Abstract
Brucella abortus is a bacterial pathogen causing bovine brucellosis worldwide. This facultative extracellular–intracellular pathogen can be transmitted to humans, leading to a zoonotic disease. The disease remains a public health concern, particularly in regions where livestock farming is present. The two-component regulatory system BvrR/BvrS was described by isolating the attenuated transposition mutants bvrR::Tn5 and bvrS::Tn5, whose characterization led to the understanding of the role of the system in bacterial survival. However, a phenotypic comparison with deletion mutants has not been performed because their construction has been unsuccessful in brucellae and difficult in phylogenetically related Rhizobiales with BvrR/BvrS orthologs. Here, we used an unmarked gene excision strategy to generate a B. abortus mutant strain lacking both genes, called B. abortus ∆bvrRS. The deletion was verified through PCR, Southern blot, Western blot, Sanger sequencing, and whole-genome sequencing, confirming a clean mutation without further alterations at the genome level. B. abortus ∆bvrRS shared attenuated phenotypic traits with both transposition mutants, confirming the role of BvrR/BvrS in pathogenesis and membrane integrity. This B. abortus ∆bvrRS with a non-antimicrobial marker is an excellent tool for continuing studies on the role of BvrR/BvrS in the B. abortus lifestyle.
1. Introduction
Brucella organisms are Gram-negative facultative extracellular–intracellular pathogens that cause brucellosis, a neglected zoonotic disease with a worldwide distribution. Brucella abortus infects cattle and exhibits a strong tropism for the reproductive system, causing abortion, infertility, decreased milk production, reproductive failure, epididymitis, and hence, economic losses. Humans are accidental hosts, initially developing an acute febrile disease that could become a chronic infection with osteoarticular, gastrointestinal, hepatobiliary, pulmonary, genitourinary, cardiovascular, and neurological complications [1].
Brucellae lack classical virulence factors, and their pathogenicity depends on their ability to invade, survive, and replicate inside host professional and non-professional phagocytes. During their intracellular lifecycle, brucellae avoid the endocytic pathway and redirect their trafficking to a replicative compartment derived from the endoplasmic reticulum [2,3]. Therefore, the adaptation to this intracellular trafficking requires extremely well-coordinated gene expression, mainly through two-component signal transduction systems (TCSs).
In brucellae, the TCS BvrR/BvrS comprises the sensor membrane protein BvrS and its cognate cytoplasmic response regulator BvrR. This TCS was described in 1998 through the isolation and phenotypic characterization of two B. abortus kanamycin-resistant transposition mutants, one in bvrR and the other in bvrS. These mutants were selected based on an association of brucellae virulence with peculiar membrane properties rarely present simultaneously in other Gram-negative pathogens [4], like a low-immunogenic LPS, permeability to hydrophobic agents, and resistance to bactericidal cationic peptides and polymyxin B. Both transposition mutants exhibited an attenuated phenotype characterized by reduced invasiveness and absence of replication in HeLa cells and macrophages, incapability to avoid the lysosomal route, and lack of virulence in the murine model [4]. According to subsequent studies, BvrS probably senses signals associated with the intracellular environment, like low pH and nutrient availability [5,6], and BvrR regulates cell envelope proteins for host–pathogen interactions, among them Omp25 [7], an outer membrane protein with structural functions [8] that is associated with bacterial persistence in vivo [9]. BvrR also regulates metabolic fitness [10], pathways for intracellular life [11], and virulence circuits for intracellular traffic and cell egress [5,6].
The use of transposition mutants encoding antimicrobial resistance, although not ideal, has being an alternative to the construction of mutants in this TCS, which has proven difficult to generate in brucellae [4,12] and in other phylogenetically related Rhizobiales having orthologs of BvrR/BvrS, like the Sinorhizobium meliloti TCS ExoS/ChvI [13,14,15,16,17], the Agrobacterium tumefacient TCS ChvG/ChvI [18,19,20], and the Bartonella henselae TCS BatR/BatS [21]. Therefore, a clean deletion mutant with non-acquired antibiotic resistance should be obtained to compare and corroborate phenotypes [22]. Here, we constructed a B. abortus double-null mutant strain in bvrR and bvrS called B. abortus ∆bvrRS. The phenotype of B. abortus ∆bvrRS was attenuated in the cell culture model like the one described for both transposition mutants, validating the role of BvrR/BvrS in virulence.
