Exploration of microRNA Biomarkers in Blood Small Extracellular Vesicles for Enzootic Bovine Leukosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Diagnosis of EBL
2.3. Hematology
2.4. Detection of Serum Antibodies against BLV
2.5. Detection of BLV Provirus by Nested PCR
2.6. Measurement of BLV Proviral Load by Quantitative Real-Time Polymerase Chain Reaction (qPCR)
2.7. LDH Analysis
2.8. Isolation and Characterization of Blood sEVs
2.9. Microarray Analysis
2.10. Quantification of Levels of miRNA Biomarker Candidates for EBL in sEVs by qPCR
2.11. Validation of miRNA Biomarker Candidates for EBL in Blood sEVs
2.12. Statistical Analysis
3. Results
3.1. BLV Infection and Clinical Status
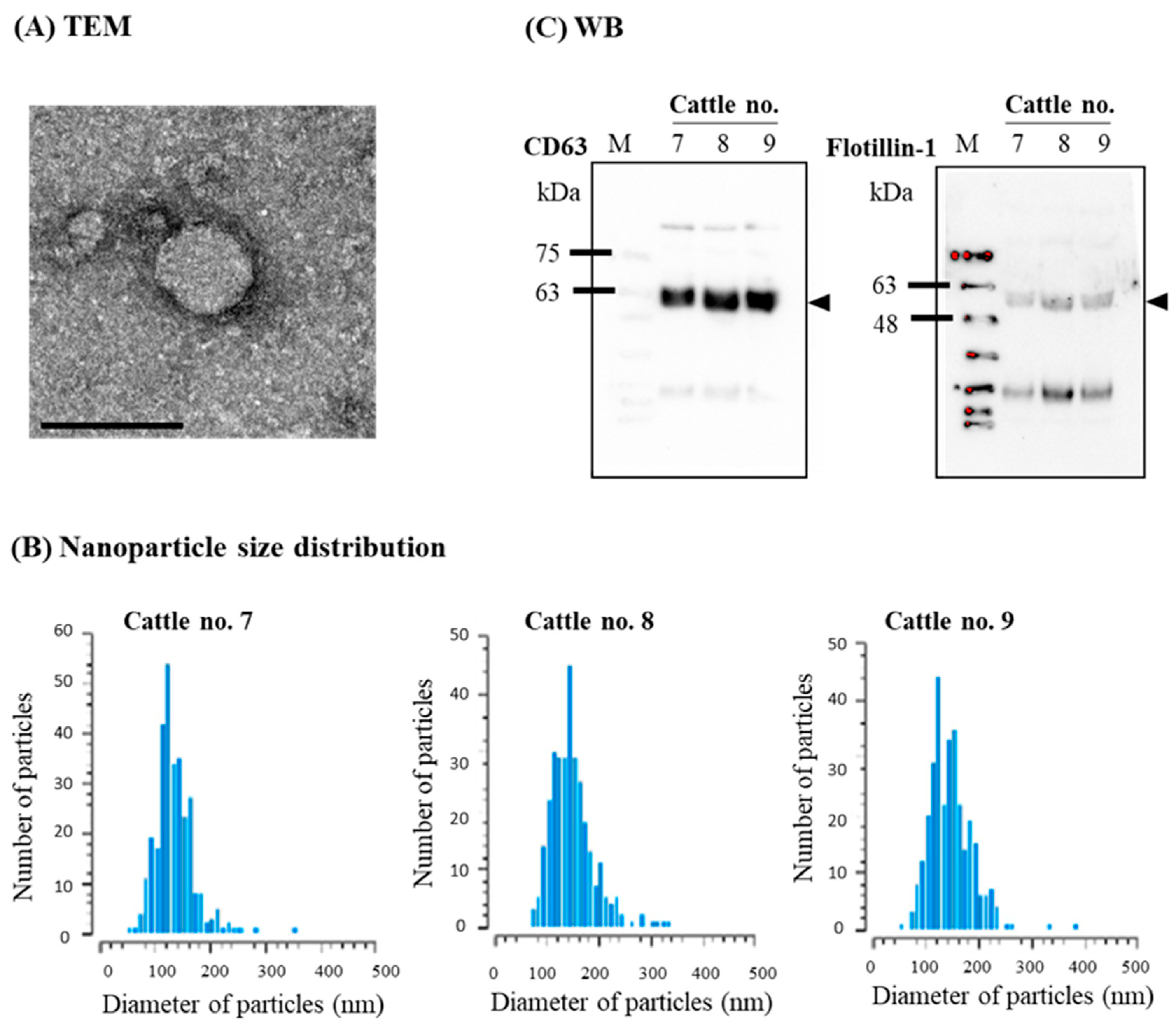
3.2. Confirmation of sEVs
3.3. Microarray Analysis
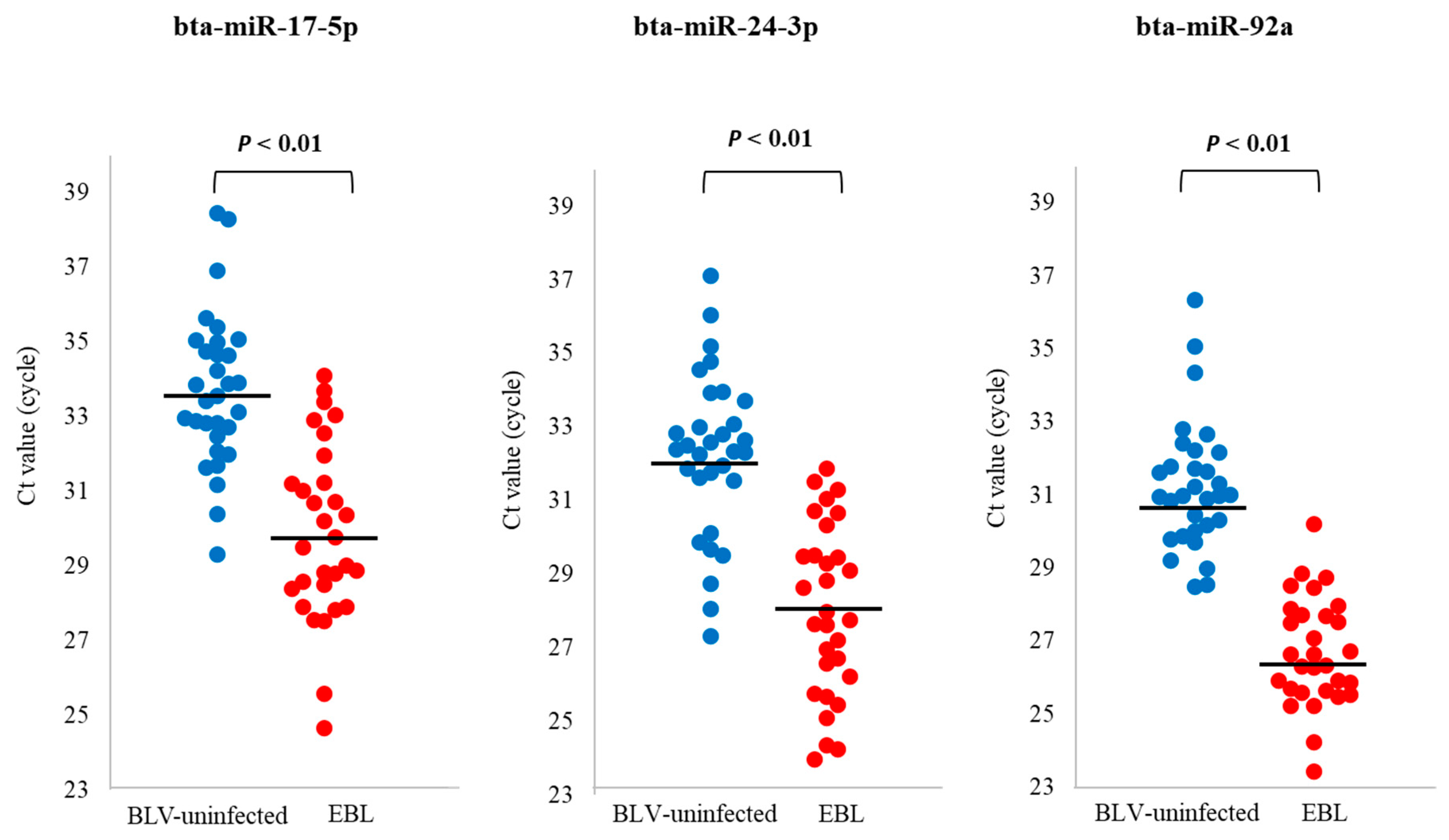
3.4. qPCR for Detection of miRNA Biomarker Candidates for EBL in sEVs
3.5. qPCR for Evaluation of the Utility of miRNA Biomarker Candidates for EBL
3.6. Correlations between Levels of miRNA Biomarker Candidates for EBL in sEVs and Several Diagnostic Criteria of EBL
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodríguez, S.M.; Florins, A.; Gillet, N.; De Brogniez, A.; Sánchez-Alcaraz, M.T.; Boxus, M.; Boulanger, F.; Gutiérrez, G.; Trono, K.; Alvarez, I.; et al. Preventive and Therapeutic Strategies for Bovine Leukemia Virus: Lessons for HTLV. Viruses 2011, 3, 1210–1248. [Google Scholar] [CrossRef] [PubMed]
- Gillet, N.; Florins, A.; Boxus, M.; Burteau, C.; Nigro, A.; Vandermeers, F.; Balon, H.; Bouzar, A.-B.; Defoiche, J.; Burny, A.; et al. Mechanisms of leukemogenesis induced by bovine leukemia virus: Prospects for novel anti-retroviral therapies in human. Retrovirology 2007, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Burny, A.; Cleuter, Y.; Kettmann, R.; Mammerickx, M.; Marbaix, G.; Portetelle, D.; Broeke, A.V.D.; Willems, L.; Thomas, R. Bovine leukaemia: Facts and hypotheses derived from the study of an infectious cancer. Veter-Microbiol. 1988, 17, 197–218. [Google Scholar] [CrossRef]
- Ministry of Agriculture, Forestry and Fisheries, Japan. Available online: https://www.maff.go.jp/j/keiei/nogyohoken/attach/pdf/kokujituuchi-182.pdf (accessed on 5 October 2022).
