Abstract
Candida albicans (C. albicans) reigns as a major cause of clinical candidiasis. C. albicans biofilms are known to increase resistance to antifungal agents, making biofilm-related infections particularly challenging to treat. Drug resistance is of particular concern due to the spread of multidrug-resistant fungal pathogens, while autophagy is crucial for the maintenance of cellular homeostasis. Therefore, this study aimed to investigate the effects of an activator and an inhibitor of autophagy on the susceptibility of C. albicans biofilms to antifungal agents and the related mechanisms. The susceptibility of C. albicans biofilms to different antifungal agents after treatment with or without the autophagy activator or inhibitor was evaluated using XTT assay. Alkaline phosphatase (ALP) activity and reactive oxygen species (ROS) level, as well as the expression of ROS-related and autophagy-related genes, were examined to evaluate the autophagic activity of C. albicans biofilms when treated with antifungal agents. The autophagosomes were observed by transmission electron microscopy (TEM). The susceptibility of C. albicans biofilms to antifungal agents changed when autophagy changed. The ALP activity and ROS level of C. albicans biofilms increased with the treatment of antifungal agents, and autophagosomes could be observed in C. albicans biofilms. Autophagy was involved in the susceptibility of C. albicans biofilms to antifungal agents.
1. Introduction
Candida albicans (C. albicans) is a common opportunistic fungal pathogen in humans that can colonize the skin, mucosal surfaces, and gastrointestinal and vaginal tract of healthy people [1,2,3], while causing superficial mucosal infections or life-threatening systemic diseases in immunocompromised or immunologically deficient individuals [4]. Candidiasis leads to high incidence rates and mortality [5]. A major virulence attribute of C. albicans is that it exists in the form of biofilms in the human body [6]. Compared with the planktonic form, the biofilm form of C. albicans can escape an immune attack of the host and lead to persistent infection, which ultimately increases the difficulty of clinical treatment [7]. So far, C. albicans infection has been an important issue related to human health.
Currently, antifungal agents are mainly employed to treat C. albicans infection, and their sensitivity is related to their therapeutic effects [8]. Among them, four major classes of antifungal agents are commonly employed, including polyenes (like amphotericin B), azoles (like fluconazole), echinocandins (like caspofungin), and pyrimidine or nucleoside analogs (like flucytosine), which are fungicidal against C. albicans [9,10]. Nevertheless, with the increasing use of antifungal agents, drug resistance to conventional antifungal therapeutics makes C. albicans biofilm-associated infections and drug resistance significant clinical challenges [11,12].
Autophagy is a conserved cellular process in eukaryotic cells, which is associated with the virulence of C. albicans [13,14]. Moreover, autophagy regulation is involved in biofilm formation and antifungal resistance of C. albicans [15]. However, the effects of antifungal agents on the autophagic activity in C. albicans biofilms remain unknown. Autophagy is an essential process through which damaged organelles from cytoplasm are recycled to sustain the development of organisms [16,17], and it is more generally classified as a basic cell survival mechanism against environmental stressors [18]. The autophagic activity of eukaryotic cells can be analyzed through alkaline phosphatase (ALP) activity, reactive oxygen species (ROS) level, and the expressions of autophagy-related genes and proteins [15]. In the present study, susceptibility tests, ALP activity, ROS level, mitochondrial membrane potential (MMP), autophagosomes, and the expressions of autophagy-related genes and proteins were used to compare the autophagy level of C. albicans biofilms under treatment with different antifungal agents. This study has shed new light on how autophagy changes the susceptibility of C. albicans biofilms to antifungal agents and its related mechanisms.
2. Materials and Methods
2.1. Strain and Growth Media
The standard strain Candida albicans SC5314 (C. albicans SC5314, ATCC® MYA-2876TM) was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The strain was stored at −80 °C until use. A yeast extract peptone dextrose (YPD) medium (2% peptone, 1% yeast extract, and 2% glucose) was prepared for C. albicans strain incubation, and RPMI-1640 medium (Gibco Ltd., Paisley, UK) was used for C. albicans biofilm formation.
2.2. Biofilm Formation
C. albicans was cultured overnight at 30 °C and 200 rpm by shaking in a 10 mL YPD medium to reach a logarithmic growth phase [19]. Cells were harvested by centrifugation at 3000 rpm and washed with phosphate buffered saline (PBS). Then, the C. albicans cells were resuspended in fresh RPMI 1640 medium (Gibco Ltd., Paisley, UK) and the cell density was adjusted with a haemocytometer (1 × 106 cells/mL) [20]. The suspensions were placed in 96-well plates or culture dishes (Thermo Fisher Scientific Inc., Waltham, MA, USA). The plates or culture dishes were then incubated at 37 °C for 24 h for C. albicans biofilm formation.
2.3. Preparation of Antifungal Agents
Stock solutions of fluconazole (10 mg/mL), itraconazole (10 mg/mL), terbinafine (10 mg/mL), amphotericin B (5 mg/mL), nystatin (10 mg/mL), and 5-fluorocytosine (10 mg/mL) (Sigma-Aldrich, St. Louis, MO, USA) were dissolved in dimethyl sulfoxide (DMSO). These antifungal agents were prepared in serial two-fold dilutions based on the protocol M27-A4 document from the Clinical and Laboratory Standards Institute (CLSI, Berwyn, PA, USA) (2017), and their final concentrations ranged from 1 to 8 μg/mL for amphotericin B and nystatin and from 64 to 1024 μg/mL for fluconazole, itraconazole, terbinafine, and 5-fluorocytosine. Then, C. albicans biofilms were submitted to incubation with these antifungal agents for 24 h and biofilm SMIC50 (Sessile Minimum Inhibitory Concentration 50%) was analyzed using XTT [2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide] assay (Sigma-Aldrich, St. Louis, MO, USA) [21], where SMIC50 was defined as 50% inhibition of C. albicans biofilm metabolic activity compared with a drug-free control group [22].