2. Materials and Methods
Bacterial strains, growth conditions, and plasmids. All procedures involving live B. abortus were carried out according to the “Reglamento de Bioseguridad de la CCSS 39975–0”, 2012, after the “Decreto Ejecutivo #30965-S”, 2002, and research protocol SIA 0652–19 approved by the National University, Costa Rica. The strains and plasmids used in this study are listed in Table 1. The culture media were Tryptic Soy Broth (TSB), Tryptic Soy Agar (TSA), SOC (2% tryptone, 0.5% yeast extract, 10 mM NaCl, 2.5 mM KCl, 10 mM MgCl2, 10 mM MgSO4, and 20 mM glucose), Columbia Agar supplemented with 5% (v/v) of sheep blood (CBA), and Luria–Bertani (LB) medium. When required, the following supplements were added to the different culture media: 10% (w/v) sucrose (Suc), 30 µg/mL kanamycin (Km), 100 µg/mL spectinomycin (Spc), and 30 µg/mL chloramphenicol (Cm). All bacterial cultures were incubated at 37 °C, under constant shaking at 200 rpm when necessary. The cultures were inoculated with 7 × 109 CFU in a final volume of 20 mL TSB to obtain growth curves. The optical densities were measured at 420 nm every 2 to 4 h.

Table 1.
Bacterial strains and plasmids used in this study.
The construction of B. abortus ΔbvrRS. The genes bvrR (BAW_12006) and bvrS (BAW_12007) of the parental strain B. abortus 2308W were mutated using a previously described [24,25], non-polar, unmarked gene excision strategy with modifications (Supplementary Figure S1). A bvrR upstream (Up) fragment from approximately 1 kb to the second codon of the bvrR coding sequence (coordinates 2010312 to 2011285) was amplified with the primers bvrRS-Up-For and bvrRS-Up-Rev (Supplementary Table S1). A bvrS downstream (Dn) fragment containing the last two codons of the bvrS coding region to approximately 1 kb downstream (coordinates 2013910 to 2014911) was amplified with the primers bvrRS-Dn-For and bvrRS-Dn-Rev (Supplementary Table S1). The Up fragment was cut with BamHI (Thermo Fisher Scientific, Vilnius, Lithuania), and the Dn fragment was cut with PstI (Thermo Fisher Scientific, Vilnius, Lithuania). Both fragments were treated with polynucleotide kinase in a ligase buffer and included in a single ligation mix with BamHI/PstI-digested pNPTS138 (Table 1), a suicide vector expressing kanamycin (Km) resistance, and the gene sacB for sucrose (Suc) counterselection. The resulting plasmid was called pΔbvrRS (Table 1) and lacked a 2623 bp region located between the Up and Dn fragments in the genome of the parental strain and corresponding to most of the coding sequences of bvrR and bvrS (coordinates 2011286 to 2013909). Subsequently, 1–3 μL of pΔbvrRS in distilled water was electroporated into 40 μL of B. abortus 2308W competent cells prepared as described in [24,25]. Electroporation was performed in a BTXTM cell electroporator using the following parameters: 2.5 kV at 400 ohms and 50 mF. After electroporation, 1 mL of SOC medium was added, and the cells were incubated overnight. Then, 100–200 μL volumes were plated on CBA + Km to select the clones harboring pΔbvrRS. The plates were incubated for 6–10 days. Single colonies were selected, transferred into TSB, and incubated overnight. Volumes of 50, 100, and 200 μL were plated on TSA + 10% Suc to counterselect clones integrating the Up–Dn construct into the chromosome of the parental strain by allelic exchange. The plates were incubated for 3–6 days. Single colonies were picked, and exact replicas were cultured on TSA + 10% Suc and TSA+ Km, to select SucR and KmS clones that were expected to have lost both bvrRS genes and the suicidal plasmid. The plates were incubated for 2–3 days. The SucR and KmS clones were screened by colony PCR with the primers bvrRS-Con-For and bvrRS-Con-Rev (Supplementary Table S1) in a reaction with DreamTaq PCR Master Mix (2X) (Thermo Fisher Scientific, Vilnius, Lithuania). The “Con” primers amplify a 3332 bp fragment in the parental strain (coordinates 2011017 to 2014349) and a 709 bp fragment in the B. abortus ΔbvrRS mutant strain. Clones giving the expected result for the mutant strain were also subjected to PCR with the bvrR_bv1 pair of primers (Supplementary Table S1). The “bv1” primers amplify a 63 bp fragment of the bvrR coding sequence in the parental strain (coordinates 2011468 to 2011531) and are not expected to amplify any PCR product in the mutant strain. Mutant clones selected based on the presence of the 709 bp Con-amplicon and the absence of the 63 bp bv1-amplicon, were subjected to biochemical identification tests and further confirmation by DNA Sanger sequencing, Southern blot, Western blot, and whole-genome sequencing, as described below.
Biochemical identification. Basic biochemical tests were performed as described in [26] using the potential mutant clones and the strain B. abortus 2308W. The performed tests included urease (1 h and 24 h), oxidase, H2S production, nitrate reduction, and sensitivity to thionine (20 µg/mL) (24 h and 72 h) and basic fuchsin (20 µg/mL) (24 h and 72 h). The presence of a smooth LPS was verified through the acriflavine agglutination test as described in [26].