- Murakami, K.; Kobayashi, S.; Konishi, M.; Kameyama, K.; Tsutsui, T. Nationwide survey of bovine leukemia virus infection among dairy and beef breeding cattle in Japan from 2009−2011. J. Vet. Med. Sci. 2013, 75, 1123–1126. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture, Forestry and Fisheries, Japan. Available online: https://www.maff.go.jp/j/syouan/douei/kansi_densen/attach/pdf/kansi_densen-27.pdf (accessed on 11 October 2022).
- Kobayashi, T.; Inagaki, Y.; Ohnuki, N.; Sato, R.; Murakami, S.; Imakawa, K. Increasing Bovine leukemia virus (BLV) proviral load is a risk factor for progression of Enzootic bovine leucosis: A prospective study in Japan. Prev. Vet. Med. 2019, 178, 104680. [Google Scholar] [CrossRef]
- Miura, S.; Inokuma, H. Evaluation of lactate dehydrogenase activity as an onset marker for enzootic bovine leukosis. Jpn. J. Large Anim. Clin. 2016, 6, 149–153. [Google Scholar] [CrossRef]
- Tawfeeq, M.M.; Miura, S.; Horiuchi, N.; Kobayashi, Y.; Furuoka, H.; Inokuma, H. Utility of Serum Thymidine Kinase Activity Measurements for Cases of Bovine Leukosis with Difficult Clinical Diagnoses. J. Vet. Med. Sci. 2013, 75, 1167–1172. [Google Scholar] [CrossRef][Green Version]
- Simons, M.; Raposo, G. Exosomes—Vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 2009, 21, 575–581. [Google Scholar] [CrossRef]
- Conde-Vancells, J.; Rodriguez-Suarez, E.; Embade, N.; Gil, D.; Matthiesen, R.; Valle, M.; Elortza, F.; Lu, S.C.; Mato, J.M.; Falcon-Perez, J.M. Characterization and Comprehensive Proteome Profiling of Exosomes Secreted by Hepatocytes. J. Proteome Res. 2008, 7, 5157–5166. [Google Scholar] [CrossRef]
- Kosaka, N.; Iguchi, H.; Ochiya, T. Circulating microRNA in body fluid: A new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010, 101, 2087–2092. [Google Scholar] [CrossRef]
- Pisitkun, T.; Shen, R.F.; Knepper, M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar] [CrossRef]
- Bard, M.P.; Hegmans, J.P.; Hemmes, A.; Luider, T.M.; Willemsen, R.; Severijnen, L.-A.A.; van Meerbeeck, J.P.; Burgers, S.A.; Hoogsteden, H.C.; Lambrecht, B.N. Proteomic Analysis of Exosomes Isolated from Human Malignant Pleural Effusions. Am. J. Respir. Cell Mol. Biol. 2004, 31, 114–121. [Google Scholar] [CrossRef]
