Association between the Respiratory Microbiome and Plasma Microbial Extracellular Vesicles in Intubated Patients
Abstract
:1. Introduction
2. Materials and Methods
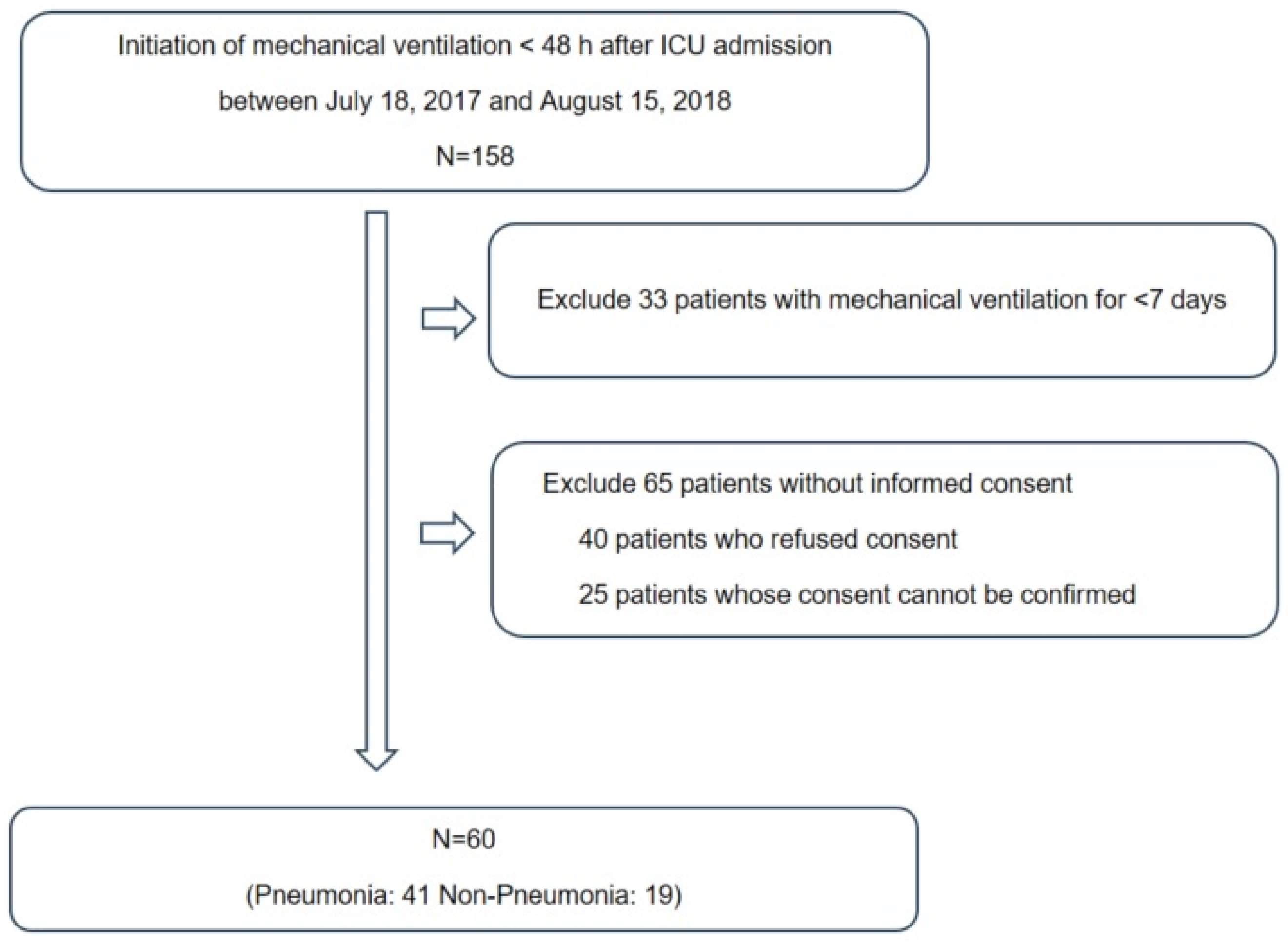
2.1. Study Subjects
2.2. EV Isolation and DNA Extraction
2.3. Paired-End Reads Sequencing and Data Processing
2.4. Statistical Analysis
3. Results
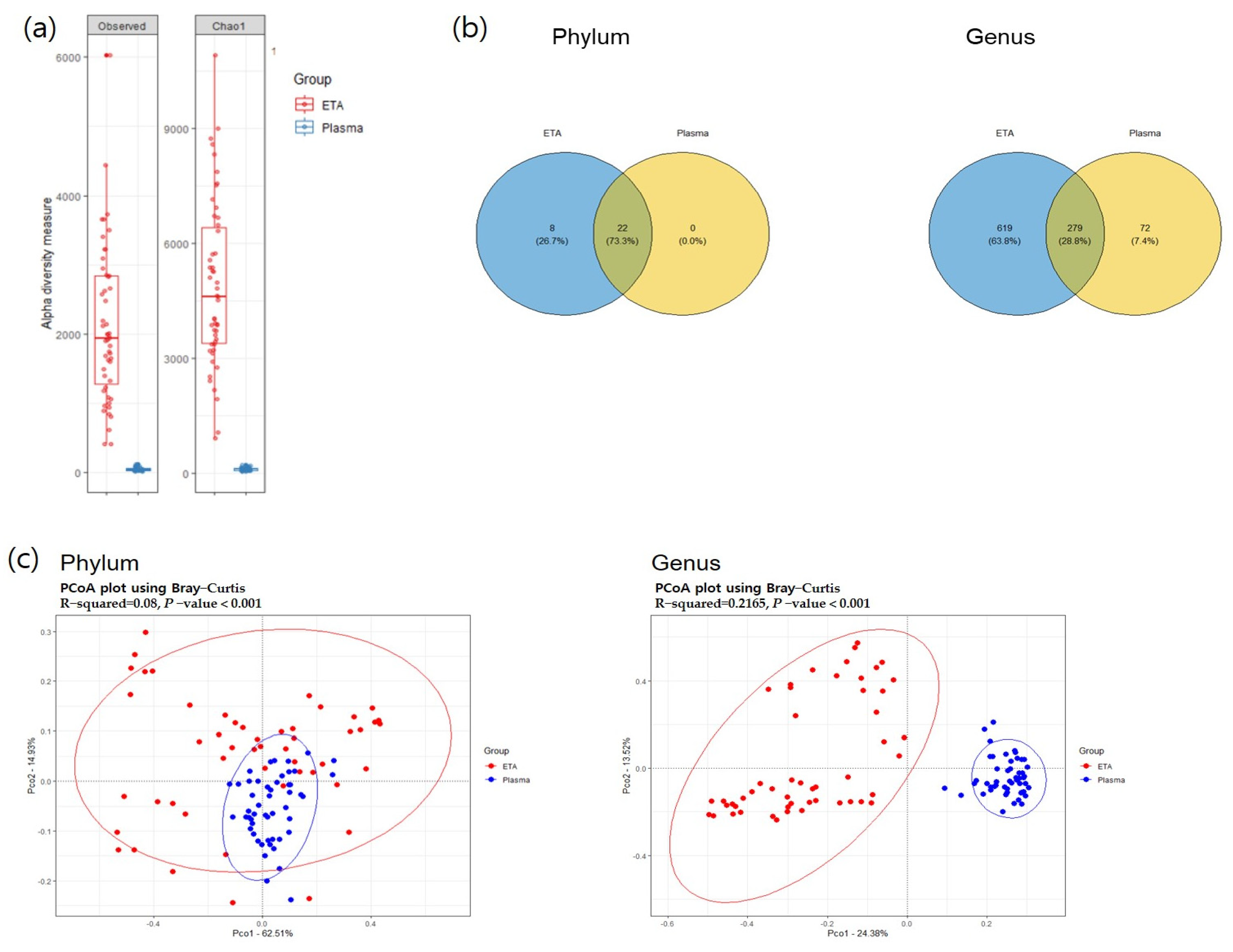
3.1. Microbial Diversity between Respiratory Microbiome and Plasma Microbial EVs
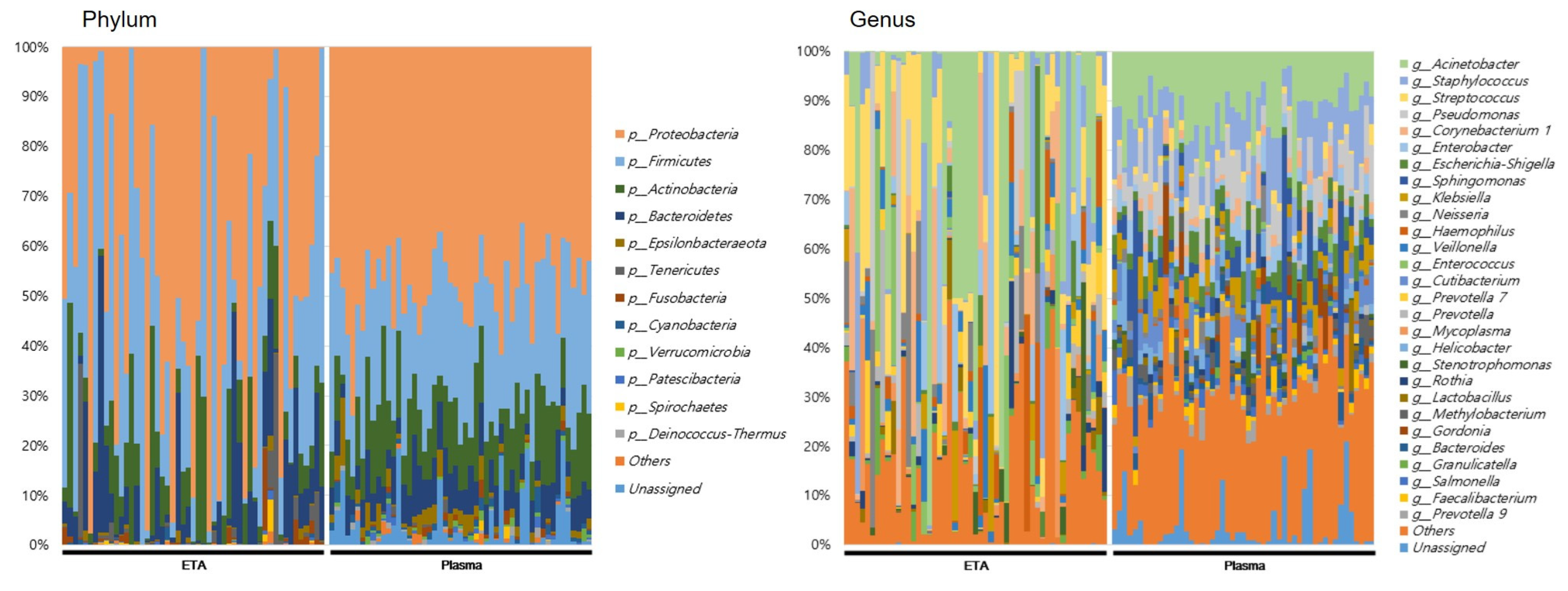
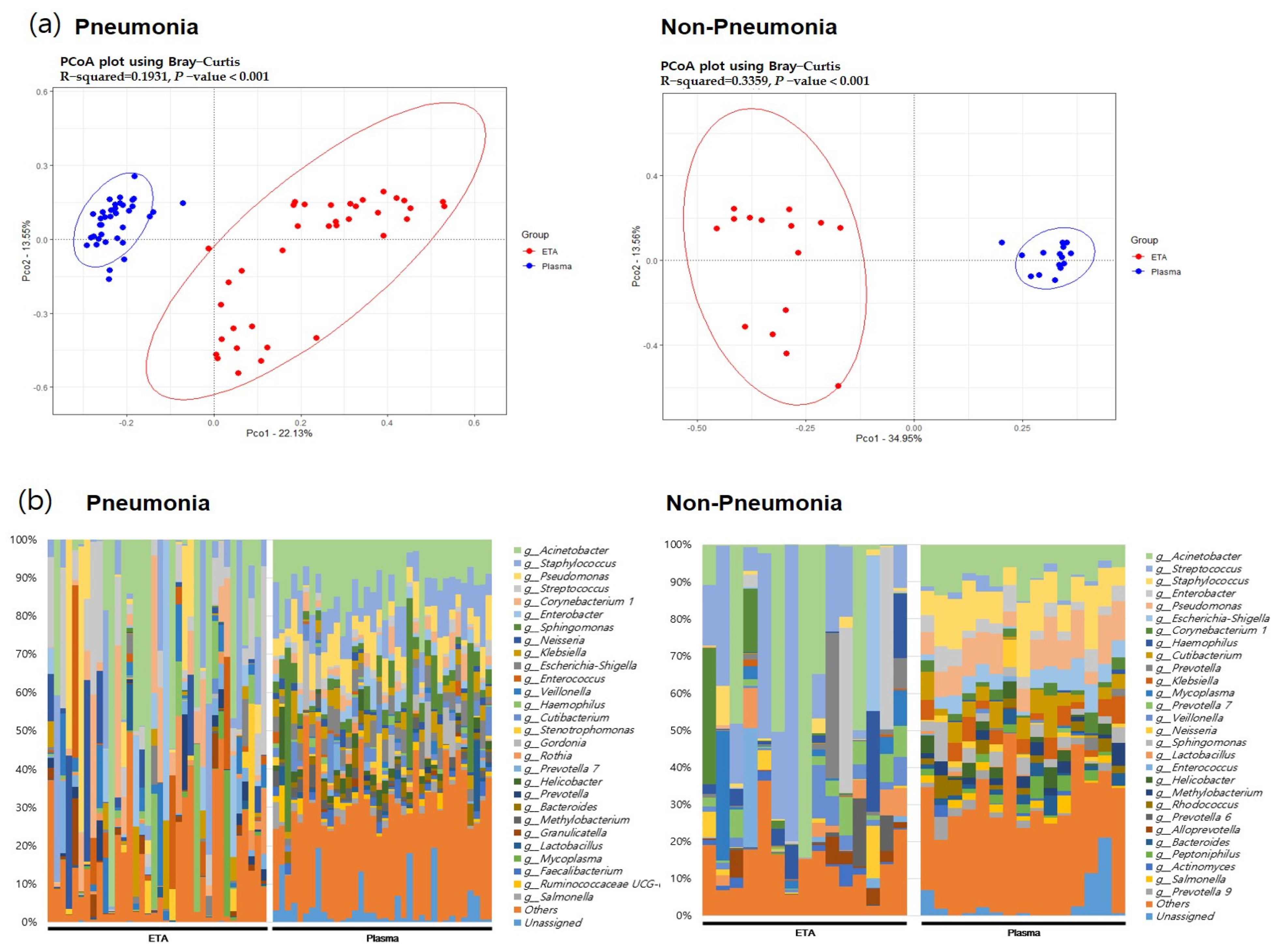
3.2. The 16S rRNA Microbiome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef]
- Fujita, Y.; Kadota, T.; Araya, J.; Ochiya, T.; Kuwano, K. Extracellular Vesicles: New Players in Lung Immunity. Am. J. Respir. Cell Mol. Biol. 2018, 58, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Schorey, J.S.; Cheng, Y.; Singh, P.P.; Smith, V.L. Exosomes and other extracellular vesicles in host–pathogen interactions. EMBO Rep. 2014, 16, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Zhang, D.; Zhu, Z.; Cruz, C.S.D.; Jin, Y. Epithelial cell-derived microvesicles activate macrophages and promote inflammation via microvesicle-containing microRNAs. Sci. Rep. 2016, 6, 35250. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Abston, E.; Zhang, D.; Rai, A.; Jin, Y. Extracellular Vesicle: An Emerging Mediator of Intercellular Crosstalk in Lung Inflammation and Injury. Front. Immunol. 2018, 9, 924. [Google Scholar] [CrossRef] [PubMed]