2.4. Susceptibility Tests
XTT and antifungal agents were prepared for susceptibility tests with reference to Section 2.3. C. albicans biofilms were treated with autophagy activator (rapamycin; 100 and 200 nM) or autophagy inhibitor (chloroquine; 100 and 200 nM) (Sigma-Aldrich, St. Louis, MO, USA) for 2 h, washed with sterile PBS twice, and then incubated with antifungal agents for 24 h. The SMIC50 of C. albicans biofilm was analyzed using XTT [21], where absorbance was measured at 490 nm using a microplate reader (SpectraMax M2, Molecular Devices, Sunnyvale, CA, USA). The tests were repeated three times.
2.5. Alkaline Phosphatase Activity Assay
Alkaline phosphatase (ALP) activity, as an indicator for autophagy, can be detected by a microplate reader [23]. C. albicans biofilms inoculated on the surface of culture dishes were treated with antifungal agents at the concentration of SMIC50 for 6 h and 24 h, respectively. Then, the biofilms were harvested for ALP activity assay, which was assessed based on previous research [15]. ALP activity was quantitatively analyzed with an alkaline phosphatase assay kit (Beyotime Biotechnology, Shanghai, China) according to the manufacturer’s instructions. Absorbance was recorded at 405 nm using a microplate reader (SpectraMax M2, Molecular Devices, Sunnyvale, CA, USA). The assay was performed in triplicate.
2.6. Reactive Oxygen Species (ROS)
ROS level was evaluated with 2′,7′-dichlorofluorescein diacetate (DCFH-DA) [24]. C. albicans biofilms that were incubated in 96-well plates were treated with antifungal agents at the concentration of SMIC50 for 24 h. The control (with no treatment) and treated biofilms were stained with 10 μM DCFH-DA (Beyotime Biotechnology, Shanghai, China) at 37 °C for 1 h in the dark, and were subsequently washed with PBS three times to remove residual dye. The fluorescence value (excitation wave 488 nm and emission wave 520 nm) was investigated via a microplate reader. In addition, the biofilms were incubated for 24 h in 96-well plates before being treated with antifungal agents for 24 h at the concentration of SMIC50 for fluorescence observation. Fluorescence images were obtained by an inverted fluorescence microscope (Leica DMI3000B, Wetzlar, Germany). The experiment was repeated in triplicate sets.
2.7. Mitochondrial Membrane Potential (MMP)
The JC-1 staining was applied to assess the changes in MMP [25]. Double fluorescence staining of mitochondria (green fluorescent J-monomers and red fluorescent J-aggregates) was employed to monitor the changes in mitochondrial membrane potential. C. albicans biofilms were treated with antifungal agents at the concentration of SMIC50 for 24 h, and then the biofilms of each group were stained with JC-1 (Beyotime Biotechnology, Shanghai, China) at 37 °C for 30 min. The fluorescence images were observed by an inverted fluorescence microscope (Leica DMI3000B, Wetzlar, Germany). Besides this, red and green fluorescence values were measured by a microplate reader, and then the relative ratio of red/green fluorescence was calculated. The test was repeated in triplicate.
2.8. Transmission Electron Microscopy
The autophagosomes in biofilm cells were observed by transmission electron microscopy (TEM; Carl Zeiss, Oberkochen, Germany). C. albicans biofilms after treatment with antifungal agents for 24 h at the concentration of SMIC50 were harvested, washed twice with PBS, and fixed in stationary liquid (glutaraldehyde) overnight at 4 °C. Then, the biofilm cells were fixed with 1% osmium tetroxide for 2 h at 4 °C. Next, the cells were dehydrated successively through ethanol (30, 50, 70, 80, 95, and 100%) and embedded into epoxy resins followed by being sliced and stained. Eventually, the prepared sections were observed using TEM.
2.9. RT-qPCR
RNA-seq data were verified by real-time quantitative PCR (RT-qPCR) analysis. Control and treated biofilms under antifungal agents for 24 h at the concentration of SMIC50 were harvested and frozen in liquid nitrogen for RNA extraction. Extraction of total RNA was carried out using an RNA extraction Kit (Vazyme, Nanjing, China). The integrity of the RNA of each group was measured using nanodrop (NanoDrop One, Thermo Fisher Scientific Inc., Waltham, MA, USA), and then the extracted RNA was submitted to be reversely transcribed into cDNA using HiScript II RT SuperMix (Vazyme, Nanjing, China) according to the manufacturer’s instructions. The cDNA was used to analyze the expressions of ROS-related genes and autophagy-related genes, which was performed on a QuantStudio™ 7 Flex (Thermo Fisher Scientific Inc., Waltham, MA, USA) using ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China). The relative expressions of genes were determined using the 2−ΔΔCt method [26]. β-actin served as an internal reference gene. The primers and sequences are listed in Table 1. Three biological replicates were prepared for each sample and the test was repeated three times.

Table 1.
Primers and sequences used in the present study.
2.10. Western Blotting
Total protein extracts from the control and treated biofilms under antifungal agents for 24 h at the concentration of SMIC50 were prepared using an immunoprecipitation protocol for western blotting [27]. Total protein concentration was quantified using a BCA protein assay kit (Beyotime Biotechnology, Shanghai, China). The protein samples were electrophoresed by 10% SDS-PAGE gels and transferred onto PVDF membranes (Bio-Rad Laboratories, Hercules, CA, USA). The membranes were incubated with primary antibodies (ATG7, ATG13, ATG17, and ATG27; 1:500 dilution) (Dia-An Biotech, Wuhan, China) overnight at 4 °C after blocking with 5% skimmed milk in TBST, followed by incubation with corresponding secondary antibodies for 1 h. Target protein bands were detected with a chemiluminescence imaging system (Merck & Co., Inc., Kenilworth, NJ, USA). GAPDH (Bioworld, Minneapolis, MN, USA) served as a reference protein. All operations were repeated in triplicate.
