A bvrR/bvrS Non-Polar Brucella abortus Mutant Confirms the Role of the Two-Component System BvrR/BvrS in Virulence and Membrane Integrity
Abstract
1. Introduction
2. Materials and Methods
3. Results
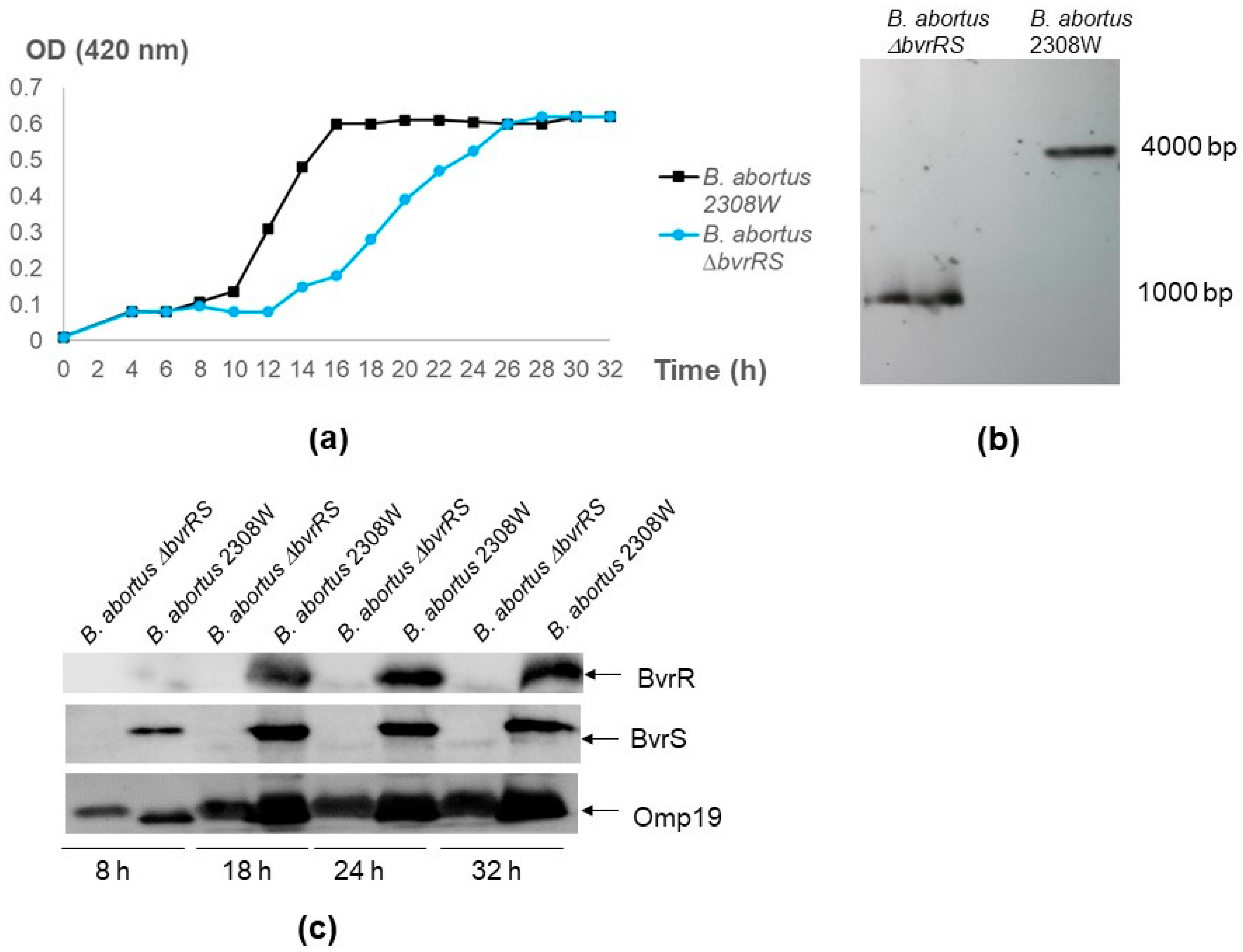
3.1. The ∆bvrRS Mutant Strain Is a Null Mutant
3.2. Whole-Genome Sequencing
3.3. The ∆bvrRS Mutant Strain Displays an Attenuated Phenotype
3.4. The ∆bvrRS Mutant Strain Exhibits Altered Membrane Integrity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corbel, M.J.; Food and Agriculture Organization of the United Nations; World Health Organization; World Organisation for Animal Health. Brucellosis in Humans and Animals; World Health Organization: Geneva, Switzerland, 2006.