- Behrens, F.; Funk-Hilsdorf, T.C.; Kuebler, W.M.; Simmons, S. Bacterial Membrane Vesicles in Pneumonia: From Mediators of Virulence to Innovative Vaccine Candidates. Int. J. Mol. Sci. 2021, 22, 3858. [Google Scholar] [CrossRef]
- Nah, G.; Park, S.C.; Kim, K.; Kim, S.; Park, J.; Lee, S.; Won, S. Type-2 Diabetics Reduces Spatial Variation of Microbiome Based on Extracellur Vesicles from Gut Microbes across Human Body. Sci. Rep. 2019, 9, 20136. [Google Scholar] [CrossRef]
- Shukla, S.D.; Budden, K.F.; Neal, R.; Hansbro, P.M. Microbiome effects on immunity, health and disease in the lung. Clin. Transl. Immunol. 2017, 6, e133. [Google Scholar] [CrossRef] [PubMed]
- Budden, K.F.; Gellatly, S.L.; Wood, D.L.A.; Cooper, M.A.; Morrison, M.; Hugenholtz, P.; Hansbro, P.M. Emerging pathogenic links between microbiota and the gut–lung axis. Nat. Rev. Microbiol. 2016, 15, 55–63. [Google Scholar] [CrossRef]
- Romano, M. Gut Microbiota as a Trigger of Accelerated Directional Adaptive Evolution: Acquisition of Herbivory in the Context of Extracellular Vesicles, MicroRNAs and Inter-Kingdom Crosstalk. Front. Microbiol. 2017, 8, 721. [Google Scholar] [CrossRef]
- Choi, Y.; Kwon, Y.; Kim, D.-K.; Jeon, J.; Jang, S.C.; Wang, T.; Ban, M.; Jeon, S.G.; Kim, M.-S.; Choi, C.S.; et al. Gut microbe-derived extracellular vesicles induce insulin resistance, thereby impairing glucose metabolism in skeletal muscle. Sci. Rep. 2015, 5, 15878. [Google Scholar] [CrossRef] [PubMed]