2.11. Statistical Analysis
After validating the equal variance assumption of the data, the statistical significance of differences in groups was compared by one-way ANOVA. Statistical analyses were performed using SPSS Statistics 22.0 Software (SPSS Inc., Chicago, IL, USA). p < 0.05 was regarded as statistically significant.
3. Results
3.1. Autophagy Activator and Inhibitor Affected the Drug Resistance of C. albicans Biofilms to Antifungal Agents
The drug resistance of C. albicans biofilms to antifungal agents changed after being autophagy activator and inhibitor treated (Table 2). Among them, fluconazole had the worst inhibitory effect on C. albicans biofilms (SMIC50 value > 512), while nystatin exhibited the best (SMIC50 value > 2) as compared to the groups without the treatment of rapamycin or chloroquine. After treatment with rapamycin (100 nM and 200 nM), the SMIC50 of C. albicans biofilms to fluconazole, itraconazole, terbinafine, 5-fluorocytosine, amphotericin B, and nystatin were significantly increased (p < 0.05). After treatment with chloroquine (200 nM), the SMIC50 of C. albicans biofilms to terbinafine, 5-fluorocytosine, amphotericin B, and nystatin significantly decreased (p < 0.05), while fluconazole and itraconazole showed no significant difference regardless of the concentration of chloroquine compared with those groups with no treatment (p > 0.05).

Table 2.
The SMIC50 of C. albicans biofilms detected by XTT reduction assay.
3.2. Antifungal Agents Changed the ALP Activity of C. albicans Biofilms
The ALP activity of C. albicans biofilms significantly increased after the biofilms were treated with fluconazole, itraconazole, terbinafine, and amphotericin B (for 6 h) and with amphotericin B and nystatin (for 24 h) at the concentration of their SMIC50 (p < 0.01), respectively, while there was no significant difference in ALP activity of the groups treated with 5-fluorocytosine (for 6 h and 24 h) (p > 0.05) compared to the control group without antifungal agent treatment. Besides this, the ALP activity of C. albicans biofilms treated with fluconazole, itraconazole, and terbinafine for 6 h was higher than that for 24 h (p < 0.01), and the ALP activity of C. albicans biofilms treated with amphotericin B and nystatin for 24 h was higher than that for 6 h (p < 0.01) (Figure 1).
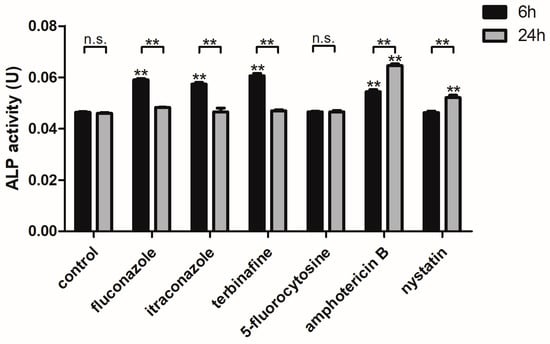
Figure 1.
The ALP activity of C. albicans biofilms treated with antifungal agents for 6 h and 24 h. ** p < 0.01: ALP activity of C. albicans biofilms treated with antifungal agents for 6 h and 24 h compared with the control group; ** p < 0.01; n.s. no significant difference: comparison of ALP activity of C. albicans biofilms treated with antifungal agents between 6 h and 24 h.
3.3. Antifungal Agents Increased the ROS Level of C. albicans Biofilms
The ROS level of C. albicans biofilms significantly increased with the treatment of fluconazole, itraconazole, amphotericin B, and nystatin at the concentration of SMIC50 (p < 0.05) compared with the control group (Figure 2). In addition, the expressions of ROS-related genes (CAT, TRR1, SOD1, and GLR1) were significantly upregulated after treatment with fluconazole, itraconazole, amphotericin B, and nystatin (Figure 3), which was consistent with the results for ROS level (Figure 2). DCFH-DA fluorescence images showed that the green fluorescence intensity of C. albicans biofilms after treatment with fluconazole, itraconazole, amphotericin B, and nystatin was stronger than that of other groups (Figure 4).
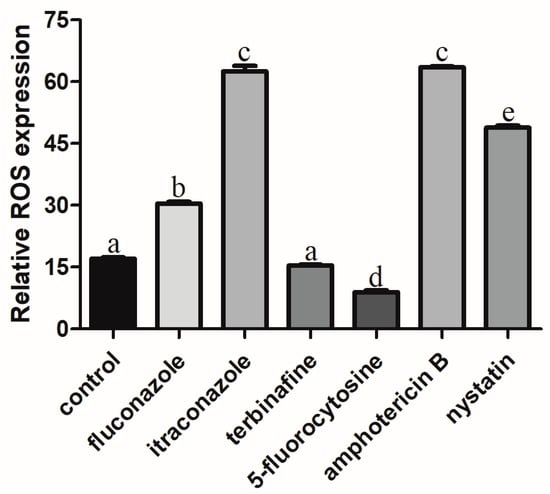
Figure 2.
The ROS level of C. albicans biofilms treated with antifungal agents for 24 h. Values labeled with the same superscript are not significantly different (p > 0.05).
