Genetic Characterization of the Acidic and Neutral Glycosphingolipid Biosynthetic Pathways in Neurospora crassa
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
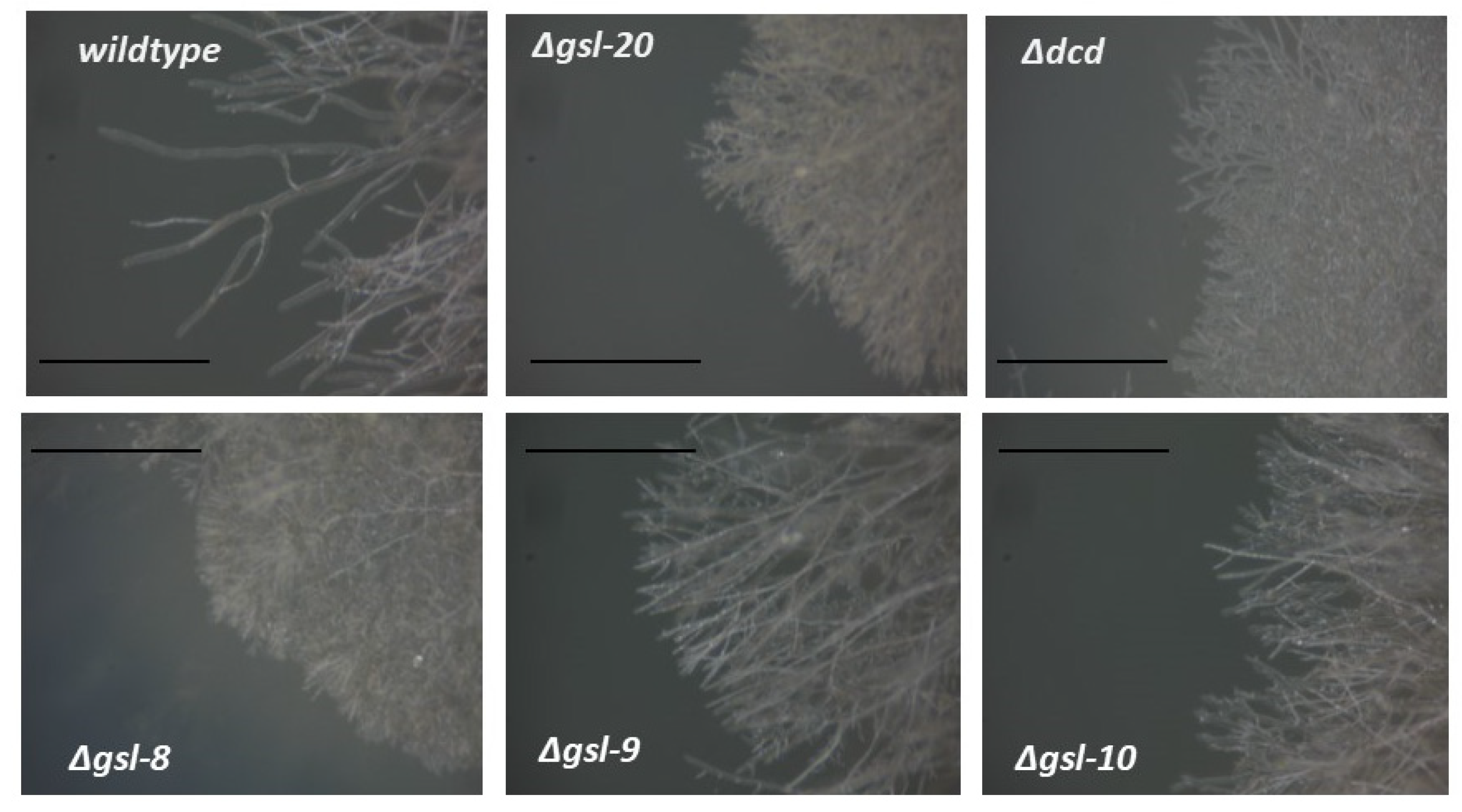
2.2. Vegetative Growth Rate Assay
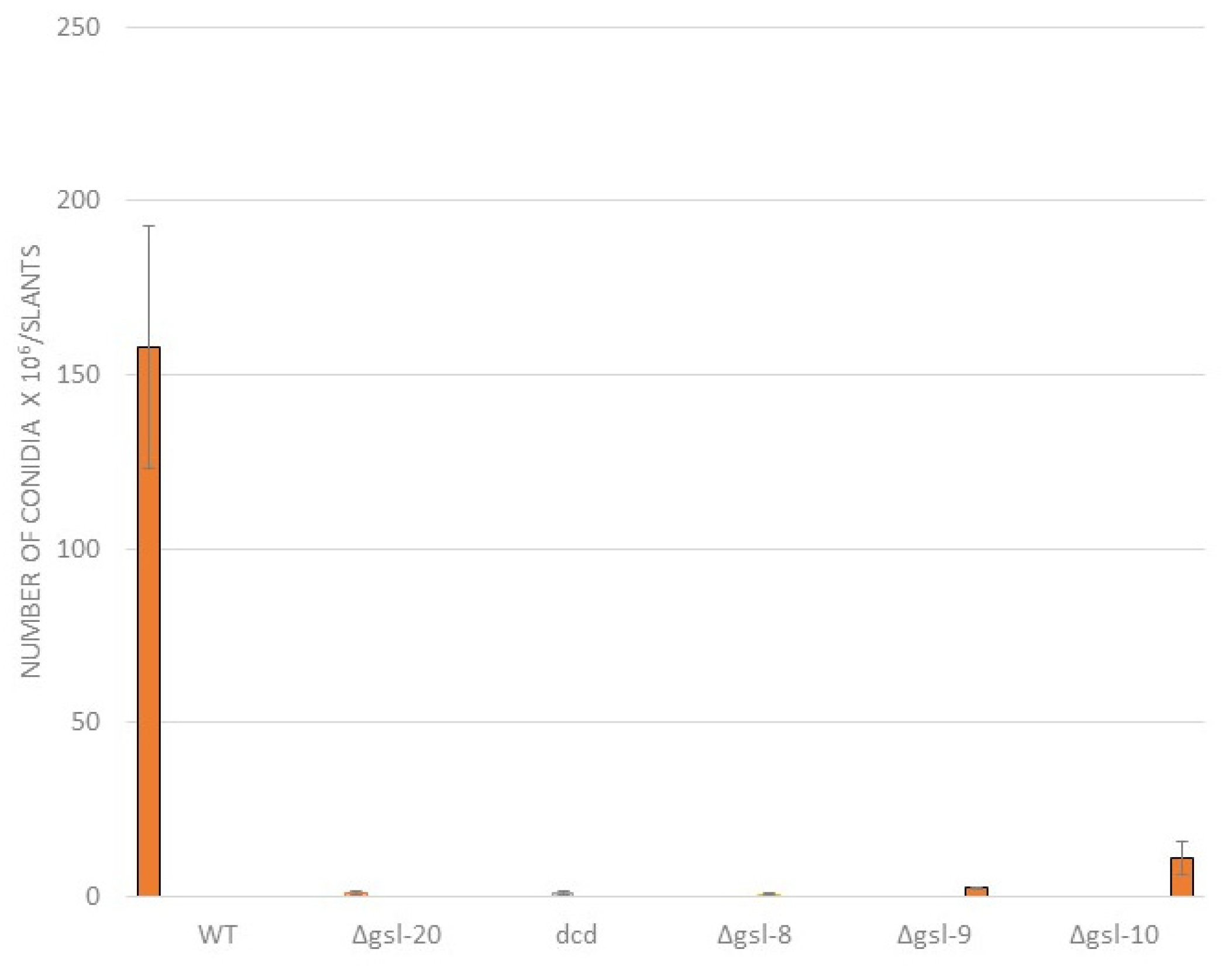
2.3. Conidia Production Assay
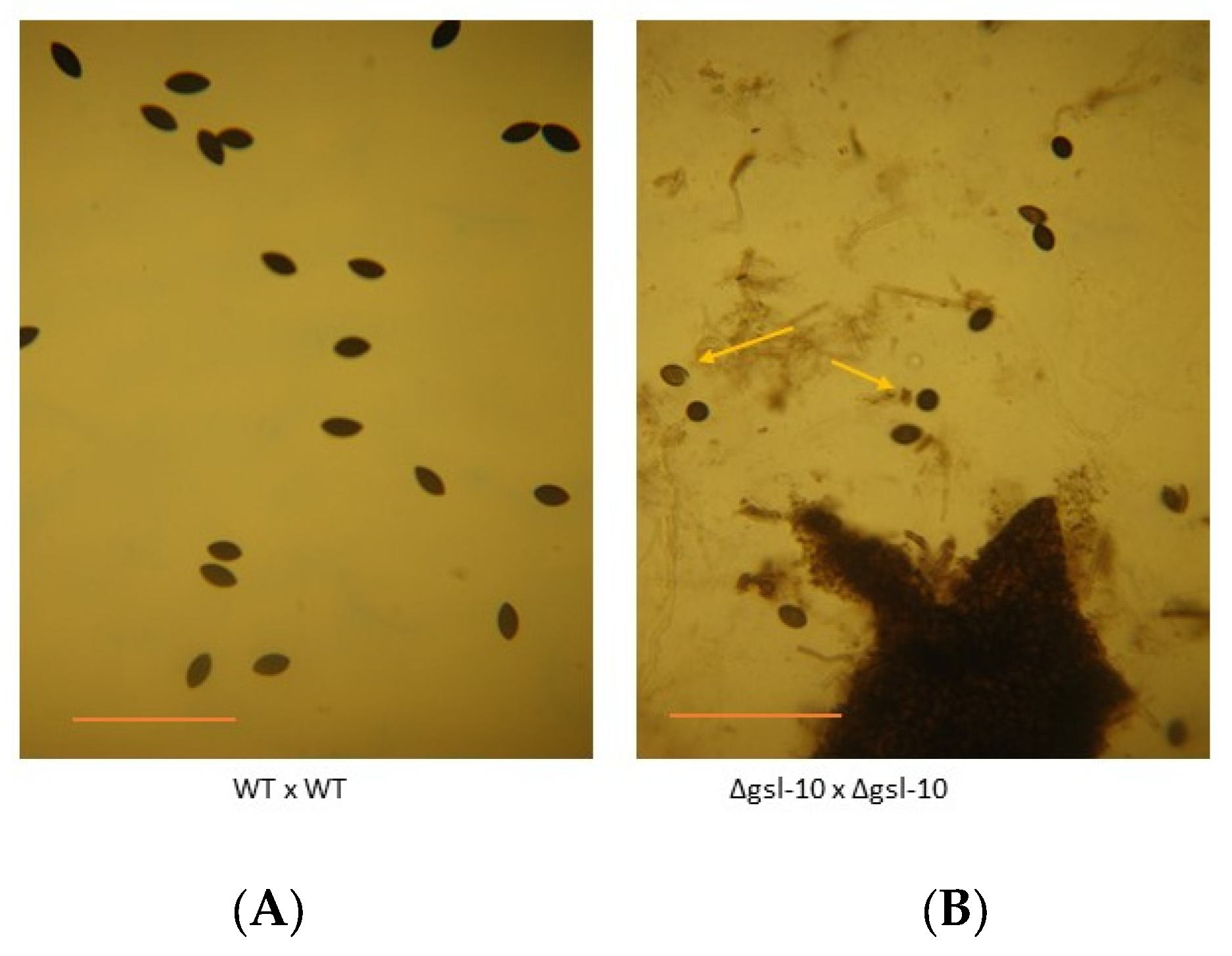
2.4. Ascospores Production Test
2.5. Characterization of Neutral GSLs
2.6. GC-MS Analysis of the Sugars Present in Purified Sphingolipid Samples
3. Results
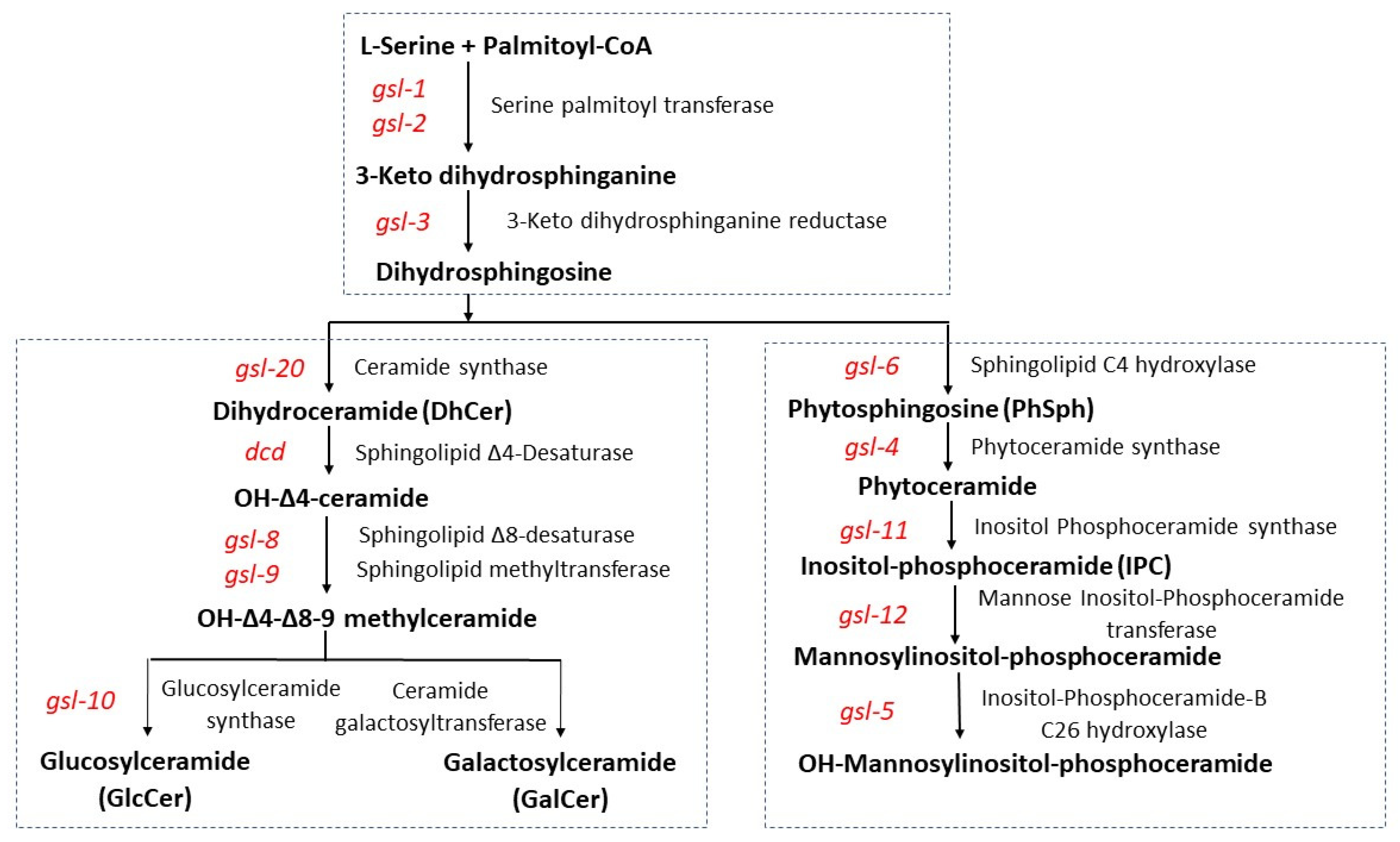
3.1. Genetic Characterization of the Entry Steps in Fungal GSL Biosynthesis
3.2. Genetic Characterization of the Acidic GSL Pathway
3.3. Genetic Characterization of the N. crassa Neutral GSL Pathway
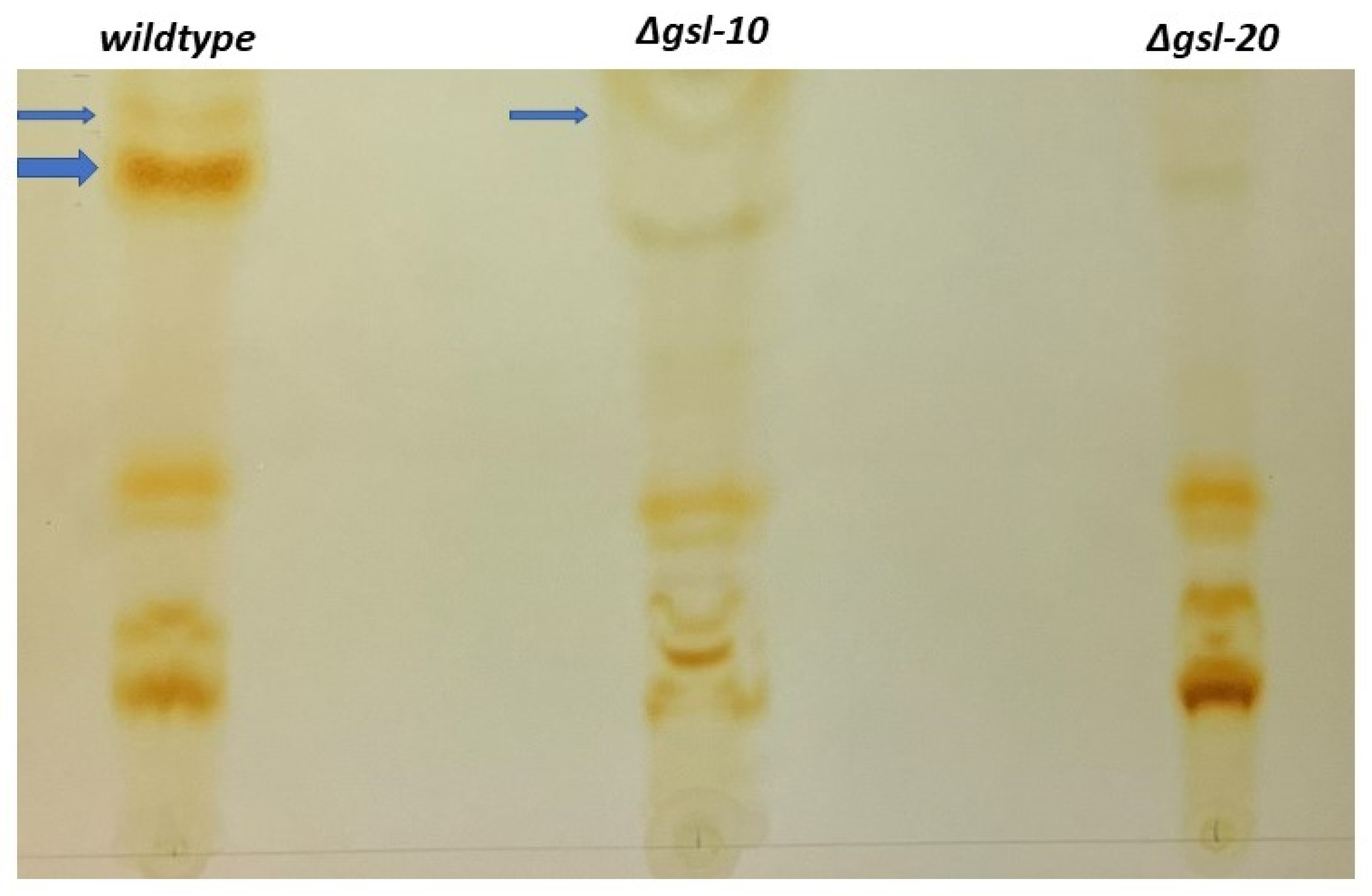
3.4. Characterization of Sphingolipids with TLC
3.5. Characterization of the Sphingolipids by GC-MS
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Guimaraes, L.L.; Toledo, M.S.; Ferreira, F.A.; Straus, A.H.; Takahashi, H.K. Structural diversity and biological significance of glycosphingolipids in pathogenic and opportunistic fungi. Front. Cell. Infect. Microbiol. 2014, 4, 138. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, T. Sphingolipids from the human fungal pathogen Aspergillus fumigatus. Biochimie 2017, 141, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Del Poeta, M.; Nimrichter, L.; Rodrigues, M.L.; Luberto, C. Synthesis and biological properties of fungal glucosylceramide. PLoS Pathog. 2014, 10, e1003832. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.M.; Goldman, G.H.; Del Poeta, M. Biological Roles Played by Sphingolipids in Dimorphic and Filamentous Fungi. mBio 2018, 9, e00642-18. [Google Scholar] [CrossRef] [PubMed]
