Intersociety Position Statement on the Prevention of Ophthalmia Neonatorum in Italy
Abstract
:1. Introduction
2. Strategies for the Prevention of Ophthalmia Neonatorum and Current Guidelines
- Screening pregnant women for sexually transmitted infections (STIs) and giving appropriate treatment as necessary; treated patients and those with persistent risk factors for STI should be regularly followed up with and re-tested.
- Ocular prophylaxis, with antiseptic or antibiotic medication, of the neonate after birth.
- Tetracycline hydrochloride 1% eye ointment;
- Erythromycin 0.5% eye ointment;
- Povidone iodine 2.5% solution (water based);
- Silver nitrate 1% solution;
- Chloramphenicol 1% eye ointment.
3. Incidence of Gonorrhea and C. trachomatis Infections in Italy
4. Intersociety Recommendations on Ophthalmia Neonatorum Prophylaxis in Italy
- Do not give conjunctival antibiotic prophylaxis against ON indiscriminately to all infants at birth.
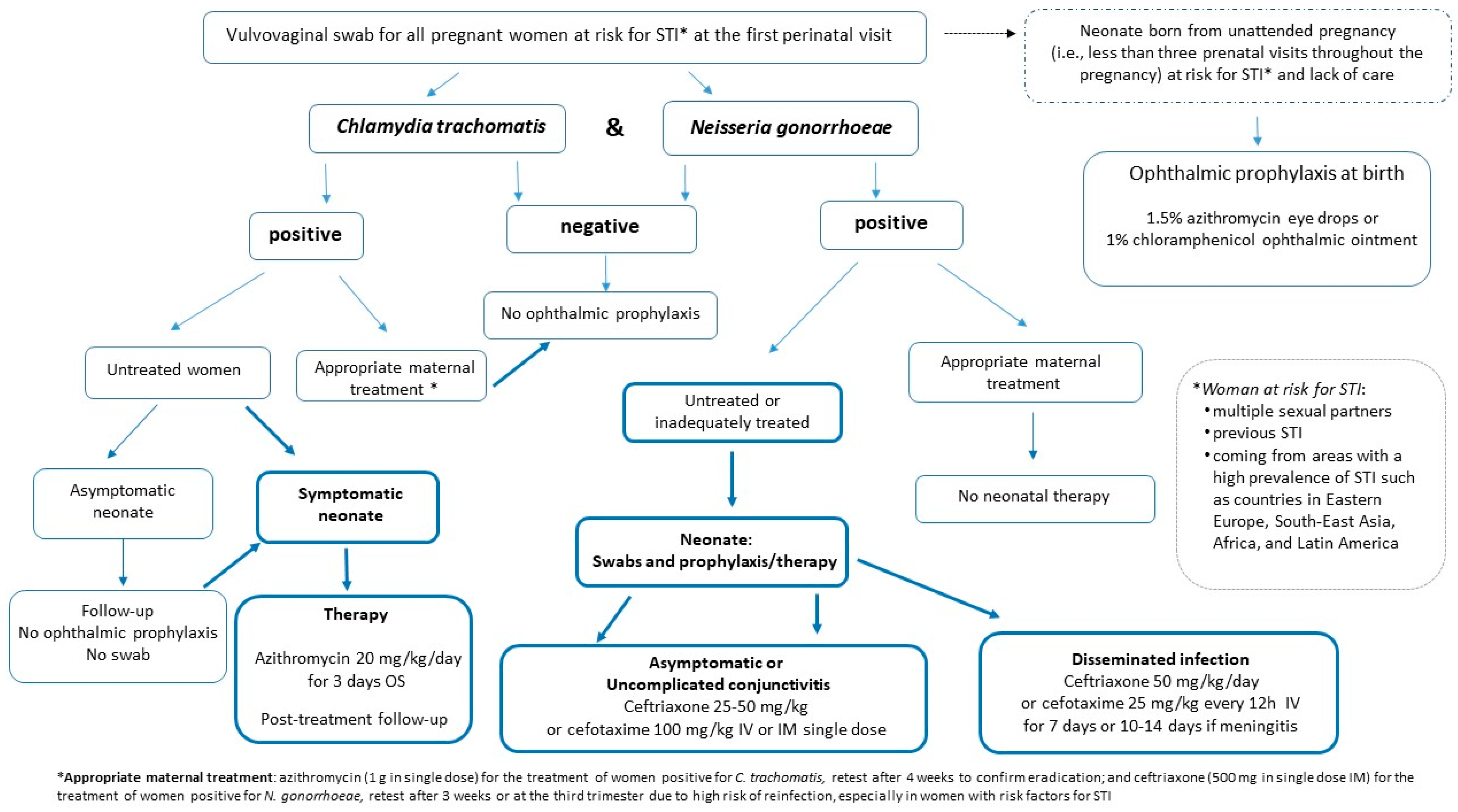
- Ophthalmic prophylaxis with 1.5% azithromycin eye drops or 1% chloramphenicol ophthalmic ointment [25,28,42] should be administered immediately after birth exclusively to neonates born from unattended pregnancies (defined as fewer than three prenatal visits throughout the pregnancy); mothers at risk of sexually transmitted diseases, or coming from areas with a high prevalence of gonococcal infections and with no access to care; and when it is assumed that no primary prevention of STI has taken place before, and during, pregnancy. Maternal screening for N. gonorrhoeae and C. trachomatis at delivery, or immediately postpartum, should be performed.