- Metlay, J.P.; Kapoor, W.N.; Fine, M.J. Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA 1997, 278, 1440–1445. [Google Scholar] [CrossRef]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratala, J.; et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef]
- Kim, M.-R.; Hong, S.-W.; Choi, E.-B.; Lee, W.-H.; Jeon, S.G.; Jang, M.H.; Gho, Y.S.; Kim, Y.-K. Staphylococcus aureus-derived extracellular vesicles induce neutrophilic pulmonary inflammation via both Th1 and Th17 cell responses. Allergy 2012, 67, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Park, H.; Park, H.S.; Kim, Y.K. Extracellular Vesicles, a Key Mediator to Link Environmental Microbiota to Airway Immunity. Allergy Asthma Immunol. Res. 2017, 9, 101–106. [Google Scholar] [CrossRef] [PubMed]
- The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gao, H.; A Mihindukulasuriya, K.; La Rosa, P.S.; Wylie, K.M.; Vishnivetskaya, T.; Podar, M.; Warner, B.; Tarr, P.I.; Nelson, D.E.; et al. Biogeography of the ecosystems of the healthy human body. Genome Biol. 2013, 14, R1. [Google Scholar] [CrossRef]
- Matthay, M.A.; Zemans, R.L. The Acute Respiratory Distress Syndrome: Pathogenesis and Treatment. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 147–163. [Google Scholar] [CrossRef]
- Muraca, M.; Putignani, L.; Fierabracci, A.; Teti, A.; Perilongo, G. Gut microbiota-derived outer membrane vesicles: Under-recognized major players in health and disease? Discov. Med. 2015, 19, 343–348. [Google Scholar]