- Gorvel, J.P.; Moreno, E. Brucella Intracellular Life: From Invasion to Intracellular Replication. Vet. Microbiol. 2002, 90, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.; Moriyón, I. Brucella melitensis: A Nasty Bug with Hidden Credentials for Virulence. Proc. Natl. Acad. Sci. USA 2002, 99, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Sola-Landa, A.; Pizarro-Cerdá, J.; Grilló, M.; Moreno, E.; Moriyón, I.; Blasco, J.; Gorvel, J.; López-Goñi, I. A Two-component Regulatory System Playing a Critical Role in Plant Pathogens and Endosymbionts Is Present in Brucella abortus and Controls Cell Invasion and Virulence. Mol. Microbiol. 1998, 29, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Altamirano-Silva, P.; Meza-Torres, J.; Castillo-Zeledón, A.; Ruiz-Villalobos, N.; Zuñiga-Pereira, A.M.; Chacón-Díaz, C.; Moreno, E.; Guzmán-Verri, C.; Chaves-Olarte, E. Brucella abortus Senses the Intracellular Environment through the BvrR/BvrS Two-Component System, Which Allows B. Abortus To Adapt to Its Replicative Niche. Infect. Immun. 2018, 86, e00713-17. [Google Scholar] [CrossRef]
- Altamirano-Silva, P.; Cordero-Serrano, M.; Méndez-Montoya, J.; Chacón-Díaz, C.; Guzmán-Verri, C.; Moreno, E.; Chaves-Olarte, E. Intracellular Passage Triggers a Molecular Response in Brucella abortus That Increases Its Infectiousness. Infect. Immun. 2021, 89, e00004-21. [Google Scholar] [CrossRef]
- Guzmán-Verri, C.; Manterola, L.; Sola-Landa, A.; Parra, A.; Cloeckaert, A.; Garin, J.; Gorvel, J.-P.; Moriyón, I.; Moreno, E.; López-Goñi, I. The Two-Component System BvrR/BvrS Essential for Brucella abortus Virulence Regulates the Expression of Outer Membrane Proteins with Counterparts in Members of the Rhizobiaceae. Proc. Natl. Acad. Sci. USA 2002, 99, 12375–12380. [Google Scholar] [CrossRef]
- Godessart, P.; Lannoy, A.; Dieu, M.; Van der Verren, S.E.; Soumillion, P.; Collet, J.-F.; Remaut, H.; Renard, P.; De Bolle, X. β-Barrels Covalently Link Peptidoglycan and the Outer Membrane in the α-Proteobacterium Brucella abortus. Nat. Microbiol. 2021, 6, 27–33. [Google Scholar] [CrossRef]
- Degos, C.; Hysenaj, L.; Gonzalez-Espinoza, G.; Arce-Gorvel, V.; Gagnaire, A.; Papadopoulos, A.; Pasquevich, K.A.; Méresse, S.; Cassataro, J.; Mémet, S.; et al. Omp25-dependent Engagement of SLAMF1 by Brucella abortus in Dendritic Cells Limits Acute Inflammation and Favours Bacterial Persistence in Vivo. Cell. Microbiol. 2020, 22, e13164. [Google Scholar] [CrossRef]
- Viadas, C.; Rodríguez, M.C.; Sangari, F.J.; Gorvel, J.-P.; García-Lobo, J.M.; López-Goñi, I. Transcriptome Analysis of the Brucella abortus BvrR/BvrS Two-Component Regulatory System. PLoS ONE 2010, 5, e10216. [Google Scholar] [CrossRef]