- Screening of women at risk of STI should be performed at the first prenatal care visit by a vulvovaginal swab for N. gonorrhoeae and C. trachomatis [22,23]. Swabs should be repeated in the third trimester in the case of ongoing exposure to risk (i.e., women with multiple sexual partners, previous STI, or coming from areas with a high prevalence of the disease such as Eastern Europe, South East Asia, Africa and Latin American countries). Women without risk factors who have not been swabbed during pregnancy and who have been regularly followed-up with by the obstetrician are to be considered negative.
- Screening for N. gonorrhoeae and C. trachomatis at delivery, or immediately postpartum, should be performed in women with risk factors for STI who, despite having access to care and being followed-up with during pregnancy, have not been screened, to ensure appropriate management of the mother–neonate in the event of a positive test [28]. Pending the result of the maternal swab, the asymptomatic newborn can be routinely discharged from the birthplace without receiving conjunctival antibiotics. The mother should be recalled to communicate the result of the vaginal swab. Neonates, even if asymptomatic, born to mothers with a vulvovaginal swab positive for N. gonorrheae, or those with conjunctival and/or respiratory symptoms born to mothers with a swab positive for C. trachomatis, must be re-evaluated by the pediatrician to ensure appropriate therapy (see recommendation numbers 7–9).
- Neonates born to women at risk for STI who were not screened during pregnancy, or to those positive for C. trachomatis, even untreated, in the absence of clinical symptoms of conjunctivitis, should not undergo an ocular swab.
- In the absence of conjunctival and/or respiratory symptoms, neonates born to women infected by C. trachomatis should not undergo ophthalmic prophylaxis or systemic therapy; instead, they should be subject to pediatrician follow-up to recognize early on any signs of conjunctivitis, which usually occurs between 5 and 14 days of life, or pneumonia, which has a later onset of between 4 and 12 weeks [28].
- Symptomatic neonates with conjunctival and/or respiratory signs born to mothers with untreated chlamydial infection should be treated with oral azithromycin at a dose of 20 mg/kg/day, once a day, for 3 days [42,43,44,45]. Follow-up is recommended to determine the effectiveness of the initial treatment because data on the efficacy of azithromycin for eye or lung disease are limited [43,44].
- Asymptomatic neonates born to women with untreated or inadequately treated N. gonorrhoeae infection [45] should be evaluated by swab (e.g., ocular, rectal, vaginal and oropharyngeal). Treatment should start immediately with 25–50 mg/kg of ceftriaxone intramuscularly (IM) or intravenously (IV), up to a maximum of 250 mg total, or cefotaxime 100 mg/kg IM or IV in a single dose when ceftriaxone is contraindicated (i.e., concomitant treatment with calcium-containing IV fluids), pending the results of the swabs [23,46,47].
- Symptomatic neonates with:
- Uncomplicated gonococcal conjunctivitis should be evaluated to rule out disseminated gonococcal infection and treated with a single dose of ceftriaxone, 25–50 mg/kg IM or IV, or a single dose of cefotaxime 100 mg/kg, IM or IV, when ceftriaxone is contraindicated (i.e., concomitant treatment with calcium-containing IV fluids) [23,46,47].