- Montefusco, D.J.; Matmati, N.; Hannun, Y.A. The yeast sphingolipid signaling landscape. Chem. Phys. Lipids 2014, 177, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Warnecke, D.; Heinz, E. Recently discovered functions of glucosylceramides in plants and fungi. Cell. Mol. Life Sci. 2003, 60, 919–941. [Google Scholar] [CrossRef] [PubMed]
- Mor, V.; Rella, A.; Farnoud, A.M.; Singh, A.; Munshi, M.; Bryan, A.; Naseem, S.; Konopka, J.B.; Ojima, I.; Bullesbach, E.; et al. Identification of a New Class of Antifungals Targeting the Synthesis of Fungal Sphingolipids. mBio 2015, 6, e00647. [Google Scholar] [CrossRef] [PubMed]
- Mota Fernandes, C.; Del Poeta, M. Fungal sphingolipids: Role in the regulation of virulence and potential as targets for future antifungal therapies. Expert Rev. Anti-Infect. Ther. 2020, 18, 1083–1092. [Google Scholar] [CrossRef]
- McEvoy, K.; Normile, T.G.; Poeta, M.D. Antifungal Drug Development: Targeting the Fungal Sphingolipid Pathway. J. Fungi 2020, 6, 142. [Google Scholar] [CrossRef]
- Rollin-Pinheiro, R.; Singh, A.; Barreto-Bergter, E.; Del Poeta, M. Sphingolipids as targets for treatment of fungal infections. Future Med. Chem. 2016, 8, 1469–1484. [Google Scholar] [CrossRef]
- Lester, R.L.; Smith, S.W.; Wells, G.B.; Rees, D.C.; Angus, W.W. The isolation and partial characterization of two novel sphingolipids from Neurospora crassa: Di(inositolphosphoryl)ceramide and ((gal)3glu)ceramide. J. Biol. Chem. 1974, 249, 3388–3394. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Bennion, B.; Francois, I.E.; Ferket, K.K.; Cammue, B.P.; Thevissen, K.; Levery, S.B. Neutral glycolipids of the filamentous fungus Neurospora crassa: Altered expression in plant defensin-resistant mutants. J. Lipid Res. 2005, 46, 759–768. [Google Scholar] [CrossRef]