- Daou, Y.; Falabrègue, M.; Pourzand, C.; Peyssonnaux, C.; Edeas, M. Host and microbiota derived extracellular vesicles: Crucial players in iron homeostasis. Front. Med. 2022, 9, 985141. [Google Scholar] [CrossRef] [PubMed]
- Codemo, M.; Muschiol, S.; Iovino, F.; Nannapaneni, P.; Plant, L.; Wai, S.N.; Henriques-Normark, B. Immunomodulatory Effects of Pneumococcal Extracellular Vesicles on Cellular and Humoral Host Defenses. mBio 2018, 9, e00559-18. [Google Scholar] [CrossRef] [PubMed]
- Tiku, V.; Kofoed, E.M.; Yan, D.; Kang, J.; Xu, M.; Reichelt, M.; Dikic, I.; Tan, M.-W. Outer membrane vesicles containing OmpA induce mitochondrial fragmentation to promote pathogenesis of Acinetobacter baumannii. Sci. Rep. 2021, 11, 618. [Google Scholar] [CrossRef] [PubMed]
- Athman, J.J.; Sande, O.J.; Groft, S.G.; Reba, S.M.; Nagy, N.; Wearsch, P.A.; Richardson, E.T.; Rojas, R.; Boom, W.H.; Shukla, S.; et al. Mycobacterium tuberculosis Membrane Vesicles Inhibit T Cell Activation. J. Immunol. 2017, 198, 2028–2037. [Google Scholar] [CrossRef] [PubMed]
- Vidakovics, M.L.A.P.; Jendholm, J.; Mörgelin, M.; Månsson, A.; Larsson, C.; Cardell, L.-O.; Riesbeck, K. B Cell Activation by Outer Membrane Vesicles—A Novel Virulence Mechanism. PLOS Pathog. 2010, 6, e1000724. [Google Scholar] [CrossRef] [PubMed]