Southern Blot. The mutation was confirmed by a Southern blot as described in [27], with a few modifications. Genomic DNA from the parental and the mutant strains was extracted with the Wizard® Genomic DNA Purification Kit (Promega, Madison, WI, USA). Purified DNA was double digested with 25 U of BshTI (AgeI) and 12.5 U of MlsI (MscI) restriction enzymes (Thermo Scientific, USA), respectively, cutting the B. abortus 2308W genomic DNA at positions 2011220 (−60 from bvrR start codon) and 2014898 (+983 from bvrS stop codon) and, respectively, generating 3679 bp and 1055 bp fragments in the parental strain and in the B. abortus ∆bvrRS mutant strains. The digested DNA of each strain was separated through electrophoresis in a 0.6% agarose gel for 2 h at 100 V, and the gel was blotted onto a nylon membrane (Roche, Mannheim, Germany). The probe was generated using the primers bvrRS-South (Supplementary Table S1), amplifying a fragment of 299 bp of the coding sequence of the bvrS downstream gene (BAW_12008, coordinates 2013977 to 2014276). Probe labeling, hybridization, and detection were performed using the DIG DNA Labeling and Detection kit (Roche, Mannheim, Germany).
Western Blot. The BvrS, BvrR, and Omp25 expressions were assessed as described in [27], with modifications. The bacterial cultures were grown for up to 32 h to test protein expression at different growth phases. The loading control was Omp19.
Sanger sequencing. The Con-amplicon of the mutant strain was Sanger sequenced to confirm the absence of bvrR and bvrS. The regions recombined during the deletion of the bvrR and bvrS genes were also sequenced. The upstream recombination region was amplified with pckA primers (Supplementary Table S1), obtaining an amplicon of 677 bp (coordinates 2009968 to 2010645). The downstream recombination region was amplified with the revtrans20682069-2.3 and revtrans20692070-2.5 primers (Supplementary Table S1), obtaining an amplicon of 628 bp (coordinates 2014621 to 2015249). All amplicons were cycle sequenced using the BigDye™ Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific, Vilnius, Lithuania) and sent to “Centro de Investigación en Biología Celular y Molecular”, at the University of Costa Rica, for capillary electrophoresis. The sequencing results were analyzed using BLAST (Basic Local Alignment Search Tool) [28], with blastn and default parameters to look for their correspondence in the genomic sequence of B. abortus 2308, chromosome I (NCBI Reference Sequence: NC_007618.1).
Whole-genome sequencing. For whole-genome sequencing, genomic DNA from B. abortus 2308W and B. abortus ∆bvrRS was obtained using the Wizard® Genomic DNA Purification Kit (Promega, Madison, WI, USA) from liquid cultures grown to the stationary phase. Genomic libraries were prepared with the rapid barcoding sequencing SQK-RBK004 kit (Oxford Nanopore Technologies, Oxford, UK) for sequencing on a minION platform using the MinKNOW software version 22.08.4, according to the manufacturers’ recommendations. Base calling and the conversion of the raw data to the FASTQ format were performed with Guppy v.3.6.0., while the reads’ quality was verified with nanoplot 1.40.2 [29]. Low-quality reads (Q < 10 and minimum length of 1000) were filtered using nanofilt 2.8.0 [29]. Adapters and barcodes were trimmed with Porechop 0.2.4. Reads that passed the quality check were used for whole-genome assembly with Unicycler v0.4.8 [30]. The assembled genomes were verified with QUAST 5.0.2 (Quality Assessment Tool for Genome Assemblies Version) [31] and CheckM v1.1.3 for completeness and contamination [32]. The project was deposited at DDBJ/ENA/GenBank under the following accession numbers: Bioproject PRJNA891361, assemblies CP109916-CP109917 and CP109914-CP109915, and raw sequencing data SRR21939256 and SRR21939255 for B. abortus 2308W and B. abortus ∆bvrRS, respectively. The resulting complete-genome assemblies at the chromosome level were compared using BLAST [33] with blastn type and default parameters to verify the genomic differences between B. abortus ∆bvrRS and B. abortus 2308W. Genome content coverage was also verified by read mapping against the assembled genome using the long-read mapping pipeline Vulcan 1.0.3 [34]. Genomic visualizations were conducted with Artemis Comparison Tool 18.2.0 [35].
Gentamicin-protection assay and intracellular replication quantification. Murine RAW 264.7 macrophages (ATCC TIB-71) or HeLa epithelial cells (ATCC clone CCl-2) were cultivated and infected with B. abortus strains in the exponential growth phase as described in [5,27]. The number of intracellular, viable B. abortus CFUs was determined at 0, 24, and 48 h after infection.
Membrane integrity tests. The sensitivity to the bactericidal action of non-immune serum was tested as described in [27], with an incubation time of 45 min with the non-immune serum instead of 90 min. The minimal inhibitory concentration of polymyxin B in TSB at pH 7.0 and 6.0 was determined by the microdilution method as described in [27].
Statistical analyses. The Kruskal–Wallis test for multiple comparisons was used as described in [27].