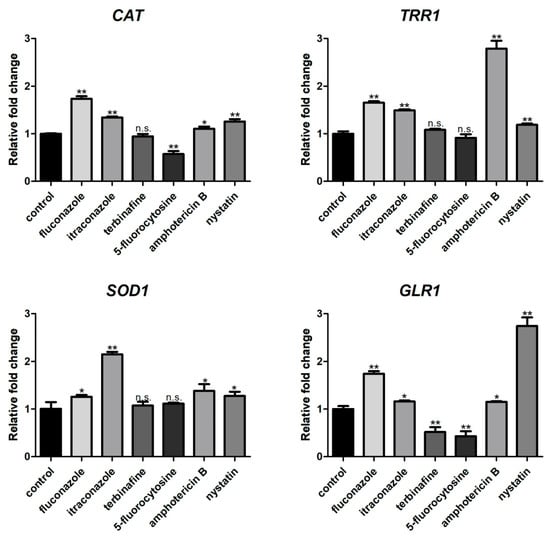
Figure 3.
Expressions of ROS-related genes (CAT, TRR1, SOD1, and GLR1). * p < 0.05; ** p < 0.01; n.s. no significant difference.
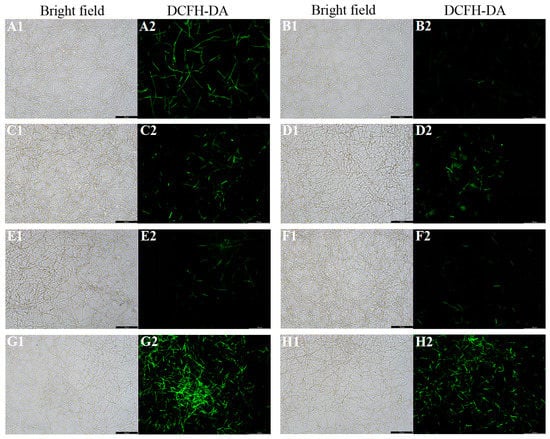
Figure 4.
Representative DCFH-DA fluorescence images of C. albicans biofilms after treatment with antifungal agents for 24 h. (A1,A2): C. albicans ros up; (B1,B2): C. albicans (control); (C1,C2): fluconazole; (D1,D2): itraconazole; (E1,E2): terbinafine; (F1,F2): 5-fluorocytosine; (G1,G2): amphotericin B; (H1,H2): nystatin. Scale bar = 100 μm.
3.4. Antifungal Agents Changed the MMP of C. albicans Biofilms
To investigate whether ROS level affects mitochondrial function, the mitochondrial membrane potential (MMP) was measured to indicate the mitochondria functional status [28]. The JC-1 fluorescence images showed that the green fluorescence intensity of C. albicans biofilms after treatment with fluconazole, itraconazole, amphotericin B, and nystatin was stronger than that of other groups (Figure 5A), which was consistent with the ROS results above. Furthermore, the relative red/green fluorescence ratio of these groups as mentioned above (treatment with fluconazole, itraconazole, amphotericin B, and nystatin) decreased significantly (p < 0.05) (Figure 5B).
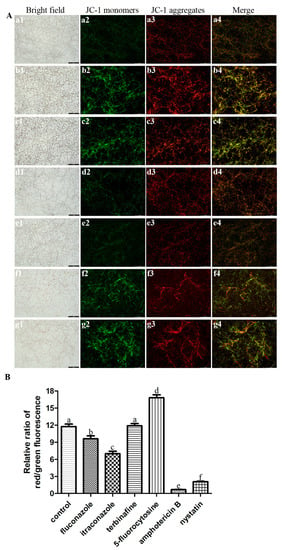
Figure 5.
(A) Representative JC-1 fluorescence images of C. albicans biofilms after treatment with antifungal agents for 24 h. a: C. albicans (control); b: fluconazole; c: itraconazole; d: terbinafine; e: 5-fluorocytosine; f: amphotericin B; g: nystatin. Scale bar = 100 μm. (B) The relative ratio of JC-1 red/green fluorescence of C. albicans biofilms after treatment with antifungal agents for 24 h. Values labeled with the same superscript are not significantly different (p > 0.05). (a1–g1) Bright field. (a2–g2) JC-1 monomers. (a3–g3) JC-1 aggregates. (a4–g4) Merge.
3.5. Antifungal Agents Increased the Autophagy Level of C. albicans Biofilms
RT-qPCR and western blotting results presented changes in the autophagy level of C. albicans biofilms, where four critical autophagy-related genes (ATG7, ATG13, ATG17, and ATG27) were selected (Figure 6). RT-qPCR showed that the gene expressions of ATG7, ATG13, ATG17, and ATG27 were significantly upregulated (p < 0.05) after treatment with different antifungal agents compared with the control group (Figure 6A). Moreover, western blotting results were consistent with RT-qPCR results that protein levels of ATG7, ATG13, ATG17, and ATG27 were higher in C. albicans biofilms treated with antifungal agents than the control group (Figure 6B).
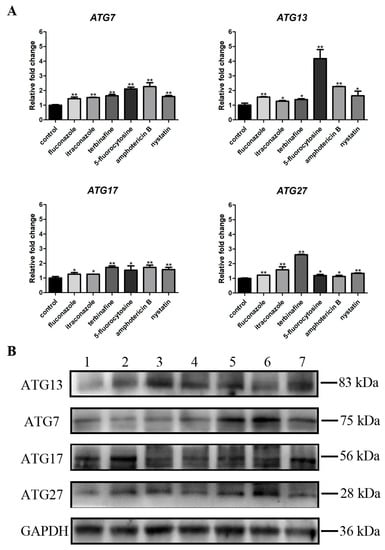
Figure 6.
(A) Expressions of autophagy-related genes (ATG7, ATG13, ATG17, and ATG27). * p < 0.05; ** p < 0.01; (B) Protein expression levels of ATG7, ATG13, ATG17, and ATG27. 1: C. albicans (control); 2: fluconazole; 3: itraconazole; 4: terbinafine; 5: 5-fluorocytosine; 6: amphotericin B; 7: nystatin.