- Tani, Y.; Amaishi, Y.; Funatsu, T.; Ito, M.; Itonori, S.; Hata, Y.; Ashida, H.; Yamamoto, K. Structural analysis of cerebrosides from Aspergillus fungi: The existence of galactosylceramide in A. oryzae. Biotechnol. Lett. 2014, 36, 2507–2513. [Google Scholar] [CrossRef] [PubMed]
- Barreto-Bergter, E.; Sassaki, G.L.; de Souza, L.M. Structural analysis of fungal cerebrosides. Front. Microbiol. 2011, 2, 239. [Google Scholar] [CrossRef] [PubMed]
- Nagiec, M.M.; Baltisberger, J.A.; Wells, G.B.; Lester, R.L.; Dickson, R.C. The LCB2 gene of Saccharomyces and the related LCB1 gene encode subunits of serine palmitoyltransferase, the initial enzyme in sphingolipid synthesis. Proc. Natl. Acad. Sci. USA 1994, 91, 7899–7902. [Google Scholar] [CrossRef] [PubMed]
- Buede, R.; Rinker-Schaffer, C.; Pinto, W.J.; Lester, R.L.; Dickson, R.C. Cloning and characterization of LCB1, a Saccharomyces gene required for biosynthesis of the long-chain base component of sphingolipids. J. Bacteriol. 1991, 173, 4325–4332. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Beeler, T.; Dunn, T. Suppressors of the Ca(2+)-sensitive yeast mutant (csg2) identify genes involved in sphingolipid biosynthesis. Cloning and characterization of SCS1, a gene required for serine palmitoyltransferase activity. J. Biol. Chem. 1994, 269, 21480–21488. [Google Scholar] [CrossRef]
- Fornarotto, M.; Xiao, L.; Hou, Y.; Koch, K.A.; Chang, E.; O’Malley, R.M.; Black, T.A.; Cable, M.B.; Walker, S.S. Sphingolipid biosynthesis in pathogenic fungi: Identification and characterization of the 3-ketosphinganine reductase activity of Candida albicans and Aspergillus fumigatus. Biochim. Biophys. Acta 2006, 1761, 52–63. [Google Scholar] [CrossRef]