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- American Academy of Pediatrics. Committee on Infectious Diseases. Neonatal ophthalmia. In Red Book: 2021–2024 Report of the Committee on Infectious Diseases, 32nd ed.; Kimberlin, D.W., Barnett, E.D., Lynfield, R., Sawyer, M.H., Eds.; American Academy of Pediatrics: Itasca, IL, USA, 2021; pp. 1023–1026. [Google Scholar]
- Kapoor, V.S.; Evans, J.R.; Vedula, S.S. Interventions for preventing ophthalmia neonatorum. Cochrane Database Syst. Rev. 2020, 9, CD001862. [Google Scholar] [CrossRef] [PubMed]
- Nolt, D.; O’Leary, S.T.; Aucott, S.W. Risks of infectious diseases in newborns exposed to alternative perinatal practices. AAP Committee on Infectious Diseases, AAP Committee on Fetus and Newborn. Pediatrics 2022, 149, e2021055554. [Google Scholar] [CrossRef] [PubMed]
- Credé, C.S. Ophthalmia neonatorum in newborn children [Die verhub rtung der augenentzub ndung der neugeborenen]. Archiv. Gynaekol. 1881, 17, 50–53. [Google Scholar]
- Forbes, G.B.; Forbes, G.M. Silver nitrate and the eye of the newborn. Credé’s contribution to preventive medicine. Am. J. Dis. Child. 1971, 121, 1–3. [Google Scholar] [CrossRef]
- Zloto, O.; Gharaibeh, A.; Mezer, E.; Stankovic, B.; Isenberg, S.; Wygnanski-Jaffe, T. Ophthalmia neonatorum treatment and prophylaxis: IPOSC global study. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 577–582. [Google Scholar] [CrossRef]
- Boadi-Kusi, S.B.; Kyei, S.; Holdbrook, S.; Abu, E.K.; Ntow, J.; Ateko, A.M. A study of Ophthalmia Neonatorum in the Central Reion of Ghana: Causative Agents and Antibiotic Susceptibility Patterns. Glob. Pediatr. Health 2021, 8, 2333794X211019700. [Google Scholar] [CrossRef]
- Guirguis-Blake, J.M.; Evans, C.V.; Rushkin, M. Ocular Prophylaxis for Gonococcal Ophthalmia Neonatorum: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2019, 321, 404–446. [Google Scholar] [CrossRef]
- Gildea, D.; Goetz, R.; Drew, R.; Chamney, S. Ophthalmia neonatorum in a tertiary referral children’s hospital: A retrospective study. Eur. J. Opthalmol. 2022, 32, 587–591. [Google Scholar] [CrossRef]
- Chang, K.; Cheng, V.Y.W.; Kwong, N.S. Neonatal haemorrhagic conjunctivitis: A specific sign of chlamydial infection. Hong Kong Med. J. 2006, 12, 27–32. [Google Scholar]
- Amini, E.; Ghasemi, M.; Daneshjou, K. A five-year study in Iran of ophthalmia neonatorum: Prevalence and etiology. Med. Sci. Monit. 2008, 14, CR90–CR96. [Google Scholar]
- Normann, E.K.; Bakken, O.; Peltola, J.; Andréasson, B.; Buhl, S.; Sigg, P.; Nilesen, K. Treatment of acute neonatal bacterial conjunctivitis: A comparison of fucidic acid to chloramphenicol eye drops. Acta Ophthalmol. Scand. 2002, 80, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolomeo, S.; Mirta, D.H.; Janer, M.; Rodríguez Fermepin, M.R.; Sauka, D.; Magariños, F.; de Torres, R.A. Incidence of Chlamydia trachomatis and other potential pathogens in neonatal conjunctivitis. Int. J. Infect. Dis. 2001, 5, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Sexually Transmitted Infection (STI). Treatment Guidelines Update Webinar, CDC 2021. Available online: https://npin.cdc.gov/sites/default/files/CDC_STI_Transcript_FINAL_0114.pdf (accessed on 1 March 2023).