3. Results
3.1. The ∆bvrRS Mutant Strain Is a Null Mutant
In this study, we constructed a null mutant with a deletion in bvrR and bvrS using an unmarked gene excision approach. The colony PCR screening of the SucR and KmS clones revealed four potential mutant clones lacking bvrR and bvrS, numbered 3, 4, 41, and 42 (Supplementary Figure S2). Clone 4 was selected for further analysis and renamed B. abortus ∆bvrRS. This strain tested positive in the following biochemical tests used for the identification of the Brucella genus: urease at 1 h and 4 h; oxidase, H2S production, and resistance to fuchsin (20 µg/mL) and thionine (20 µg/mL) at 72 h; and negative reactions to nitrate reduction and resistance to fuchsin (20 µg/mL) and thionine (20 µg/mL) at 24 h.
Like the parental strain, B. abortus ∆bvrRS excluded crystal violet, did not agglutinate with acriflavine, and agglutinated with antibodies against smooth LPS, indicating the conservation of the S-LPS phenotype as the parental strain. In TSB, B. abortus ∆bvrRS consistently exhibited a prolonged lag phase in all independent replicas, retarding entry into the log phase and catching up with B. abortus 2308 W in the stationary phase (Figure 1a). The Southern blot results demonstrated a deletion of approximately 3000 bp (Figure 1b); the Western blot results confirmed a lack of expression of BvrR and BvrS (Figure 1c), and the Sanger sequencing results showed an in-frame deletion of the bvrRS genes and the correct recombination of the Up and the Dn fragments.
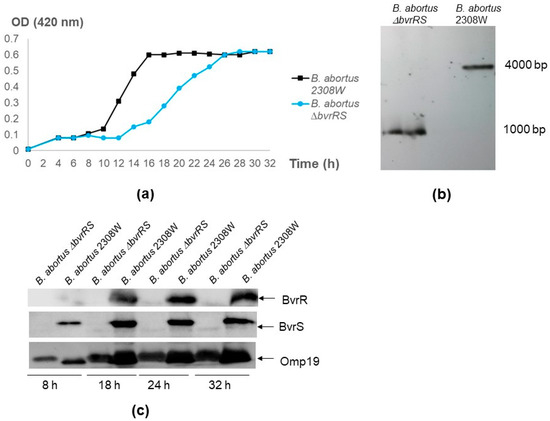
Figure 1.
Confirmation of the B. abortus ∆bvrRS double-mutant strain. (a) Growth curve of B. abortus ∆bvrRS compared to B. abortus 2308W. An inoculum of 7 × 109 CFU/mL was incubated in TSB at 37 °C and 200 rpm. Optical density (OD) at 420 nm was measured every 2–3 h, up to 32 h. (b) Southern blot assay. Genomic DNA of B. abortus ∆bvrRS and B. abortus 2308W was extracted and digested with BshTI (AgeI) and MlsI (MscI). A probe targeting the coding sequence of BAW_12008 downstream of bvrS, hybridized with a ~1000 bp band in B. abortus ∆bvrRS and a ~4000 bp band in B. abortus 2308W. The difference in molecular weight between these two bands matched the size of the excised genes (2635 bp), confirming the deletion of the bvrRS genes. (c) Western blot analysis of BvrR and BvrS expression according to growth phase in B. abortus ∆bvrRS and B. abortus 2308W. Both strains were grown in TSB for 32 h, and representative time points of the different growth phases were analyzed according to the growth curves shown in the first panel. Equal amounts (20 μg) of whole-bacterium lysates were separated through 12.5% SDS-PAGE, blotted, and analyzed with anti-BvrR, anti-BvrS, and anti-Omp19 as the loading control. The results are representative of at least three independent experiments.
3.2. Whole-Genome Sequencing
The obtained coverage was over 68×; the GC content was 57.22% GC, and over 97.99% predicted completeness was achieved with contamination below 0.71% (complete sequencing and assembly results in Supplementary Table S2). The genome assembly resulted in two contigs for both strains, representing the complete chromosomes of B. abortus composed of 2.1 and 1.2 Mb, respectively.
BLAST comparison between the strain 2308W and the mutant strain confirmed the deletion of the bvrRS genes (coordinates 2011286 to 2013909) without further alterations in the genome (Figure 2).

Figure 2.
BLAST comparison of the genomic sequences obtained from the strains B. abortus ∆bvrRS and B. abortus 2308W, confirming the deletion of the bvrRS genes in B. abortus ∆bvrRS. The orange lines show the 2.1 Mb chromosome of B. abortus, with the reads’ coverage across the genome shown by a mapping visualization in the upper and lower graphs. The section of the chromosome with alignment gaps is highlighted and amplified, showing gray lines as the genome sequences and blue boxes as coding sequences (CDSs), and the red in the middle represents 99% identity among regions compared. Deletion of the genes bvrR and bvrS is shown by the absence of similarity among both regions (white triangle). Up- and downstream CDSs show no additional alterations in B. abortus ∆bvrRS.