- Li, S.; Bao, D.; Yuen, G.; Harris, S.D.; Calvo, A.M. basA regulates cell wall organization and asexual/sexual sporulation ratio in Aspergillus nidulans. Genetics 2007, 176, 243–253. [Google Scholar] [CrossRef][Green Version]
- Haak, D.; Gable, K.; Beeler, T.; Dunn, T. Hydroxylation of Saccharomyces cerevisiae ceramides requires Sur2p and Scs7p. J. Biol. Chem. 1997, 272, 29704–29710. [Google Scholar] [CrossRef]
- Cheon, S.A.; Bal, J.; Song, Y.; Hwang, H.M.; Kim, A.R.; Kang, W.K.; Kang, H.A.; Hannibal-Bach, H.K.; Knudsen, J.; Ejsing, C.S.; et al. Distinct roles of two ceramide synthases, CaLag1p and CaLac1p, in the morphogenesis of Candida albicans. Mol. Microbiol. 2011, 83, 728–745. [Google Scholar] [CrossRef] [PubMed]
- Ternes, P.; Wobbe, T.; Schwarz, M.; Albrecht, S.; Feussner, K.; Riezman, I.; Cregg, J.M.; Heinz, E.; Riezman, H.; Feussner, I.; et al. Two pathways of sphingolipid biosynthesis are separated in the yeast Pichia pastoris. J. Biol. Chem. 2011, 286, 11401–11414. [Google Scholar] [CrossRef] [PubMed]
- Heidler, S.A.; Radding, J.A. Inositol phosphoryl transferases from human pathogenic fungi. Biochim. Biophys. Acta 2000, 1500, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Kotz, A.; Wagener, J.; Engel, J.; Routier, F.; Echtenacher, B.; Pich, A.; Rohde, M.; Hoffmann, P.; Heesemann, J.; Ebel, F. The mitA gene of Aspergillus fumigatus is required for mannosylation of inositol-phosphorylceramide, but is dispensable for pathogenicity. Fungal Genet. Biol. 2010, 47, 169–178. [Google Scholar] [CrossRef]