- Hu, Y.; Thaler, J.; Nieuwland, R. Extracellular Vesicles in Human Milk. Pharmaceuticals 2021, 14, 1050. [Google Scholar] [CrossRef]
- Sato-Kuwabara, Y.; Melo, S.A.; Soares, F.A.; Calin, G.A. The fusion of two worlds: Non-coding RNAs and extracellular vesicles—Diagnostic and therapeutic implications (Review). Int. J. Oncol. 2014, 46, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, S.; Yao, J.; Lowery, F.J.; Zhang, Q.; Huang, W.-C.; Li, P.; Li, M.; Wang, X.; Zhang, C.; et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nat. Cell. Biol. 2015, 17, 816–826. [Google Scholar] [CrossRef]
- Paggetti, J.; Haderk, F.; Seiffert, M.; Janji, B.; Distler, U.; Ammerlaan, W.; Kim, Y.J.; Adam, J.; Lichter, P.; Solary, E.; et al. Exosomes released by chronic lymphocytic leukemia cells induce the transition of stromal cells into cancer-associated fibroblasts. Blood 2015, 126, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Ali Syeda, Z.; Langden, S.S.S.; Munkhzul, C.; Lee, M.; Song, S.J. Regulatory Mechanism of MicroRNA Expression in Cancer. Int. J. Mol. Sci. 2020, 21, 1723. [Google Scholar] [CrossRef]
- Hussen, B.M.; Hidayat, H.J.; Salihi, A.; Sabir, D.K.; Taheri, M.; Ghafouri-Fard, S. MicroRNA: A signature for cancer progression. Biomed Pharmacother. 2021, 138, 111528. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yuan, T.; Liang, M.; Du, M.; Xia, S.; Dittmar, R.; Wang, D.; See, W.; Costello, B.A.; Quevedo, F.; et al. Exosomal miR-1290 and miR-375 as prognostic markers in castration-resistant prostate cancer. Eur. Urol. 2015, 67, 33–41. [Google Scholar] [CrossRef]
- Fu, F.; Jiang, W.; Zhou, L.; Chen, Z. Circulating Exosomal miR-17-5p and miR-92a-3p Predict Pathologic Stage and Grade of Colorectal Cancer. Transl. Oncol. 2018, 11, 221–232. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, W.; Wang, F.; Yang, S.; Hu, J.; Lu, B.; Pan, Z.; Ma, Y.; Zheng, M.; Zhou, L.; et al. Plasma-derived exosomal miR-15a-5p as a promising diagnostic biomarker for early detection of endometrial carcinoma. Mol. Cancer 2021, 20, 57. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Deshayes, L.; Guillemain, B.; Parodi, A.