- Moore, D.L.; MacDonald, N.E. Canadian Paediatric Society, Infectious Diseases and Immunization Committee. Preventing ophthalmia neonatorum. Paediatr. Child. Health 2015, 20, 93–96. [Google Scholar] [PubMed]
- US Preventive Services Task Force; Curry, S.J.; Krist, A.H.; Owens, D.K.; Barry, M.J.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W.; Kemper, A.R.; et al. Ocular Prophylaxis for gonococcal Ophthalmia Neonatorum: US preventive Services Task Force reaffirmation Recommendation statement. JAMA 2019, 321, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Woods, C.R. Gonococcal infections in neonates and young children. Semin. Pediatr. Infect. Dis. 2005, 16, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Darville, T. Chlamydia trachomatis infections in neonates and young children. Semin. Pediatr. Infect. Dis. 2005, 16, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Whitcher, J.P.; Srinivasan, M.; Upadhyay, M.P. Corneal blindness: A global perspective. Bull. World Health Organ. 2001, 79, 214–221. [Google Scholar]
- Smith-Norowitz, T.A.; Ukaegbu, C.; Kohlhoff, S.; Hammerschlag, M.R. Neonatal prophylaxis with antibiotic containing ointments does not reduce incidence of chlamydial conjunctivitis in newborns. BMC Infect. Dis. 2021, 21, 270. [Google Scholar] [CrossRef]
- LeFevre, M.L.; US Preventive Services Task Force. Screening for chlamydia and gonorrhea: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2014, 161, 902–910. [Google Scholar] [CrossRef]
- Cantor, A.; Dana, T.; Griffin, J.C.; Nelson, H.D.; Atchison, C.; Winthrop, K.L.; Chou, R. Screening for Chlamydial and Gonococcal Infections: A Systematic Review Update for the U.S. Preventive Services Task Force; Evidence Synthesis No. 206; AHRQ Publication No. 21-05275-EF-1; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2021.
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.M.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M.; Bollan, G.A. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm. Rep. 2021, 70, 1–187. [Google Scholar] [CrossRef]
- Ministero Della Salute, Istituto Superiore di Sanità e Centro per la Valutazione Dell’efficacia Dell’assistenza Sanitaria. Linee Guida Gravidanza Fisiologica. 2011. Available online: https://www.epicentro.iss.it/itoss/pdf/gravidanza%20fisiologica_allegato.pdf (accessed on 22 October 2022).
- WHO Guidelines for the Treatment of Neisseria Gonorrhoeae; World Health Organization: Geneva, Switzerland, 2016; (WHO Guidelines Approved by the Guidelines Review Committee). Available online: http://www.ncbi.nlm.nih.gov/books/NBK379221/ (accessed on 22 October 2022).
- Nishida, H.; Risenberg, H.M. Silver nitrate ophthalmic solution and chemical conjunctivities. Pediatrics 1975, 56, 368–373. [Google Scholar] [CrossRef]
- Departamento Científico de Neonatologia—Sociedade Brasileira de Pediatria. Profilaxia da Oftalmia Neonatal por Transmissão Vertical. No 9, Dezembro de 2020. Available online: https://institutomacabi.com/wp-content/uploads/2021/02/22851d-DC-Profilaxia_da_Oftalmia_Neonatal_TransmVert.pdf (accessed on 1 March 2023).
- Guidelines for Perinatal Care. American Academy of Pediatrics, American College of Obstetricians and Gynecologists, 8th ed.; Kilpatrick, S., Papile, L., Macones, G., Watterberg, K., Eds.; American Academy of Pediatrics: Elk Grove Village, IL, USA; American College of Obstetricians and Gynecologists: Washington, DC, USA, 2017. [Google Scholar]
- Ministerio De Sanidad, Servicios Sociales E Igualdad Spain. Guía de Práctica Clínica de Atención en el Embarazo y Puerperio. Available online: http://portal.guiasalud.es/completa-pdf/ (accessed on 23 October 2022).
- Jug Došler, A.; Petročnik, P.; Mivšek, A.P.; Zakšek, T.; Skubic, M. Neonatal prophylaxis: Prevention of Vitamin K deficiency haemorrhage and neonatal ophthalmia. Zdr. Varst. 2015, 54, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Celik, M.; Koroglu, O.A. Ocular prophylaxis in the newborn. Eur. Eye Res. 2022, 2, 80–83. [Google Scholar] [CrossRef]
- Consultivo de Infecciones Neonatales, Sociedad Chilena de Infectología. Rationale of ocular prophylaxis of neonatal ophtalmia in the newborn infant. Rev. Chil. Infectol. 2017, 34, 257–258. [Google Scholar]
- Wahlberg, V. Reconsideration of Crede´ prophylaxis. A study of maternity and neonatal care. Acta Paediatr. Scand. 1982, 295, 1–73. [Google Scholar]
- Darling, E.K.; McDonald, H. A meta-analysis of the efficacy of ocular prophylactic agents used for the prevention of gonococcal and chlamydial ophthalmia neonatorum. J. Midwifery Womens Health 2010, 55, 319–327. [Google Scholar] [CrossRef]
- Gazzetta Ufficiale del Regno d’Italia, n. 249, 23-X-1940, XVIII, Decreto Ministeriale 11 Ottobre 1940-XVIII. Available online: https://www.gazzettaufficiale.it/eli/gu/1940/10/23/249/sg/pdf (accessed on 22 October 2022).