- Dunn, T.M.; Haak, D.; Monaghan, E.; Beeler, T.J. Synthesis of monohydroxylated inositolphosphorylceramide (IPC-C) in Saccharomyces cerevisiae requires Scs7p, a protein with both a cytochrome b5-like domain and a hydroxylase/desaturase domain. Yeast 1998, 14, 311–321. [Google Scholar] [CrossRef]
- Huber, A.; Oemer, G.; Malanovic, N.; Lohner, K.; Kovacs, L.; Salvenmoser, W.; Zschocke, J.; Keller, M.A.; Marx, F. Membrane Sphingolipids Regulate the Fitness and Antifungal Protein Susceptibility of Neurospora crassa. Front. Microbiol. 2019, 10, 605. [Google Scholar] [CrossRef] [PubMed]
- Rittenour, W.R.; Chen, M.; Cahoon, E.B.; Harris, S.D. Control of glucosylceramide production and morphogenesis by the Bar1 ceramide synthase in Fusarium graminearum. PLoS ONE 2011, 6, e19385. [Google Scholar] [CrossRef] [PubMed]
- Ternes, P.; Franke, S.; Zahringer, U.; Sperling, P.; Heinz, E. Identification and characterization of a sphingolipid delta 4-desaturase family. J. Biol. Chem. 2002, 277, 25512–25518. [Google Scholar] [CrossRef]
- Ternes, P.; Sperling, P.; Albrecht, S.; Franke, S.; Cregg, J.M.; Warnecke, D.; Heinz, E. Identification of fungal sphingolipid C9-methyltransferases by phylogenetic profiling. J. Biol. Chem. 2006, 281, 5582–5592. [Google Scholar] [CrossRef]
- Fernandes, C.M.; de Castro, P.A.; Singh, A.; Fonseca, F.L.; Pereira, M.D.; Vila, T.V.; Atella, G.C.; Rozental, S.; Savoldi, M.; Del Poeta, M.; et al. Functional characterization of the Aspergillus nidulans glucosylceramide pathway reveals that LCB Delta8-desaturation and C9-methylation are relevant to filamentous growth, lipid raft localization and Psd1 defensin activity. Mol. Microbiol. 2016, 102, 488–505. [Google Scholar] [CrossRef]