- Rivas-Solano, O.; Van der Henst, M.; Castillo-Zeledón, A.; Suárez-Esquivel, M.; Muñoz-Vargas, L.; Capitan-Barrios, Z.; Thomson, N.R.; Chaves-Olarte, E.; Moreno, E.; De Bolle, X.; et al. The Regulon of Brucella abortus Two-Component System BvrR/BvrS Reveals the Coordination of Metabolic Pathways Required for Intracellular Life. PLoS ONE 2022, 17, e0274397. [Google Scholar] [CrossRef] [PubMed]
- Barbier, T.; Nicolas, C.; Letesson, J.J. Brucella Adaptation and Survival at the Crossroad of Metabolism and Virulence. FEBS Lett. 2011, 585, 2929–2934. [Google Scholar] [CrossRef] [PubMed]
- Doherty, D.; Leigh, J.A.; Glazebrook, J.; Walker, G.C. Rhizobium Meliloti Mutants That Overproduce the R. meliloti Acidic Calcofluor-Binding Exopolysaccharide. J. Bacteriol. 1988, 170, 4249–4256. [Google Scholar] [CrossRef] [PubMed]
- Osterås, M.; Stanley, J.; Finan, T.M. Identification of Rhizobium-Specific Intergenic Mosaic Elements within an Essential Two-Component Regulatory System of Rhizobium Species. J. Bacteriol. 1995, 177, 5485–5494. [Google Scholar] [CrossRef]
- Cheng, H.-P.; Walker, G.C. Succinoglycan Production by Rhizobium meliloti Is Regulated through the ExoS-ChvI Two-Component Regulatory System. J. Bacteriol. 1998, 180, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.H.; Chen, E.J.; Fisher, R.F.; Long, S.R. ExoR Is Genetically Coupled to the ExoS?ChvI Two-Component System and Located in the Periplasm of Sinorhizobium meliloti. Mol. Microbiol. 2007, 64, 647–664. [Google Scholar] [CrossRef]
- Bélanger, L.; Dimmick, K.A.; Fleming, J.S.; Charles, T.C. Null Mutations in Sinorhizobium meliloti ExoS and ChvI Demonstrate the Importance of This Two-Component Regulatory System for Symbiosis. Mol. Microbiol. 2009, 74, 1223–1237. [Google Scholar] [CrossRef]
- Charles, T.C.; Nester, E.W. A Chromosomally Encoded Two-Component Sensory Transduction System Is Required for Virulence of Agrobacterium tumefaciens. J. Bacteriol. 1993, 175, 6614–6625. [Google Scholar] [CrossRef]
- Mantis, N.J.; Winans, S.C. The Chromosomal Response Regulatory Gene ChvI of Agrobacterium tumefaciens Complements an Escherichia coli PhoB Mutation and Is Required for Virulence. J. Bacteriol. 1993, 175, 6626–6636. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-F.; Lin, J.-S.; Shaw, G.-C.; Lai, E.-M. Acid-Induced Type VI Secretion System Is Regulated by ExoR-ChvG/ChvI Signaling Cascade in Agrobacterium tumefaciens. PLoS Pathog. 2012, 8, e1002938. [Google Scholar] [CrossRef]