- Asensio, C.J.; Gaillard, M.E.; Moreno, G.; Bottero, D.; Zurita, E.; Rumbo, M.; van der Ley, P.; van der Ark, A.; Hozbor, D. Outer membrane vesicles obtained from Bordetella pertussis Tohama expressing the lipid A deacylase PagL as a novel acellular vaccine candidate. Vaccine 2011, 29, 1649–1656. [Google Scholar] [CrossRef]
- Olaya-Abril, A.; Prados-Rosales, R.; McConnell, M.J.; Martín-Peña, R.; González-Reyes, J.A.; Jiménez-Munguía, I.; Gómez-Gascón, L.; Fernández, J.; Luque-García, J.L.; García-Lidón, C.; et al. Characterization of protective extracellular membrane-derived vesicles produced by Streptococcus pneumoniae. J. Proteom. 2014, 106, 46–60. [Google Scholar] [CrossRef]
- Lee, W.-H.; Choi, H.-I.; Hong, S.-W.; Kim, K.-S.; Gho, Y.S.; Jeon, S.G. Vaccination with Klebsiella pneumoniae-derived extracellular vesicles protects against bacteria-induced lethality via both humoral and cellular immunity. Exp. Mol. Med. 2015, 47, e183. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Q.; Li, W.; Chen, Y.; Shu, C.; Li, Q.; Zhou, J.; Ye, C.; Bai, H.; Sun, W.; et al. Anti-outer Membrane Vesicle Antibodies Increase Antibiotic Sensitivity of Pan-Drug-Resistant Acinetobacter baumannii. Front. Microbiol. 2019, 10, 1379. [Google Scholar] [CrossRef]
- Pulido, M.R.; García-Quintanilla, M.; Pachón, J.; McConnell, M.J. A lipopolysaccharide-free outer membrane vesicle vaccine protects against Acinetobacter baumannii infection. Vaccine 2019, 38, 719–724. [Google Scholar] [CrossRef]