- Singh, A.; Wang, H.; Silva, L.C.; Na, C.; Prieto, M.; Futerman, A.H.; Luberto, C.; Del Poeta, M. Methylation of glycosylated sphingolipid modulates membrane lipid topography and pathogenicity of Cryptococcus neoformans. Cell. Microbiol. 2011, 14, 500–516. [Google Scholar] [CrossRef] [PubMed]
- Oura, T.; Kajiwara, S. Candida albicans sphingolipid C9-methyltransferase is involved in hyphal elongation. Microbiol. Read. 2010, 156, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, V.; Cahoon, E.B.; Thokala, M.; Kaur, J.; Li, J.; Shah, D.M. Sphingolipid C-9 methyltransferases are important for growth and virulence but not for sensitivity to antifungal plant defensins in Fusarium graminearum. Eukaryot. Cell 2009, 8, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Bredeweg, E.L.; McCluskey, K.; Baker, S.E. Phenotype to genotype in Neurospora crassa: Association of the scumbo phenotype with mutations in the gene encoding ceramide C9-methyltransferase. Curr. Res. Microb. Sci. 2022, 3, 100117. [Google Scholar] [CrossRef] [PubMed]
- Barratt, R.W.; Newmeyer, D.; Perkins, D.D.; Garnjobst, L. Map construction in Neurospora crassa. Adv. Genet. 1954, 6, 1–93. [Google Scholar] [CrossRef] [PubMed]
- Levery, S.B.; Momany, M.; Lindsey, R.; Toledo, M.S.; Shayman, J.A.; Fuller, M.; Brooks, K.; Doong, R.L.; Straus, A.H.; Takahashi, H.K. Disruption of the glucosylceramide biosynthetic pathway in Aspergillus nidulans and Aspergillus fumigatus by inhibitors of UDP-Glc:ceramide glucosyltransferase strongly affects spore germination, cell cycle, and hyphal growth. FEBS Lett. 2002, 525, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, V.; Cahoon, E.B.; Li, J.; Thokala, M.; Minto, R.E.; Shah, D.M. Glucosylceramide synthase is essential for alfalfa defensin-mediated growth inhibition but not for pathogenicity of Fusarium graminearum. Mol. Microbiol. 2007, 66, 771–786. [Google Scholar] [CrossRef] [PubMed]