- Quebatte, M.; Dehio, M.; Tropel, D.; Basler, A.; Toller, I.; Raddatz, G.; Engel, P.; Huser, S.; Schein, H.; Lindroos, H.L.; et al. The BatR/BatS Two-Component Regulatory System Controls the Adaptive Response of Bartonella henselae during Human Endothelial Cell Infection. J. Bacteriol. 2010, 192, 3352–3367. [Google Scholar] [CrossRef]
- Maksymiuk, C.; Ioerger, T.; Balakrishnan, A.; Bryk, R.; Rhee, K.; Sacchettini, J.; Nathan, C. Comparison of Transposon and Deletion Mutants in Mycobacterium tuberculosis: The Case of Rv1248c, Encoding 2-Hydroxy-3-Oxoadipate Synthase. Tuberculosis 2015, 95, 689–694. [Google Scholar] [CrossRef]
- Suárez-Esquivel, M.; Ruiz-Villalobos, N.; Castillo-Zeledón, A.; Jiménez-Rojas, C.; Roop II, R.M.; Comerci, D.J.; Barquero-Calvo, E.; Chacón-Díaz, C.; Caswell, C.C.; Baker, K.S.; et al. Brucella abortus Strain 2308 Wisconsin Genome: Importance of the Definition of Reference Strains. Front. Microbiol. 2016, 7, 1557. [Google Scholar] [CrossRef] [PubMed]
- Caswell, C.C.; Gaines, J.M.; Roop, R.M. The RNA Chaperone Hfq Independently Coordinates Expression of the VirB Type IV Secretion System and the LuxR-Type Regulator BabR in Brucella abortus 2308. J. Bacteriol. 2012, 194, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Caswell, C.C.; Gaines, J.M.; Ciborowski, P.; Smith, D.; Borchers, C.H.; Roux, C.M.; Sayood, K.; Dunman, P.M.; Roop II, R.M. Identification of Two Small Regulatory RNAs Linked to Virulence in Brucella abortus 2308: Brucella Small RNAs Are Required for Virulence. Mol. Microbiol. 2012, 85, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Alton, G.G.; Jones, L.M.; Angus, R.D.; Verger, J.M. Techniques for the Brucellosis Laboratory; INRA: Paris, France, 1988; ISBN 978-2-7380-0042-2.
- Castillo-Zeledón, A.; Ruiz-Villalobos, N.; Altamirano-Silva, P.; Chacón-Díaz, C.; Barquero-Calvo, E.; Chaves-Olarte, E.; Guzmán-Verri, C. A Sinorhizobium meliloti and Agrobacterium tumefaciens ExoR Ortholog Is Not Crucial for Brucella abortus Virulence. PLoS ONE 2021, 16, e0254568. [Google Scholar] [CrossRef]
- Altschu, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- De Coster, W.; D’Hert, S.; Schultz, D.T.; Cruts, M.; Van Broeckhoven, C. NanoPack: Visualizing and Processing Long-Read Sequencing Data. Bioinformatics 2018, 34, 2666–2669. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving Bacterial Genome Assemblies from Short and Long Sequencing Reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality Assessment Tool for Genome Assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the Quality of Microbial Genomes Recovered from Isolates, Single Cells, and Metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef]
- BLAST Help Page. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi?CMD=Web&PAGE_TYPE=BlastDocs& (accessed on 24 October 2022).