- Gazzetta Ufficiale Della Repubblica Italiana, n. 147, 6-VI-1975, Decreto del Presidente della Repubblica 7 Marzo 1975, n. 163. Available online: https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=1975-06-06&atto.codiceRedazionale=075U0163&elenco30giorni=false (accessed on 22 October 2022).
- Istituto Superiore di Sanità. Le Infezioni sessualmente trasmesse: Aggiornamento dei dati dei due Sistemi di sorveglianza sentinella attivi in Italia al 31 dicembre 2021. Not. ISS 2023, 36, 1–39. Available online: https://www.iss.it/documents/20126/6683812/Vol.+36,+n.+5.pdf/c7178c1e-667a-a0f5-134ce22a1c30939d?t=1689688348936 (accessed on 1 June 2023).
- ECDC (European Centre for Disease Prevention and Control). Gonorrhoea. In ECDC; Annual Epidemiological Report for 2019; ECDC: Stockholm, Sweden, 2023; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/GONO_AER_2019_Report.pdf (accessed on 29 November 2023).
- Camera dei Deputati; Parlamento Italiano. Interrogazione a Risposta Scritta 4/05397 Presentata da Valpiana Tiziana in Data 17/11/1994. Available online: http://documenti.camera.it/apps/commonServices/getDocumento.ashx?sezione=lavori&tipoDoc=sicross&idlegislatura=12&ramo=CAMERA&stile=9&idDocumento=4-05397 (accessed on 22 October 2022).
- Mondì, V.; Tzialla, C.; Aversa, S.; Merazzi, D.; Martinelli, S.; Araimo, G.; Massenzi, L.; Cavallaro, G.; Gagliardi, L.; Piersigilli, F.; et al. Antibiotic prophylaxis for ophthalmia neonatorum in Italy: Results from a national survey and the Italian intersociety new position statements. Ital. J. Pediatr. 2023, 49, 117. [Google Scholar] [CrossRef]
- Tavole Certificato di Assistenza al Parto (CeDAP). Anno 2020. Available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_3149_0_alleg.xlsx (accessed on 22 September 2022).
- WHO. WHO Recommendations on Newborn Health: Guidelines Approved by the WHO. Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2017; (WHO/MCA/17.07). Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- American Academy of Pediatrics. Chlamydia trachomatis. In Red Book: 2021–2024 Report of the Committee on Infectious Diseases, 32nd ed.; Kimberlin, D.W., Barnett, E.D., Lynfield, R., Sawyer, M.H., Eds.; American Academy of Pediatrics: Itasca, IL, USA, 2021; pp. 260–266. [Google Scholar]
- Hammerschlag, M.R. Chlamydial and gonococcal infections in infants and children. Clin. Infect. Dis. 2011, 53 (Suppl. S3), S99–S102. [Google Scholar] [CrossRef]
- Bolan, G.A.; Sparling, P.F.; Wasserheit, J.N. The emerging threat of untreatable gonococcal infection. N. Engl. J. Med. 2012, 366, 485–487. [Google Scholar] [CrossRef]
- Società Italiana di Neonatologia. Neofarm SIN, app di Farmacoterapia Neonatale. Available online: https://www.sin-neonatologia.it/app-neofarm-sin/ (accessed on 1 March 2023).