-L. Bovine leukemia virus specific antibodies among French cattle. I. Comparison of complement fixation and hematological tests. Int. J. Cancer 1977, 19, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Mammerickx, M.; Lorenz, R.J.; Straub, O.C.; Donnelly, W.J.; Flensburg, J.C.; Gentile, G.; Markson, L.M.; Ressang, A.; Taylor, S.M. Bovine hematology. III. Comparative breed studies on the leukocyte parameters of several European cattle breeds as determined in the common reference laboratory. Zentralblatt Veterinärmedizin Reihe B 1978, 25, 257–267. [Google Scholar]
- Murakami, K.; Okada, K.; Ikawa, Y.; Aida, Y. Bovine leukemia virus induces CD5-B cell lymphoma in sheep despite temporarily increasing CD5+ B cells in asymptomatic stage. Virology 1994, 202, 458–465. [Google Scholar] [CrossRef]
- Fechner, H.; Blankensteina, P.; Looman, A.C.; Elwerta, J.; Geuec, L.; Albrechta, C.; Kurgd, A.; Beierc, D.; Marquardte, O.; Ebnera, D. Provirus Variants of the Bovine Leukemia Virus and Their Relation to the Serological Status of Naturally Infected Cattle. Virology 1997, 237, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Nakanishi, R.; Takashima, S.; Wakihara, Y.; Kamatari, Y.O.; Kitamura, Y.; Shimizu, K.; Okada, A.; Inoshima, Y. Comparing microRNA in milk small extracellular vesicles among healthy cattle and cattle at high risk for bovine leukemia virus transmission. J. Dairy Sci. 2022, 105, 5370–5380. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, F.; Takashima, S.; Wakihara, Y.; Kamatari, Y.O.; Shimizu, K.; Okada, A.; Inoshima, Y. Characterization of miRNAs in Milk Small Extracellular Vesicles from Enzootic Bovine Leukosis Cattle. Int. J. Mol. Sci. 2022, 23, 10782. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Ma, J.; Zhu, Y.; Zan, J.; Wang, Z.; Ling, L.; Li, Q.; Lv, J.; Qi, S.; Cao, Y.; et al. miR-24-3p promotes cell migration and proliferation in lung cancer by targeting SOX7. J. Cell. Biochem. 2018, 119, 3989–3998. [Google Scholar] [CrossRef]
- Iwamoto, H.; Kanda, Y.; Sejima, T.; Osaki, M.; Okada, F.; Takenaka, A. Serum miR-210 as a potential biomarker of early clear cell renal cell carcinoma. Int. J. Oncol. 2013, 44, 53–58. [Google Scholar] [CrossRef]
- Liu, S.; Chu, L.; Xie, M.; Ma, L.; An, H.; Zhang, W.; Deng, J. miR-92a-3p promoted EMT via targeting LATS1 in cervical cancer stem cells. Front. Cell Dev. Biol. 2021, 9, 757747. [Google Scholar] [CrossRef]