- Fu, Y.; Mahmoud, M.; Muraliraman, V.V.; Sedlazeck, F.J.; Treangen, T.J. Vulcan: Improved Long-Read Mapping and Structural Variant Calling via Dual-Mode Alignment. GigaScience 2021, 10, giab063. [Google Scholar] [CrossRef] [PubMed]
- Carver, T.J.; Rutherford, K.M.; Berriman, M.; Rajandream, M.-A.; Barrell, B.G.; Parkhill, J. ACT: The Artemis Comparison Tool. Bioinformatics 2005, 21, 3422–3423. [Google Scholar] [CrossRef] [PubMed]
- Sternon, J.-F.; Godessart, P.; Gonçalves de Freitas, R.; Van der Henst, M.; Poncin, K.; Francis, N.; Willemart, K.; Christen, M.; Christen, B.; Letesson, J.-J.; et al. Transposon Sequencing of Brucella abortus Uncovers Essential Genes for Growth In Vitro and Inside Macrophages. Infect. Immun. 2018, 86, e00312-18. [Google Scholar] [CrossRef] [PubMed]
- Manterola, L.; Moriyón, I.; Moreno, E.; Sola-Landa, A.; Weiss, D.S.; Koch, M.H.J.; Howe, J.; Brandenburg, K.; López-Goñi, I. The Lipopolysaccharide of Brucella abortus BvrS/BvrR Mutants Contains Lipid A Modifications and Has Higher Affinity for Bactericidal Cationic Peptides. J. Bacteriol. 2005, 187, 5631–5639. [Google Scholar] [CrossRef] [PubMed]
| Bacterial Strains/Plasmids | Phenotype/Characteristics | Source/Reference |
|---|---|---|
| B. abortus 2308W | Parental strain, smooth LPS, NalR, virulent | [23] |
| B. abortus bvrS::Tn5 | 2308-derivative, bvrS::Tn5, smooth LPS, attenuated | [4] |
| B. abortus bvrR::Tn5 | 2308-derivative, bvrR::Tn5, smooth LPS, attenuated | [4] |
| B. abortus ΔbvrRS | 2308W-derivative, ΔbvrRS, smooth LPS, attenuated | This study |
| pNTPS138 | Suicide vector, oriT, sacB KmR | M.R.K. Alley, unpublished |
| pΔbvrRS | pNTPS138 derivative, ΔbvrRS, KmR | Courtesy of Clayton C. Caswell |
| Strain | MIC (µg/mL) | |
|---|---|---|
| pH 7.0 | pH 6.0 | |
| B. abortus 2308W | 4–8 | 64 |
| B. abortus ΔbvrRS | 1–4 | 4–8 |
| B. abortus bvrR::Tn5 | 2 | 8–16 |
| B. abortus bvrS::Tn5 | 2 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivas-Solano, O.; Núñez-Montero, K.; Altamirano-Silva, P.; Ruiz-Villalobos, N.; Barquero-Calvo, E.; Moreno, E.; Chaves-Olarte, E.; Guzmán-Verri, C. A bvrR/bvrS Non-Polar Brucella abortus Mutant Confirms the Role of the Two-Component System BvrR/BvrS in Virulence and Membrane Integrity. Microorganisms 2023, 11, 2014. https://doi.org/10.3390/microorganisms11082014
Rivas-Solano O, Núñez-Montero K, Altamirano-Silva P, Ruiz-Villalobos N, Barquero-Calvo E, Moreno E, Chaves-Olarte E, Guzmán-Verri C. A bvrR/bvrS Non-Polar Brucella abortus Mutant Confirms the Role of the Two-Component System BvrR/BvrS in Virulence and Membrane Integrity. Microorganisms. 2023; 11(8):2014. https://doi.org/10.3390/microorganisms11082014
Chicago/Turabian StyleRivas-Solano, Olga, Kattia Núñez-Montero, Pamela Altamirano-Silva, Nazareth Ruiz-Villalobos, Elías Barquero-Calvo, Edgardo Moreno, Esteban Chaves-Olarte, and Caterina Guzmán-Verri. 2023. "A bvrR/bvrS Non-Polar Brucella abortus Mutant Confirms the Role of the Two-Component System BvrR/BvrS in Virulence and Membrane Integrity" Microorganisms 11, no. 8: 2014. https://doi.org/10.3390/microorganisms11082014
APA StyleRivas-Solano, O., Núñez-Montero, K., Altamirano-Silva, P., Ruiz-Villalobos, N., Barquero-Calvo, E., Moreno, E., Chaves-Olarte, E., & Guzmán-Verri, C. (2023). A bvrR/bvrS Non-Polar Brucella abortus Mutant Confirms the Role of the Two-Component System BvrR/BvrS in Virulence and Membrane Integrity. Microorganisms, 11(8), 2014. https://doi.org/10.3390/microorganisms11082014