- Huang, W.; Yao, Y.; Long, Q.; Yang, X.; Sun, W.; Liu, C.; Jin, X.; Li, Y.; Chu, X.; Chen, B.; et al. Immunization against Multidrug-Resistant Acinetobacter baumannii Effectively Protects Mice in both Pneumonia and Sepsis Models. PLoS ONE 2014, 9, e100727. [Google Scholar] [CrossRef]
- McConnell, M.J.; Rumbo, C.; Bou, G.; Pachón, J. Outer membrane vesicles as an acellular vaccine against Acinetobacter baumannii. Vaccine 2011, 29, 5705–5710. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Kesavan, D.K.; Cheng, J.; Vasudevan, A.; Wang, H.; Wan, J.; Abdelaziz, M.H.; Su, Z.; Wang, S.; Xu, H. Vesicle-Mediated Dendritic Cell Activation in Acinetobacter baumannii Clinical Isolate, which Contributes to Th2 Response. J. Immunol. Res. 2019, 2019, 2835256. [Google Scholar] [CrossRef] [PubMed]
- Dominguez Rubio, A.P.; D’Antoni, C.L.; Piuri, M.; Perez, O.E. Probiotics, Their Extracellular Vesicles and Infectious Diseases. Front. Microbiol. 2022, 13, 864720. [Google Scholar] [CrossRef] [PubMed]
- Al-Nedawi, K.; Mian, M.F.; Hossain, N.; Karimi, K.; Mao, Y.-K.; Forsythe, P.; Min, K.K.; Stanisz, A.M.; Kunze, W.A.; Bienenstock, J. Gut commensal microvesicles reproduce parent bacterial signals to host immune and enteric nervous systems. FASEB J. 2015, 29, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lee, K.; Hsu, M.; Nau, G.; Mylonakis, E.; Ramratnam, B. Lactobacillus-derived extracellular vesicles enhance host immune responses against vancomycin-resistant enterococci. BMC Microbiol. 2017, 17, 66. [Google Scholar] [CrossRef]