3.3. The ∆bvrRS Mutant Strain Displays an Attenuated Phenotype
To characterize the virulence phenotype of B. abortus ∆bvrRS, we assessed its ability to replicate intracellularly in HeLa cells and RAW macrophages. As shown in Figure 3, B. abortus 2308W was able to replicate intracellularly in both types of cells, but B. abortus ∆bvrRS and the transposition mutants, B. abortus bvrR::Tn5 and B. abortus bvrS::Tn5, did not, confirming attenuation in the cell model.
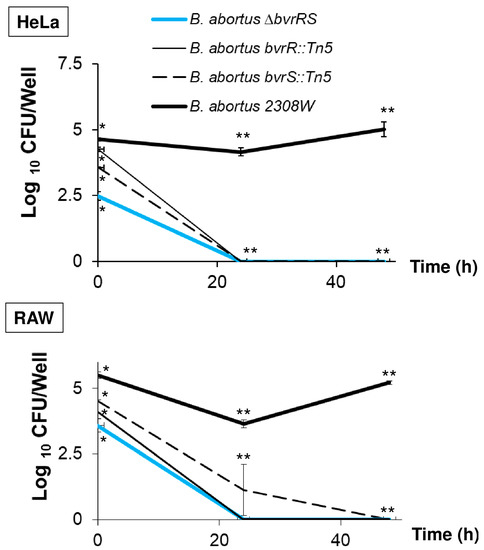
Figure 3.
B. abortus ∆bvrRS displays lack of replication in HeLa cells and RAW macrophages. Cell cultures were grown in DMEM until approximately 80% of confluence and then inoculated in triplicate with an MOI of 100 for HeLa and 500 for the RAW macrophages. The extracellular bacteria were killed with gentamicin, and the infected cells were incubated for 0, 24, and 48 h, and bacterial counts were performed in triplicate at the three post-infection times. The results are representative of at least three independent experiments. * Statistically significant differences (p < 0.05) between the four strains (Kruskal–Wallis test for multiple comparisons). ** Statistically significant differences (p < 0.05) between B. abortus 2308W and the other three strains (Kruskal–Wallis test for multiple comparisons).
3.4. The ∆bvrRS Mutant Strain Exhibits Altered Membrane Integrity
To assess the membrane integrity of B. abortus ∆bvrRS, we first performed a susceptibility to polymyxin B test. As shown in Table 2, at pH 7.0 and pH 6.0, the MIC values of B. abortus ∆bvrRS and both transposition mutants, B. abortus bvrR::Tn5 and B. abortus bvrS::Tn5, were lower by at least two dilutions of difference than those of the parental strain, suggesting altered membrane integrity. All the MICs increased by at least two dilutions of difference compared to that at pH 7.0, indicating a higher resistance under acidic conditions.

Table 2.
Results of the susceptibility test for polymyxin B.
Then, we confirmed that B. abortus ∆bvrRS was susceptible to the action of a non-immune serum, unlike the parental strain (Figure 4a), and we evaluated the expression of Omp25, known to be directly regulated by the TCS BvrR/BvrS, confirming that B. abortus ∆bvrRS displayed a lack of expression of this outer membrane protein, unlike B. abortus 2308W (Figure 4b).

Figure 4.
Characterization of the membrane integrity of B. abortus ∆bvrRS. (a) Loss of resistance to non-immune serum in B. abortus ∆bvrRS compared to B. abortus 2308W. Both strains were exposed to non-immune human serum at 37 °C for 45 min, and the survival percentage was calculated. (b) Western blot analysis of Omp25 expression, according to growth phase in B. abortus ∆bvrRS and B. abortus 2308W. Both strains were grown in TSB for 32 h, and representative time points of the different growth phases were analyzed according to the growth curves shown in Figure 1a. Equal amounts (20 μg) of whole-bacterium lysates were separated through 12.5% SDS-PAGE, blotted, and analyzed with anti-Omp25 and Omp19 as the loading control. These results are representative of at least three independent experiments. * p < 0.05 (Kruskal–Wallis test for multiple comparisons).
4. Discussion
The role of the TCS BvrR/BvrS in the virulence of brucellae has been studied in transposition mutants with a kanamycin-resistance marker in the inserted transposon [4]. Construction and characterization of a null mutant was necessary to compare and corroborate phenotypic traits and to obtain an antibiotic-marker-free mutant strain. However, previous attempts to construct clean mutants were unsuccessful, prompting the designation of the bvrRS genes as essential [12]. Later it was shown that they did not seem to be essential for extracellular growth [36], which implied that they could be mutated. Since the construction of single mutants in several orthologs of BvrR/BvrS in other cell-associated Rhizobiales was consistently reported as difficult [13,14,15,16,17,18,19,20,21], we employed a non-polar, unmarked gene excision strategy [24,25] to construct a B. abortus mutant strain with a deletion in both bvrRS genes. Different experimental approaches confirmed the in-frame double deletion, and the whole-genome sequencing results demonstrated a clean null mutation without further alterations in the genome.