- Rittershaus, P.C.; Kechichian, T.B.; Allegood, J.C.; Merrill, A.H., Jr.; Hennig, M.; Luberto, C.; Del Poeta, M. Glucosylceramide synthase is an essential regulator of pathogenicity of Cryptococcus neoformans. J. Clin. Investig. 2006, 116, 1651–1659. [Google Scholar] [CrossRef]
- Collopy, P.D.; Colot, H.V.; Park, G.; Ringelberg, C.; Crew, C.M.; Borkovich, K.A.; Dunlap, J.C. High-throughput construction of gene deletion cassettes for generation of Neurospora crassa knockout strains. Methods Mol. Biol. 2010, 638, 33–40. [Google Scholar] [CrossRef]
- Colot, H.V.; Park, G.; Turner, G.E.; Ringelberg, C.; Crew, C.M.; Litvinkova, L.; Weiss, R.L.; Borkovich, K.A.; Dunlap, J.C. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc. Natl. Acad. Sci. USA 2006, 103, 10352–10357. [Google Scholar] [CrossRef]
- Davis, R.H.; DeSerres, F.J. Genetic and microbiological research techniques for Neurospora crassa. Meth. Enzymol. 1970, 27, 79–143. [Google Scholar]
- Jones, D.R.; Xing, X.; Tingley, J.P.; Klassen, L.; King, M.L.; Alexander, T.W.; Abbott, D.W. Analysis of Active Site Architecture and Reaction Product Linkage Chemistry Reveals a Conserved Cleavage Substrate for an Endo-alpha-mannanase within Diverse Yeast Mannans. J. Mol. Biol. 2020, 432, 1083–1097. [Google Scholar] [CrossRef]
- Santander, J.; Martin, T.; Loh, A.; Pohlenz, C.; Gatlin, D.M.; Curtiss, R. Mechanisms of intrinsic resistance to antimicrobial peptides of Edwardsiella ictaluri and its influence on fish gut inflammation and virulence. Microbiol. Read. 2013, 159, 1471–1486. [Google Scholar] [CrossRef]
