (0) | |
| Mean | 0.86 | 0.52 | 111.48 | 0.061 | 0.009 | 0.324 | ||
| N° of N.C. | 0 | 0 | 0 | 0 | 0 | 0 | ||
| SWINE | N° of samples | 47 | 38 | 5 | 38 | 38 | 38 | |
| <LOD | 27 | 30 | 0 | 29 | 27 | 25 | 47 | |
| Min–Max | 0.50–5.71 | 0.3–5.0 | 99.96–179.14 | 0.004–0.510 | 0.006–0.035 | 0.10–1.75 | (6) | |
| Mean | 1.06 | 0.5 | 125.26 | 0.179 | 0.010 | 0.26 | ||
| N° of N.C. | 1 (5.71) | 4 (0.9–1.3–1.6–5.0) | 0 | 1 (0.510) | 0 | 0 | ||
| TOTAL | 691 | 545 | 101 | 545 | 545 | 545 | ||
| % samples > LOD | 49.6% | 20.4% | 85.1% | 30.3% | 29.4% | 37.6% | ||
| N° of N.C. (%) | 4 (0.6%) | 5 (0.9%) | 0 (0%) | 3 (0.5%) | 0 (0%) | 0 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franchino, C.; Vita, V.; Iammarino, M.; De Pace, R. Monitoring of Animal Feed Contamination by Mycotoxins: Results of Five Years of Official Control by an Accredited Italian Laboratory. Microorganisms 2024, 12, 173. https://doi.org/10.3390/microorganisms12010173
Franchino C, Vita V, Iammarino M, De Pace R. Monitoring of Animal Feed Contamination by Mycotoxins: Results of Five Years of Official Control by an Accredited Italian Laboratory. Microorganisms. 2024; 12(1):173. https://doi.org/10.3390/microorganisms12010173
Chicago/Turabian StyleFranchino, Cinzia, Valeria Vita, Marco Iammarino, and Rita De Pace. 2024. "Monitoring of Animal Feed Contamination by Mycotoxins: Results of Five Years of Official Control by an Accredited Italian Laboratory" Microorganisms 12, no. 1: 173. https://doi.org/10.3390/microorganisms12010173
APA StyleFranchino, C., Vita, V., Iammarino, M., & De Pace, R. (2024). Monitoring of Animal Feed Contamination by Mycotoxins: Results of Five Years of Official Control by an Accredited Italian Laboratory. Microorganisms, 12(1), 173. https://doi.org/10.3390/microorganisms12010173