3.6. Antifungal Agents Increased the Autophagosomes of C. albicans Biofilms
Autophagosomes can be detected when autophagy occurs. Representative TEM images showed that C. albicans biofilms treated with antifungal agents exhibited more autophagosomes with distinct membranes and edges (Figure 7B1–G2), while fewer autophagosomes, despite having indistinct structures, could be observed in the control group (Figure 7A1,A2), indicating that treatment with antifungal agents increased the autophagosomes of C. albicans biofilms.
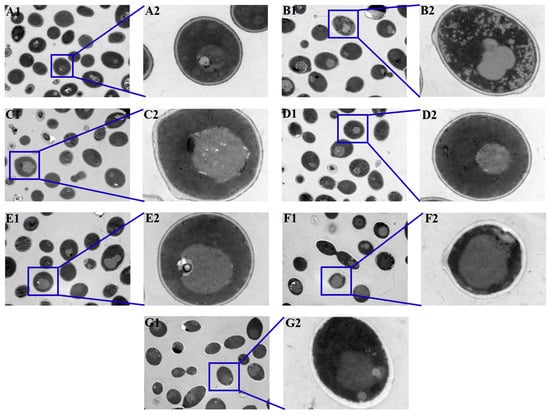
Figure 7.
Observation of the autophagosomes in C. albicans biofilms after treatment with antifungal agents for 24 h by TEM. There were more autophagosomes in C. albicans antifungal-agent-treated biofilms, while fewer autophagosomes could be detected in the control group. (A1,A2): C. albicans (control); (B1,B2): fluconazole; (C1,C2): itraconazole; (D1,D2): terbinafine; (E1,E2): 5-fluorocytosine; (F1,F2): amphotericin B; (G1,G2): nystatin. ((A1–G1), 1.5 k× magnification; (A2–G2), 10 k × magnification).
4. Discussion
Autophagy is a cellular degradation and recycling process through which damaged organelles and cellular components are recycled [18]. It plays an important role in maintaining intracellular homeostasis under nutrient depletion and stress conditions [29]. Nevertheless, the effects of autophagy on the susceptibility of C. albicans biofilms to antifungal drugs remain unclear, as well as the related autophagic molecular mechanism. The present study compared the susceptibility of C. albicans biofilms to different antifungal agents after treatment with or without an autophagy activator (rapamycin) or inhibitor (chloroquine), indicating that autophagy can affect the susceptibility of C. albicans to antifungal agents. The SMIC50 of C. albicans biofilms to all the antifungal agents used in this study increased after rapamycin treatment (p < 0.05); however, the SMIC50 of C. albicans biofilms to most antifungals (terbinafine, 5-fluorocytosine, amphotericin B, and nystatin) decreased after chloroquine treatment (p < 0.05). Autophagy activators (such as rapamycin), which can activate autophagy at low doses [30], may contribute to the resistance of C. albicans biofilms to antifungal agents. C. albicans engulfs damaged organelles through autophagy, thereby improving cell viability. Conversely, chloroquine inhibits autophagy by impairing autophagosome fusion with lysosomes [31,32,33], which may affect the efficacy of antifungal agents and decrease or not alter the drug resistance to some extent. Terbinafine has an antifungal effect by inhibiting squalene epoxidase, a key enzyme involved in ergosterol biosynthesis in the cell membrane of C. albicans [34]. The presence of terbinafine can result in the excessive accumulation of squalene and inhibit the synthesis of ergosterol [34]. Chloroquine destroys the lysosomal function of C. albicans, causing a faster accumulation of squalene. Consequently, the efficacy of terbinafine can be enhanced, which contributes to decreased SMIC50. Furthermore, 5-fluorocytosine can exert fungistatic effects by penetrating cells and interfering with the synthesis of nucleic acids [35]. After treatment with chloroquine, the permeability of lysosomes and mitochondria increases, and 5-fluorocytosine can more easily enter cells, which enhances its fungicidal effects, leading to a decrease in SMIC50. Several previous studies have reported that autophagy inhibitors (e.g., chloroquine) can result in a synergistic effect with chemotherapy in treating cancers [36]. The susceptibility results suggest that synergism between autophagy inhibitors and antifungal agents may be a potential way to treat C. albicans infections.
ALP activity was used to characterize autophagy levels [37]. In Saccharomyces cerevisiae, the precursor of ALP in cytoplasm is encapsulated in autophagosomes and then transported to vacuoles, in which it can be degraded by enzymes. Therefore, a subsequent increase in ALP activity will be detected when autophagy occurs [38]. The ALP activity of C. albicans biofilms increased after treatment with antifungal agents. Furthermore, the ALP activity of C. albicans biofilms treated with fluconazole, itraconazole, and terbinafine for 6 h was higher than that for 24 h, which may be due to the increased autophagy and adaptability of cells to these three agents after 24 h of treatment, ultimately resulting in the decrease in ALP activity. Amphotericin B and nystatin selectively bind to ergosterol in the cell membrane of C. albicans and increase its permeability [39,40]. With the prolongation of drug action time (for 24 h in this study), the permeability of cell membranes increases, which can be conducive to an increase in autophagy level. The findings suggested that the ALP activity of C. albicans biofilms may be affected by antifungal agents.
In addition, although the ALP activity value of C. albicans biofilms treated with 5-fluorocytosine was not statistically significant, the occurrence of autophagy could not be completely denied. The analysis of autophagy levels still needs to be determined with other detection methods such as ROS and MMP. Autophagy in yeast cells can be induced under stress stimulation or nutrient-deprived conditions, accompanied by changes in intracellular ROS levels [41,42]. Mitochondria is one of the main organelles of oxidative stress, in which mitochondrial respiratory complex I is one of the prominent sources of intracellular ROS [43,44]. ROS are highly reactive molecules containing oxygen, including peroxides, superoxides, and hydroxyl radicals (OH) [45,46]. High levels of ROS can activate autophagy in somatic cells, while autophagy can eliminate cell damage caused by ROS [47]. The ROS level of C. albicans biofilms treated with fluconazole, itraconazole, amphotericin B, and nystatin significantly increased compared with the control group, which was also clarified by previous studies that showed that antifungal treatment can induce ROS in fungal cells [48]. In Aspergillus fumigatus, antifungal agents affect the activity of mitochondrial respiratory complex I, resulting in excessive production of ROS, which indicates that ROS production is conducive to inhibiting the growth of fungi to some extent [49].