- Seo, M.; Park, E.; Ko, S.; Choi, E.; Kim, S. Therapeutic effects of kefir grain Lactobacillus-derived extracellular vesicles in mice with 2,4,6-trinitrobenzene sulfonic acid-induced inflammatory bowel disease. J. Dairy Sci. 2018, 101, 8662–8671. [Google Scholar] [CrossRef]
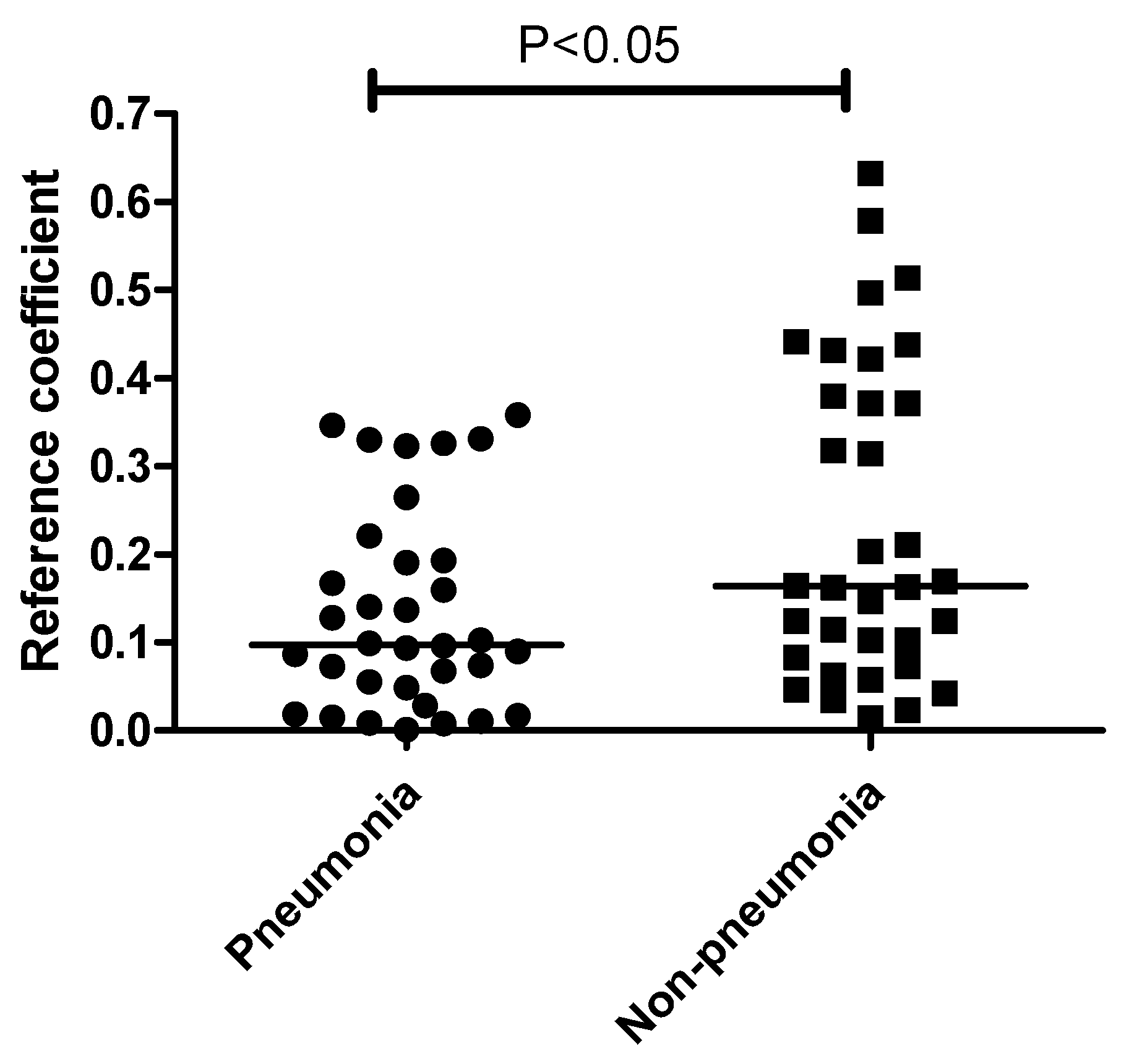
| Pneumonia | Non-pneumonia | p Value | |
|---|---|---|---|
| N = 41 | N = 19 | ||
| Age | 73 (59.5, 80.8) | 76 (59, 81) | 0.732 |
| Male | 30 (73.2%) | 13 (68.4%) | 0.763 |
| ARDS | 8 (19.5%) | 0 (0%) | 0.047 |
| Charlson Comorbidity Index | 3 (1, 4) | 2 (1, 2) | 0.051 |
| Reason for intubation | <0.001 | ||
| Cardiac arrest | 1 (2.4%) | 3 (15.8%) | |
| Neurosurgical condition | 5 (12.2%) | 14 (73.7%) | |
| Postoperative status | 0 (0%) | 1 (5.3%) | |
| Respiratory distress | 35 (85.4%) | 1 (5.3%) | |
| PaO2/FiO2 | 235.5 (143.8, 304.8) | 430.8 (321.0, 457.5) | <0.001 |
| Severity | |||
| APACHE score | 20 (16,24) | 22 (17.25) | 0.165 |
| SOFA score | 7 (5.8, 9.0) | 6 (4, 9) | 0.227 |
| GCS | 8 (6, 11) | 6 (5, 9) | 0.074 |
| 28-day mortality | 12 (29.3%) | 6 (31.6%) | 0.99 |
| In-hospital mortality | 19 (46.3%) | 6 (31.6%) | 0.4 |
| MV duration | 12 (7.8, 18.0) | 10 (7, 14) | 0.238 |
| CRP (mg/L) | 122.5 (44.0, 201.3) | 61 (4.6, 132.1) | 0.012 |
| Procalcitonin (ng/mL) | 0.59 (0.28, 3.32) | 0.23 (0.04, 0.46) | 0.012 |
| Site | Genus | Coefficient | FDR p-Value | |
|---|---|---|---|---|
| Total | ETA-serum EV | Acinetobacter | 0.368 | 0.008 |