The phenotypic characterization of B. abortus ∆bvrRS revealed a smooth LPS, unaltered biochemical characteristics, retarded lag growth phase defects, inability to replicate intracellularly in cell models, susceptibility to polymyxin B and to human non-immune serum, and lack of expression of Omp25. These results are consistent with previous studies about the role of the TCS BvrR/BvrS that were performed using the transposition mutants B. abortus bvrR::Tn5 and B. abortus bvrS::Tn5 [4,7,37].
In conclusion, a B. abortus null mutant in the bvrR/bvrS genes was successfully obtained through allelic replacement. This novel mutant does not harbor any resistant marker gene and will be useful for further studies on the TCS BvrR/BvrS.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/microorganisms11082014/s1: Figure S1: Unmarked gene excision approach used in this study to generate the ΔbvrRS mutant; Figure S2: Colony PCR screening of the SucR and KmS clones to look for potential mutants; Table S1: List of primers used in this study; Table S2: Quality results of B. abortus sequencing and whole-genome assembly.
Author Contributions
Conceptualization, C.G.-V.; methodology, O.R.-S. and K.N.-M.; validation, P.A.-S., N.R.-V. and E.B.-C.; formal analysis, O.R.-S., K.N.-M. and P.A.-S.; investigation, O.R.-S., K.N.-M., P.A.-S., N.R.-V., E.B.-C., E.M., E.C.-O. and C.G.-V.; resources, E.C.-O. and K.N.-M.; data curation, K.N.-M.; writing—original draft preparation, O.R.-S. and C.G.-V.; writing—review and editing, O.R.-S., K.N.-M., P.A.-S., N.R.-V., E.B.-C., E.M., E.C.-O. and C.G.-V.; visualization, O.R.-S., K.N.-M. and P.A.-S.; supervision, C.G.-V., E.M. and E.C.-O.; project administration, C.G.-V., E.C.-O., O.R.-S. and K.N.-M.; funding acquisition, C.G.-V., E.C.-O., O.R.-S. and K.N.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FEES-CONARE, grant 02-2020 to C.G.-V.; FIDA-UNA, grant SIA 0047-17 to C.G.-V.; UCREA-UCR, grant B8762 and C0456 to E.C.-O.; ITCR, grant 15-15-D to O.R.-S. and 1510171 to K.N.-M.; PINN-MICITT, grant PND-137-15-1 to O.R.-S.; and ANID-CHILE, grant FONDECYT 1210563 to K.N.-M.
Data Availability Statement
The data presented in this study are openly available in GenBank, accession numbers [CP109914, CP109915, CP109916, CP109917], and the SRA accession numbers for the raw reads are SRR21939256 and SRR21939255.
Acknowledgments
To Clayton C. Caswell and Roy Martin Roop II for the pΔbvrRS. To Axel Cloeckaert for providing monoclonal antibodies against Omp19 and Omp25. To Danilo Solano Quesada for his technical help with Western blot assays.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of this manuscript; or in the decision to publish the results.
References
- Corbel, M.J.; Food and Agriculture Organization of the United Nations; World Health Organization; World Organisation for Animal Health. Brucellosis in Humans and Animals; World Health Organization: Geneva, Switzerland, 2006.
- Gorvel, J.P.; Moreno, E. Brucella Intracellular Life: From Invasion to Intracellular Replication. Vet. Microbiol. 2002, 90, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.; Moriyón, I. Brucella melitensis: A Nasty Bug with Hidden Credentials for Virulence. Proc. Natl. Acad. Sci. USA 2002, 99, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Sola-Landa, A.; Pizarro-Cerdá, J.; Grilló, M.; Moreno, E.; Moriyón, I.; Blasco, J.; Gorvel, J.; López-Goñi, I. A Two-component Regulatory System Playing a Critical Role in Plant Pathogens and Endosymbionts Is Present in Brucella abortus and Controls Cell Invasion and Virulence. Mol. Microbiol. 1998, 29, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Altamirano-Silva, P.; Meza-Torres, J.; Castillo-Zeledón, A.; Ruiz-Villalobos, N.; Zuñiga-Pereira, A.M.; Chacón-Díaz, C.; Moreno, E.; Guzmán-Verri, C.; Chaves-Olarte, E. Brucella abortus Senses the Intracellular Environment through the BvrR/BvrS Two-Component System, Which Allows B. Abortus To Adapt to Its Replicative Niche. Infect. Immun. 2018, 86, e00713-17. [Google Scholar] [CrossRef]