- Santos, F.C.; Marques, J.T.; Bento-Oliveira, A.; de Almeida, R.F.M. Sphingolipid-enriched domains in fungi. FEBS Lett. 2020, 594, 3698–3718. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.C.; Lobo, G.M.; Fernandes, A.S.; Videira, A.; de Almeida, R.F.M. Changes in the Biophysical Properties of the Cell Membrane Are Involved in the Response of Neurospora crassa to Staurosporine. Front. Physiol. 2018, 9, 1375. [Google Scholar] [CrossRef]
- Li, S.; Du, L.; Yuen, G.; Harris, S.D. Distinct ceramide synthases regulate polarized growth in the filamentous fungus Aspergillus nidulans. Mol. Biol. Cell 2006, 17, 1218–1227. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoma, J.F.; Ernan, B.; Keiser, G.; Heiss, C.; Azadi, P.; Free, S.J. Genetic Characterization of the Acidic and Neutral Glycosphingolipid Biosynthetic Pathways in Neurospora crassa. Microorganisms 2023, 11, 2093. https://doi.org/10.3390/microorganisms11082093
Shoma JF, Ernan B, Keiser G, Heiss C, Azadi P, Free SJ. Genetic Characterization of the Acidic and Neutral Glycosphingolipid Biosynthetic Pathways in Neurospora crassa. Microorganisms. 2023; 11(8):2093. https://doi.org/10.3390/microorganisms11082093
Chicago/Turabian StyleShoma, Jannatul F., Ben Ernan, Griffin Keiser, Christian Heiss, Parastoo Azadi, and Stephen J. Free. 2023. "Genetic Characterization of the Acidic and Neutral Glycosphingolipid Biosynthetic Pathways in Neurospora crassa" Microorganisms 11, no. 8: 2093. https://doi.org/10.3390/microorganisms11082093
APA StyleShoma, J. F., Ernan, B., Keiser, G., Heiss, C., Azadi, P., & Free, S. J. (2023). Genetic Characterization of the Acidic and Neutral Glycosphingolipid Biosynthetic Pathways in Neurospora crassa. Microorganisms, 11(8), 2093. https://doi.org/10.3390/microorganisms11082093