| Corynebacterium | 0.326 | 0.019 | ||
| Neisseria | 0.288 | 0.041 | ||
| Bifidobacterium | 0.318 | 0.023 | ||
| Lautropia | 0.276 | 0.049 | ||
| Pneumonia | ETA-serum EV | Acinetobacter | 0.346 | 0.039 |
| Corynebacterium | 0.358 | 0.032 | ||
| Alloprevotella | −0.033 | 0.049 | ||
| Non-pneumonia | ETA-serum EV | Acinetobacter | 0.632 | 0.013 |
| Lactobacillus | −0.578 | 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Woo, S.J.; Hong, Y.; Lee, J.J.; Hong, J.Y. Association between the Respiratory Microbiome and Plasma Microbial Extracellular Vesicles in Intubated Patients. Microorganisms 2023, 11, 2128. https://doi.org/10.3390/microorganisms11092128
Park J, Woo SJ, Hong Y, Lee JJ, Hong JY. Association between the Respiratory Microbiome and Plasma Microbial Extracellular Vesicles in Intubated Patients. Microorganisms. 2023; 11(9):2128. https://doi.org/10.3390/microorganisms11092128
Chicago/Turabian StylePark, Jinkyeong, Seong Ji Woo, Yoonki Hong, Jae Jun Lee, and Ji Young Hong. 2023. "Association between the Respiratory Microbiome and Plasma Microbial Extracellular Vesicles in Intubated Patients" Microorganisms 11, no. 9: 2128. https://doi.org/10.3390/microorganisms11092128
APA StylePark, J., Woo, S. J., Hong, Y., Lee, J. J., & Hong, J. Y. (2023). Association between the Respiratory Microbiome and Plasma Microbial Extracellular Vesicles in Intubated Patients. Microorganisms, 11(9), 2128. https://doi.org/10.3390/microorganisms11092128