- Altamirano-Silva, P.; Cordero-Serrano, M.; Méndez-Montoya, J.; Chacón-Díaz, C.; Guzmán-Verri, C.; Moreno, E.; Chaves-Olarte, E. Intracellular Passage Triggers a Molecular Response in Brucella abortus That Increases Its Infectiousness. Infect. Immun. 2021, 89, e00004-21. [Google Scholar] [CrossRef]
- Guzmán-Verri, C.; Manterola, L.; Sola-Landa, A.; Parra, A.; Cloeckaert, A.; Garin, J.; Gorvel, J.-P.; Moriyón, I.; Moreno, E.; López-Goñi, I. The Two-Component System BvrR/BvrS Essential for Brucella abortus Virulence Regulates the Expression of Outer Membrane Proteins with Counterparts in Members of the Rhizobiaceae. Proc. Natl. Acad. Sci. USA 2002, 99, 12375–12380. [Google Scholar] [CrossRef]
- Godessart, P.; Lannoy, A.; Dieu, M.; Van der Verren, S.E.; Soumillion, P.; Collet, J.-F.; Remaut, H.; Renard, P.; De Bolle, X. β-Barrels Covalently Link Peptidoglycan and the Outer Membrane in the α-Proteobacterium Brucella abortus. Nat. Microbiol. 2021, 6, 27–33. [Google Scholar] [CrossRef]
- Degos, C.; Hysenaj, L.; Gonzalez-Espinoza, G.; Arce-Gorvel, V.; Gagnaire, A.; Papadopoulos, A.; Pasquevich, K.A.; Méresse, S.; Cassataro, J.; Mémet, S.; et al. Omp25-dependent Engagement of SLAMF1 by Brucella abortus in Dendritic Cells Limits Acute Inflammation and Favours Bacterial Persistence in Vivo. Cell. Microbiol. 2020, 22, e13164. [Google Scholar] [CrossRef]
- Viadas, C.; Rodríguez, M.C.; Sangari, F.J.; Gorvel, J.-P.; García-Lobo, J.M.; López-Goñi, I. Transcriptome Analysis of the Brucella abortus BvrR/BvrS Two-Component Regulatory System. PLoS ONE 2010, 5, e10216. [Google Scholar] [CrossRef]
- Rivas-Solano, O.; Van der Henst, M.; Castillo-Zeledón, A.; Suárez-Esquivel, M.; Muñoz-Vargas, L.; Capitan-Barrios, Z.; Thomson, N.R.; Chaves-Olarte, E.; Moreno, E.; De Bolle, X.; et al. The Regulon of Brucella abortus Two-Component System BvrR/BvrS Reveals the Coordination of Metabolic Pathways Required for Intracellular Life. PLoS ONE 2022, 17, e0274397. [Google Scholar] [CrossRef] [PubMed]
- Barbier, T.; Nicolas, C.; Letesson, J.J. Brucella Adaptation and Survival at the Crossroad of Metabolism and Virulence. FEBS Lett. 2011, 585, 2929–2934. [Google Scholar] [CrossRef] [PubMed]
- Doherty, D.; Leigh, J.A.; Glazebrook, J.; Walker, G.C. Rhizobium Meliloti Mutants That Overproduce the R. meliloti Acidic Calcofluor-Binding Exopolysaccharide. J. Bacteriol. 1988, 170, 4249–4256. [Google Scholar] [CrossRef] [PubMed]
- Osterås, M.; Stanley, J.; Finan, T.M. Identification of Rhizobium-Specific Intergenic Mosaic Elements within an Essential Two-Component Regulatory System of Rhizobium Species. J. Bacteriol. 1995, 177, 5485–5494. [Google Scholar] [CrossRef]
- Cheng, H.-P.; Walker, G.C. Succinoglycan Production by Rhizobium meliloti Is Regulated through the ExoS-ChvI Two-Component Regulatory System. J. Bacteriol. 1998, 180, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.H.; Chen, E.J.; Fisher, R.F.; Long, S.R. ExoR Is Genetically Coupled to the ExoS?ChvI Two-Component System and Located in the Periplasm of Sinorhizobium meliloti. Mol. Microbiol. 2007, 64, 647–664. [Google Scholar] [CrossRef]
- Bélanger, L.; Dimmick, K.A.; Fleming, J.S.; Charles, T.C. Null Mutations in Sinorhizobium meliloti ExoS and ChvI Demonstrate the Importance of This Two-Component Regulatory System for Symbiosis. Mol. Microbiol. 2009, 74, 1223–1237. [Google Scholar] [CrossRef]
- Charles, T.C.; Nester, E.W. A Chromosomally Encoded Two-Component Sensory Transduction System Is Required for Virulence of Agrobacterium tumefaciens. J. Bacteriol. 1993, 175, 6614–6625. [Google Scholar] [CrossRef]
- Mantis, N.J.; Winans, S.C. The Chromosomal Response Regulatory Gene ChvI of Agrobacterium tumefaciens Complements an Escherichia coli PhoB Mutation and Is Required for Virulence. J. Bacteriol. 1993, 175, 6626–6636. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-F.; Lin, J.-S.; Shaw, G.-C.; Lai, E.-M. Acid-Induced Type VI Secretion System Is Regulated by ExoR-ChvG/ChvI Signaling Cascade in Agrobacterium tumefaciens. PLoS Pathog. 2012, 8, e1002938. [Google Scholar] [CrossRef]