- American Academy of Pediatrics; Committee on Infectious Diseases. Gonococcal infections. In Red Book: 2021–2024 Report of the Committee on Infectious Diseases, 32nd ed.; Kimberlin, D.W., Barnett, E.D., Lynfield, R., Sawyer, M.H., Eds.; American Academy of Pediatrics: Itasca, IL, USA, 2021; pp. 338–340. [Google Scholar]
| Northern Italy | Central Italy | Southern Italy and Islands | Total | |
|---|---|---|---|---|
| Birth centers | 173 | 89 | 157 | 419 |
| Respondent centers (%) | 137 (79.2) | 63 (70.8) | 102 (65) | 302 (72.3) |
| N° of birth centers with <500 births/year | 38 | 24 | 41 | 103 |
| N° of respondent centers with <500 births/year (%) | 19 (50) | 13 (54.2) | 18 (43.9) | 50 (48.5) |
| N° of birth centers with 500–999 births/year | 67 | 37 | 66 | 170 |
| N° of respondent centers with 500–999 births/year (%) | 56 (83.6) | 26 (70.3) | 41 (62.1) | 123 (72.3) |
| N° of birth centers with 1000–2499 births/year | 55 | 23 | 49 | 127 |
| N° of respondent centers with 1000–2499 births/year (%) | 51 (92.7) | 19 (82.6) | 42 (85.7) | 112 (88.2) |
| N° of birth centers with >2500 births/year | 13 | 5 | 1 | 19 |
| N° of respondent centers with >2500 births/year (%) | 11 (84.6) | 5 (100) | 1 (100) | 17 (89.5) |
| % of Neonates | Preparation | Composition | WHO Recommendations | |
|---|---|---|---|---|
| Active ingredient | ||||
| Tobramycin 0.3% | 45.6% | Eye drops | Single active drug | Not recommended |
| Gentamicin 0.3% | 19.5% | Eye drops | Single active drug | Not recommended |
| Netylmycine 0.3% | 11.3% | Eye drops | Single active drug | Not recommended |
| Ofloxacin 0.3% | 11% | Eye drops | Single active drug | Not recommended |
| Chloramphenicol | 3.6% | Eye drops | Combination of active drugs | Recommended ointment as single active drug |
| Tetracycline 1% | 4.4% | Ointment | Combination of active drugs | Recommended as single active drug |
| Fusidic acid 1% | 3.1% | Eye drops | Single active drug | Not recommended |
| Povidone iodine 2.5% | 0.4% | Water solution | Single active drug | Recommended |
| Auromycin 1% | 0.1% | Ointment | Single active drug | Not recommended |
| Pheniramine maleate 0.3% + tetrazoline hydrochloride 0.05% | 0.6% | Eye drops | Combination of active drugs | Not recommended |
| Azithromycin 1.5% | 0.4% | Eye drops | Single active drug | Not recommended |
| Packaging | ||||
| Single-use package | 54% of treated neonates | |||
| Multi-use package | 46% of treated neonates | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzialla, C.; Auriti, C.; Aversa, S.; Merazzi, D.; Martinelli, S.; Araimo, G.; Massenzi, L.; Cavallaro, G.; Gagliardi, L.; Giuffrè, M.; et al. Intersociety Position Statement on the Prevention of Ophthalmia Neonatorum in Italy. Microorganisms 2024, 12, 15. https://doi.org/10.3390/microorganisms12010015
Tzialla C, Auriti C, Aversa S, Merazzi D, Martinelli S, Araimo G, Massenzi L, Cavallaro G, Gagliardi L, Giuffrè M, et al. Intersociety Position Statement on the Prevention of Ophthalmia Neonatorum in Italy. Microorganisms. 2024; 12(1):15. https://doi.org/10.3390/microorganisms12010015
Chicago/Turabian StyleTzialla, Chryssoula, Cinzia Auriti, Salvatore Aversa, Daniele Merazzi, Stefano Martinelli, Gabriella Araimo, Luca Massenzi, Giacomo Cavallaro, Luigi Gagliardi, Mario Giuffrè, and et al. 2024. "Intersociety Position Statement on the Prevention of Ophthalmia Neonatorum in Italy" Microorganisms 12, no. 1: 15. https://doi.org/10.3390/microorganisms12010015
APA StyleTzialla, C., Auriti, C., Aversa, S., Merazzi, D., Martinelli, S., Araimo, G., Massenzi, L., Cavallaro, G., Gagliardi, L., Giuffrè, M., Mosca, F., Cetin, I., Trojano, V., Valensise, H., Colacurci, N., Orfeo, L., Mondì, V., & on behalf of their respective Scientific Societies. (2024). Intersociety Position Statement on the Prevention of Ophthalmia Neonatorum in Italy. Microorganisms, 12(1), 15. https://doi.org/10.3390/microorganisms12010015