- Mogilyansky, E.; Rigoutsos, I. The miR-17/92 cluster: A comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ. 2013, 20, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Ota, A.; Tagawa, H.; Karnan, S.; Tsuzuki, S.; Karpas, A.; Kira, S.; Yoshida, Y.; Seto, M. Identification and Characterization of a Novel Gene, C13orf25, as a Target for 13q31-q32 Amplification in Malignant Lymphoma. Cancer Res. 2004, 64, 3087–3095. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Thomson, J.M.; Hemann, M.T.; Hernando-Monge, E.; Mu, D.; Goodson, S.; Powers, S.; Cordon-Cardo, C.; Lowe, S.W.; Hannon, G.J.; et al. A microRNA polycistron as a potential human oncogene. Nature 2005, 435, 828–833. [Google Scholar] [CrossRef]
- Ventura, A.; Young, A.G.; Winslow, M.M.; Lintault, L.; Meissner, A.; Erkeland, S.J.; Newman, J.; Bronson, R.T.; Crowley, D.; Stone, J.R.; et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell 2008, 132, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Srinivasan, L.; Calado, D.P.; Patterson, H.C.; Zhang, B.; Wang, J.; Henderson, J.; Kutok, J.; Rajewsky, K. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat. Immunol. 2009, 9, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Inomata, M.; Tagawa, H.; Guo, Y.-M.; Kameoka, Y.; Takahashi, N.; Sawada, K. MicroRNA-17-92 down-regulates expression of distinct targets in different B-cell lymphoma subtypes. Blood 2009, 113, 396–402. [Google Scholar] [CrossRef]
- O’Donnell, K.A.; Wentzel, E.A.; Zeller, K.I.; Dang, C.V.; Mendell, J.T. c-Myc-regulated microRNAs modulate E2F1 expression. Nature 2005, 435, 839–843. [Google Scholar] [CrossRef]
- Yuan, Y.; Kluiver, J.; Koerts, J.; de Jong, D.; Rutgers, B.; Razak, R.A.; Terpstra, M.; Plaat, B.; Nolte, I.; Diepstra, A.; et al. miR-24-3p is overexpressed in Hodgkin lymphoma and protects Hodgkin and reed-sternberg cells from apoptosis. Am. J. Pathol. 2017, 187, 1343–1355. [Google Scholar] [CrossRef]
- Wang, J.; Yin, K.; Lv, X.; Yang, Q.; Shao, M.; Liu, X.; Sun, H. MicroRNA-24-3p regulates Hodgkin’s lymphoma cell proliferation, migration and invasion by targeting DEDD. Oncol. Lett. 2019, 17, 365–371. [Google Scholar] [CrossRef]