MMP depends on sequential intramitochondrial biochemical reactions [50]. Changes in MMP are one of the characteristics of mitochondrial dysfunction, which affects mitochondrial homeostasis [51]. In this study, a JC-1 fluorescent probe was used for evaluating MMP, in which aggregate (red) and monomer (green) forms allow dual-color, ratiometric assessment of MMP [52]. The relative red/green fluorescence ratio of C. albicans biofilms treated with fluconazole, itraconazole, amphotericin B, and nystatin decreased significantly (p < 0.05), indicating a decreased MMP for these groups. MMP regulates respiration rates and is associated with altered ROS levels [53,54], manifested as an increase in ROS production leading to a decrease in MMP [55,56]. Some antifungal agents like azoles can cause oxidative damage to the mitochondria of fungal cells such as Cryptococcus neoformans by increasing ROS levels [57,58].
Autophagosomes inside cells represent the occurrence of autophagy. TEM can observe autophagosomes in C. albicans, which is recognized as the “gold standard” in autophagy-related research [59]. In the present study, there were more autophagosomes in the C. albicans biofilms treated with different antifungal agents, while fewer autophagosomes were present in the control group, which indicated that the treatment with antifungal agents enhanced the autophagy response. Autophagosomes can be observed by high-precision TEM, which can identify intracellular structures as well as the internal environment and the surrounding cell components of autophagosomes [60].
Autophagy-related genes and proteins are also an important indicator of autophagic activity. Some autophagy-related genes (ATG7, ATG13, ATG17, and ATG27) have been identified in C. albicans, which were investigated in this research [15]. The expressions of these genes and proteins were upregulated in C. albicans biofilms when treated with antifungal agents compared with those in the control group, indicating that autophagic activity increased with treatment with antifungal agents.
In summary, autophagy changes affect the susceptibility of C. albicans biofilms to antifungal agents and also other parameters, such as ALP, ROS, and MMP. Autophagy levels (ALP) may be upregulated with an increase in ROS to reduce cellular stress and enhance the survival rate of C. albicans. Two C. albicans parameters (stress and survival) may exhibit upregulation or downregulation, depending on the treatment (activator versus inhibitor of the autophagy process). The regulation of autophagy levels in C. albicans biofilms may be a promising strategy to reduce the occurrence of clinical C. albicans drug resistance and improve the susceptibility to antifungal agents.
5. Conclusions
In the present study, the effects of autophagy on the drug resistance of C. albicans biofilms were preliminarily discussed. The autophagy level increased when C. albicans biofilms were treated with antifungal agents, and the autophagy was related to the drug susceptibility of C. albicans, suggesting that autophagy may be a regulatory mechanism of C. albicans drug resistance. Further studies are still needed to elucidate the prospective regulatory mechanisms underlying autophagy and drug resistance in C. albicans, as well as the molecular targets of antifungal agents.
Author Contributions
Conceptualization, J.S. and X.W.; software, M.M.; methodology, J.S. and W.D.; data curation, W.D.; validation, Y.H.; investigation, B.S.; formal analysis, Q.W.; writing—original draft preparation, J.S.; writing—review and editing, J.S. and X.W.; supervision, X.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant numbers 81970945 and 81371156. The recipient of these funds was Xin Wei.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Sobel, J.D.; White, T.C. A Combination Fluorescence Assay Demonstrates Increased Efflux Pump Activity as a Resistance Mechanism in Azole-Resistant Vaginal Candida albicans Isolates. Antimicrob. Agents Chemother. 2016, 60, 5858–5866. [Google Scholar] [CrossRef] [PubMed]
- Alikhani, T.; Daie Ghazvini, R.; Mirzaii, M.; Hashemi, S.J.; Fazli, M.; Rafat, Z.; Roostaei, D.; Ardi, P.; Kamali Sarvestani, H.; Zareei, M. Drug Resistance and Biofilm Formation in Candida Species of Vaginal Origin. Iran. J. Public Health 2022, 51, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Nobile, C.J. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microbes Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef]
- Koshikawa, T.; Abe, M.; Nagi, M.; Miyazaki, Y.; Takemura, H. Biofilm-formation capability depends on environmental oxygen concentrations in Candida species. J. Infect. Chemother. 2022, 28, 643–650. [Google Scholar] [CrossRef]
- Das, S.; Goswami, A.M.; Saha, T. An insight into the role of protein kinases as virulent factors, regulating pathogenic attributes in Candida albicans. Microb. Pathog. 2022, 164, 105418. [Google Scholar] [CrossRef]
- Perrine-Walker, F. Caspofungin resistance in Candida albicans: Genetic factors and synergistic compounds for combination therapies. Braz. J. Microbiol. 2022, 53, 1101–1113. [Google Scholar] [CrossRef]
- Campoy, S.; Adrio, J.L. Antifungals. Biochem. Pharmacol. 2017, 133, 86–96. [Google Scholar] [CrossRef]