- Quebatte, M.; Dehio, M.; Tropel, D.; Basler, A.; Toller, I.; Raddatz, G.; Engel, P.; Huser, S.; Schein, H.; Lindroos, H.L.; et al. The BatR/BatS Two-Component Regulatory System Controls the Adaptive Response of Bartonella henselae during Human Endothelial Cell Infection. J. Bacteriol. 2010, 192, 3352–3367. [Google Scholar] [CrossRef]
- Maksymiuk, C.; Ioerger, T.; Balakrishnan, A.; Bryk, R.; Rhee, K.; Sacchettini, J.; Nathan, C. Comparison of Transposon and Deletion Mutants in Mycobacterium tuberculosis: The Case of Rv1248c, Encoding 2-Hydroxy-3-Oxoadipate Synthase. Tuberculosis 2015, 95, 689–694. [Google Scholar] [CrossRef]
- Suárez-Esquivel, M.; Ruiz-Villalobos, N.; Castillo-Zeledón, A.; Jiménez-Rojas, C.; Roop II, R.M.; Comerci, D.J.; Barquero-Calvo, E.; Chacón-Díaz, C.; Caswell, C.C.; Baker, K.S.; et al. Brucella abortus Strain 2308 Wisconsin Genome: Importance of the Definition of Reference Strains. Front. Microbiol. 2016, 7, 1557. [Google Scholar] [CrossRef] [PubMed]
- Caswell, C.C.; Gaines, J.M.; Roop, R.M. The RNA Chaperone Hfq Independently Coordinates Expression of the VirB Type IV Secretion System and the LuxR-Type Regulator BabR in Brucella abortus 2308. J. Bacteriol. 2012, 194, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Caswell, C.C.; Gaines, J.M.; Ciborowski, P.; Smith, D.; Borchers, C.H.; Roux, C.M.; Sayood, K.; Dunman, P.M.; Roop II, R.M. Identification of Two Small Regulatory RNAs Linked to Virulence in Brucella abortus 2308: Brucella Small RNAs Are Required for Virulence. Mol. Microbiol. 2012, 85, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Alton, G.G.; Jones, L.M.; Angus, R.D.; Verger, J.M. Techniques for the Brucellosis Laboratory; INRA: Paris, France, 1988; ISBN 978-2-7380-0042-2.
- Castillo-Zeledón, A.; Ruiz-Villalobos, N.; Altamirano-Silva, P.; Chacón-Díaz, C.; Barquero-Calvo, E.; Chaves-Olarte, E.; Guzmán-Verri, C. A Sinorhizobium meliloti and Agrobacterium tumefaciens ExoR Ortholog Is Not Crucial for Brucella abortus Virulence. PLoS ONE 2021, 16, e0254568. [Google Scholar] [CrossRef]
- Altschu, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- De Coster, W.; D’Hert, S.; Schultz, D.T.; Cruts, M.; Van Broeckhoven, C. NanoPack: Visualizing and Processing Long-Read Sequencing Data. Bioinformatics 2018, 34, 2666–2669. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving Bacterial Genome Assemblies from Short and Long Sequencing Reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality Assessment Tool for Genome Assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the Quality of Microbial Genomes Recovered from Isolates, Single Cells, and Metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef]
- BLAST Help Page. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi?CMD=Web&PAGE_TYPE=BlastDocs& (accessed on 24 October 2022).
- Fu, Y.; Mahmoud, M.; Muraliraman, V.V.; Sedlazeck, F.J.; Treangen, T.J. Vulcan: Improved Long-Read Mapping and Structural Variant Calling via Dual-Mode Alignment. GigaScience 2021, 10, giab063. [Google Scholar] [CrossRef] [PubMed]
- Carver, T.J.; Rutherford, K.M.; Berriman, M.; Rajandream, M.-A.; Barrell, B.G.; Parkhill, J. ACT: The Artemis Comparison Tool. Bioinformatics 2005, 21, 3422–3423. [Google Scholar] [CrossRef] [PubMed]
- Sternon, J.-F.; Godessart, P.; Gonçalves de Freitas, R.; Van der Henst, M.; Poncin, K.; Francis, N.; Willemart, K.; Christen, M.; Christen, B.; Letesson, J.-J.; et al. Transposon Sequencing of Brucella abortus Uncovers Essential Genes for Growth In Vitro and Inside Macrophages. Infect. Immun. 2018, 86, e00312-18. [Google Scholar] [CrossRef] [PubMed]
- Manterola, L.; Moriyón, I.; Moreno, E.; Sola-Landa, A.; Weiss, D.S.; Koch, M.H.J.; Howe, J.; Brandenburg, K.; López-Goñi, I. The Lipopolysaccharide of Brucella abortus BvrS/BvrR Mutants Contains Lipid A Modifications and Has Higher Affinity for Bactericidal Cationic Peptides. J. Bacteriol. 2005, 187, 5631–5639. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).