- Whiteside, T.L. Exosome and mesenchymal stem cell cross-talk in the tumor microenvironment. Semin. Immunol. 2017, 35, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, G.; Rodríguez, S.M.; De Brogniez, A.; Gillet, N.; Golime, R.; Burny, A.; Jaworski, J.-P.; Alvarez, I.; Vagnoni, L.; Trono, K.; et al. Vaccination against δ-Retroviruses: The Bovine Leukemia Virus Paradigm. Viruses 2014, 6, 2416–2427. [Google Scholar] [CrossRef] [PubMed]
- Maezawa, M.; Kayou, S.; Miura, S.; Oguma, K.; Sentsui, H.; Horiuchi, N.; Kobayashi, Y.; Furuoka, H.; Inokuma, H. A clinical case of enzootic bovine leukosis with BLV-provirus monoclonal integration in 21-month-old Japanese Black beef cattle. Jpn. J. Large Anim. Clin. 2016, 6, 161–164. [Google Scholar] [CrossRef]
- Oguma, K.; Suzuki, M.; Sentsui, H. Enzootic bovine leukosis in a two-month-old calf. Virus Res. 2017, 233, 120–124. [Google Scholar] [CrossRef]
- Nishimori, A.; Andoh, K.; Matsuura, Y.; Kumagai, A.; Hatama, S. Establishment of a simplified inverse polymerase chain reaction method for diagnosis of enzootic bovine leukosis. Arch. Virol. 2021, 166, 841–851. [Google Scholar] [CrossRef]
- Bendixen, H.J. Preventive measures in cattle leukemia: Leukosis enzootica bovis. Ann. N. Y. Acad. Sci. 1963, 108, 1241–1267. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takada, A.; Kamatari, Y.O.; Shimizu, K.; Okada, A.; Inoshima, Y. Exploration of microRNA Biomarkers in Blood Small Extracellular Vesicles for Enzootic Bovine Leukosis. Microorganisms 2023, 11, 2173. https://doi.org/10.3390/microorganisms11092173
Takada A, Kamatari YO, Shimizu K, Okada A, Inoshima Y. Exploration of microRNA Biomarkers in Blood Small Extracellular Vesicles for Enzootic Bovine Leukosis. Microorganisms. 2023; 11(9):2173. https://doi.org/10.3390/microorganisms11092173
Chicago/Turabian StyleTakada, Akane, Yuji O. Kamatari, Kaori Shimizu, Ayaka Okada, and Yasuo Inoshima. 2023. "Exploration of microRNA Biomarkers in Blood Small Extracellular Vesicles for Enzootic Bovine Leukosis" Microorganisms 11, no. 9: 2173. https://doi.org/10.3390/microorganisms11092173
APA StyleTakada, A., Kamatari, Y. O., Shimizu, K., Okada, A., & Inoshima, Y. (2023). Exploration of microRNA Biomarkers in Blood Small Extracellular Vesicles for Enzootic Bovine Leukosis. Microorganisms, 11(9), 2173. https://doi.org/10.3390/microorganisms11092173