- Prasad, R.; Shah, A.H.; Rawal, M.K. Antifungals: Mechanism of Action and Drug Resistance. Adv. Exp. Med. Biol. 2016, 892, 327–349. [Google Scholar] [CrossRef]
- Fiori, B.; Posteraro, B.; Torelli, R.; Tumbarello, M.; Perlin, D.S.; Fadda, G.; Sanguinetti, M. In vitro activities of anidulafungin and other antifungal agents against biofilms formed by clinical isolates of different Candida and Aspergillus species. Antimicrob. Agents Chemother. 2011, 55, 3031–3035. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Zhou, X.; Li, D.; Li, X.; Liu, W. A Proteomic Landscape of Candida albicans in the Stepwise Evolution to Fluconazole Resistance. Antimicrob. Agents Chemother. 2022, 66, e0210521. [Google Scholar] [CrossRef] [PubMed]
- Palmer, G.E. Autophagy in the invading pathogen. Autophagy 2007, 3, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Jia, C.; Dong, Y.; Zhang, B.; Xiao, C.; Chen, Y.; Wang, Y.; Li, X.; Wang, L.; Zhang, B.; et al. Candida albicans autophagy, no longer a bystander: Its role in tolerance to ER stress-related antifungal drugs. Fungal Genet. Biol. 2015, 81, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jiang, L.; Miao, H.; Lv, Y.; Zhang, Q.; Ma, M.; Duan, W.; Huang, Y.; Wei, X. Autophagy regulation of ATG13 and ATG27 on biofilm formation and antifungal resistance in Candida albicans. Biofouling 2022, 38, 926–939. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Jiang, Q.; Yang, S.; Zhang, C.; Liu, J.; Liu, W.; Lin, P.; Chen, H.; Zhou, D.; Tang, K.; et al. The endoplasmic reticulum stress-mediated unfolded protein response protects against infection of goat endometrial epithelial cells by Trueperella pyogenes via autophagy. Virulence 2022, 13, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, T.; Ishii, T.; Kamada, Y.; Funakoshi, M.; Kobayashi, H.; Furuno, N. Involvement of Gtr1p in the oxidative stress response in yeast Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 2022, 598, 107–112. [Google Scholar] [CrossRef]
- Hale, A.N.; Ledbetter, D.J.; Gawriluk, T.R.; Rucker, E.B., 3rd. Autophagy: Regulation and role in development. Autophagy 2013, 9, 951–972. [Google Scholar] [CrossRef]
- Reuss, O.; Vik, Å.; Kolter, R.; Morschhäuser, J. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene 2004, 341, 119–127. [Google Scholar] [CrossRef]
- Wang, T.; Shao, J.; Da, W.; Li, Q.; Shi, G.; Wu, D.; Wang, C. Strong Synergism of Palmatine and Fluconazole/Itraconazole against Planktonic and Biofilm Cells of Candida Species and Efflux-Associated Antifungal Mechanism. Front. Microbiol. 2018, 9, 2892. [Google Scholar] [CrossRef]
- Kuhn, D.M.; George, T.; Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Antifungal susceptibility of Candida biofilms: Unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob. Agents Chemother. 2002, 46, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xia, J.; Li, C.; Zuo, L.; Wei, X. The possible molecular mechanisms of farnesol on the antifungal resistance of C. albicans biofilms: The regulation of CYR1 and PDE2. BMC Microbiol. 2018, 18, 203. [Google Scholar] [CrossRef] [PubMed]
- Nothwehr, S.F.; Bryant, N.J.; Stevens, T.H. The newly identified yeast GRD genes are required for retention of late-Golgi membrane proteins. Mol. Cell. Biol. 1996, 16, 2700–2707. [Google Scholar] [CrossRef][Green Version]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free. Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Smiley, S.T.; Reers, M.; Mottola-Hartshorn, C.; Lin, M.; Chen, A.; Smith, T.W.; Steele, G.D., Jr.; Chen, L.B. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc. Natl. Acad. Sci. USA 1991, 88, 3671–3675. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.H.; Wei, X.; Ma, M.; Chen, X.J.; Xu, S.B. Possible inhibitory molecular mechanism of farnesol on the development of fluconazole resistance in Candida albicans biofilm. Antimicrob. Agents Chemother. 2012, 56, 770–775. [Google Scholar] [CrossRef]
- Singh, S.D.; Robbins, N.; Zaas, A.K.; Schell, W.A.; Perfect, J.R.; Cowen, L.E. Hsp90 governs echinocandin resistance in the pathogenic yeast Candida albicans via calcineurin. PLoS Pathog. 2009, 5, e1000532. [Google Scholar] [CrossRef] [PubMed]
- Simon, H.U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef]
- Xu, D.D.; Du, L.L. Fission Yeast Autophagy Machinery. Cells 2022, 11, 1086. [Google Scholar] [CrossRef]
- Gu, X.; Zhang, L.; Sun, W.; Liu, K.; Xu, H.; Wu, P.; Gui, M.; Qu, W.A.-O. Autophagy Promotes α-Amanitin-Induced Apoptosis of Hepa1-6 Liver Cells. Chem. Res. Toxicol. 2022, 35, 392–401. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Choe, H.C.; Kim, B.H.; Ahn, S.G. A new link between apoptosis induced by the metformin derivative HL156A and autophagy in oral squamous cell carcinoma. Eur. J. Pharmacol. 2022, 920, 174859. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.P.; Hu, L.F.; Zheng, H.F.; Mao, C.J.; Hu, W.D.; Xiong, K.P.; Wang, F.; Liu, C.F. Application and interpretation of current autophagy inhibitors and activators. Acta Pharmacol. Sin. 2013, 34, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef] [PubMed]
- Muhaj, F.F.; George, S.J.; Nguyen, C.D.; Tyring, S.K. Antimicrobials and resistance part II: Antifungals, antivirals, and antiparasitics. J. Am. Acad. Dermatol. 2022, 86, 1207–1226. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.B.; Gulati, M.; Johnson, A.D.; Nobile, C.J. Development and regulation of single- and multi-species Candida albicans biofilms. Nat. Rev. Microbiol. 2018, 16, 19–31. [Google Scholar] [CrossRef]
- Cocco, S.; Leone, A.; Roca, M.S.; Lombardi, R.; Piezzo, M.; Caputo, R.; Ciardiello, C.; Costantini, S.; Bruzzese, F.; Sisalli, M.J.; et al. Inhibition of autophagy by chloroquine prevents resistance to PI3K/AKT inhibitors and potentiates their antitumor effect in combination with paclitaxel in triple negative breast cancer models. J. Transl. Med. 2022, 20, 290. [Google Scholar] [CrossRef] [PubMed]
- Raiyan, S.; Rahman, M.A.; Al Mamun, M.A.; Asim, M.M.H.; Makki, A.; Hajjar, D.; Alelwani, W.; Tangpong, J.; Mathew, B. Natural compounds from Leea macrophylla enhance phagocytosis and promote osteoblasts differentiation by alkaline phosphatase, type 1 collagen, and osteocalcin gene expression. J. Biomed. Mater. Res. A 2021, 109, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Ruan, C.; Fu, S.; He, C.; Song, J.; Li, H.; Tu, Y.; Tang, D.; Yao, L.; Lin, S.; et al. Atg9-centered multi-omics integration reveals new autophagy regulators in Saccharomyces cerevisiae. Autophagy 2021, 17, 4453–4476. [Google Scholar] [CrossRef]
- Fotis, D.; Liu, J.; Dalamaga, M. Could gut mycobiome play a role in NAFLD pathogenesis? Insights and therapeutic perspectives. Metabol. Open 2022, 14, 100178. [Google Scholar] [CrossRef]
- Kinoshita, H.; Yoshioka, M.; Ihara, F.; Nihira, T. Cryptic antifungal compounds active by synergism with polyene antibiotics. J. Biosci. Bioeng. 2016, 121, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Bartoszewska, M.; Kiel, J.A. The role of macroautophagy in development of filamentous fungi. Antioxid. Redox Signal. 2011, 14, 2271–2287. [Google Scholar] [CrossRef]
- Innokentev, A.; Kanki, T. Mitophagy in Yeast: Molecular Mechanism and Regulation. Cells 2021, 10, 3569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.B.; Wang, D.G.; Guo, F.F.; Xuan, C. Mitochondrial membrane potential and reactive oxygen species in cancer stem cells. Fam. Cancer 2015, 14, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Hirst, J.; King, M.S.; Pryde, K.R. The production of reactive oxygen species by complex I. Biochem. Soc. Trans. 2008, 36, 976–980. [Google Scholar] [CrossRef] [PubMed]
- Pallichankandy, S.; Rahman, A.; Thayyullathil, F.; Galadari, S. ROS-dependent activation of autophagy is a critical mechanism for the induction of anti-glioma effect of sanguinarine. Free Radic. Biol. Med. 2015, 89, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Scherz-Shouval, R.; Elazar, Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 2007, 17, 422–427. [Google Scholar] [CrossRef]
- Shekhova, E.; Kniemeyer, O.; Brakhage, A.A. Induction of Mitochondrial Reactive Oxygen Species Production by Itraconazole, Terbinafine, and Amphotericin B as a Mode of Action against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2017, 61, e00978-17. [Google Scholar] [CrossRef]
- Li, L.; Tan, J.; Miao, Y.; Lei, P.; Zhang, Q. ROS and Autophagy: Interactions and Molecular Regulatory Mechanisms. Cell. Mol. Neurobiol. 2015, 35, 615–621. [Google Scholar] [CrossRef]
- Lee, Y.; Puumala, E.; Robbins, N.; Cowen, L.E. Antifungal Drug Resistance: Molecular Mechanisms in Candida albicans and Beyond. Chem. Rev. 2021, 121, 3390–3411. [Google Scholar] [CrossRef]
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial dysfunction in cell senescence and aging. J. Clin. Investig. 2022, 132, e158447. [Google Scholar] [CrossRef]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.W.; Norman, J.P.; Barbieri, J.; Brown, E.B.; Gelbard, H.A. Mitochondrial membrane potential probes and the proton gradient: A practical usage guide. Biotechniques 2011, 50, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Vyssokikh, M.Y.; Holtze, S.; Averina, O.A.; Lyamzaev, K.G.; Panteleeva, A.A.; Marey, M.V.; Zinovkin, R.A.; Severin, F.F.; Skulachev, M.V.; Fasel, N.; et al. Mild depolarization of the inner mitochondrial membrane is a crucial component of an anti-aging program. Proc. Natl. Acad. Sci. USA 2020, 117, 6491–6501. [Google Scholar] [CrossRef] [PubMed]
- Levraut, J.; Iwase, H.; Shao, Z.H.; Vanden Hoek, T.L.; Schumacker, P.T. Cell death during ischemia: Relationship to mitochondrial depolarization and ROS generation. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H549–H558. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Youle, R.J.; Finkel, T. The Mitochondrial Basis of Aging. Mol. Cell 2016, 61, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Hwang, J.W.; Yun, C.K.; Lee, Y.; Choi, Y.S. Delivery of exogenous mitochondria via centrifugation enhances cellular metabolic function. Sci. Rep. 2018, 8, 3330. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Oliveira, N.K.; Savitt, A.G.; Silva, V.K.A.; Krausert, R.B.; Ghebrehiwet, B.; Fries, B.C. Low Glucose Mediated Fluconazole Tolerance in Cryptococcus neoformans. J. Fungi 2021, 7, 489. [Google Scholar] [CrossRef]
- Lang, Y.; Zhang, X.; Li, X.; Xu, Y. The mitophagosome, a novel ultrastructure of mitophagy in the alcoholic steatohepatitis mouse model: A transmission electron microscope study. Ultrastruct. Pathol. 2022, 46, 251–258. [Google Scholar] [CrossRef]
- Parzych, K.R.; Klionsky, D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).