Individual and Group-Based Effects of In Vitro Fiber Interventions on the Fecal Microbiota
Abstract
:1. Introduction
2. Materials and Methods
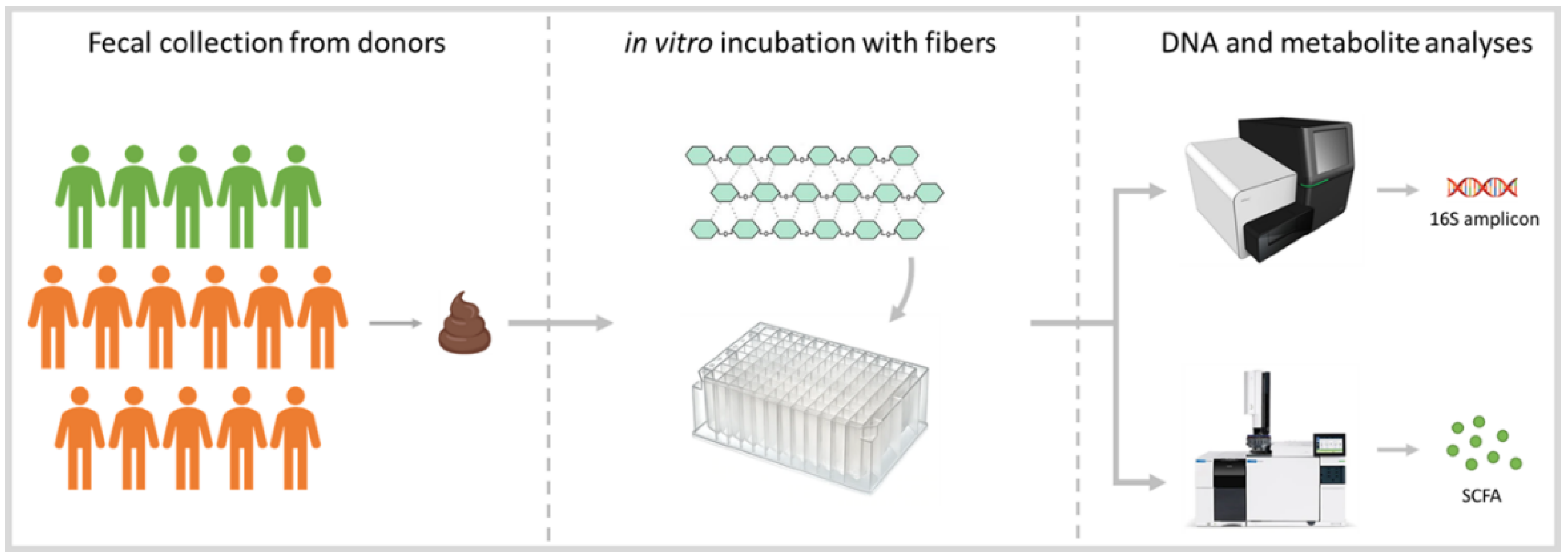
2.1. Fecal Collection
2.2. Fiber Selection and Preparation
2.3. Anaerobic Incubations
2.4. DNA Isolation
2.5. Amplicon Sequencing
2.6. Metabolite Analysis
2.7. Data Analysis—16S
2.8. Statistics
3. Results
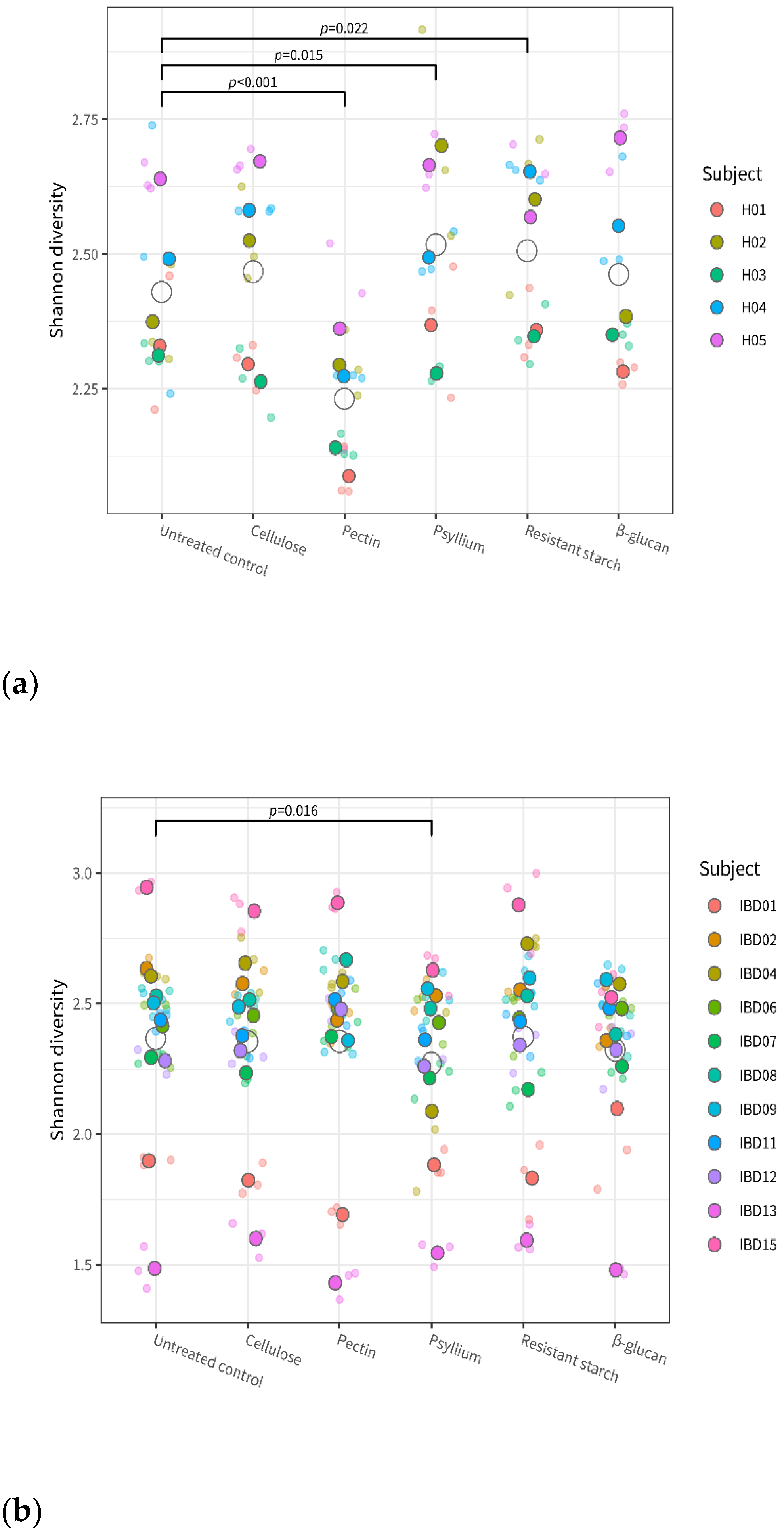
3.1. Fiber’s Effects on α-Diversity
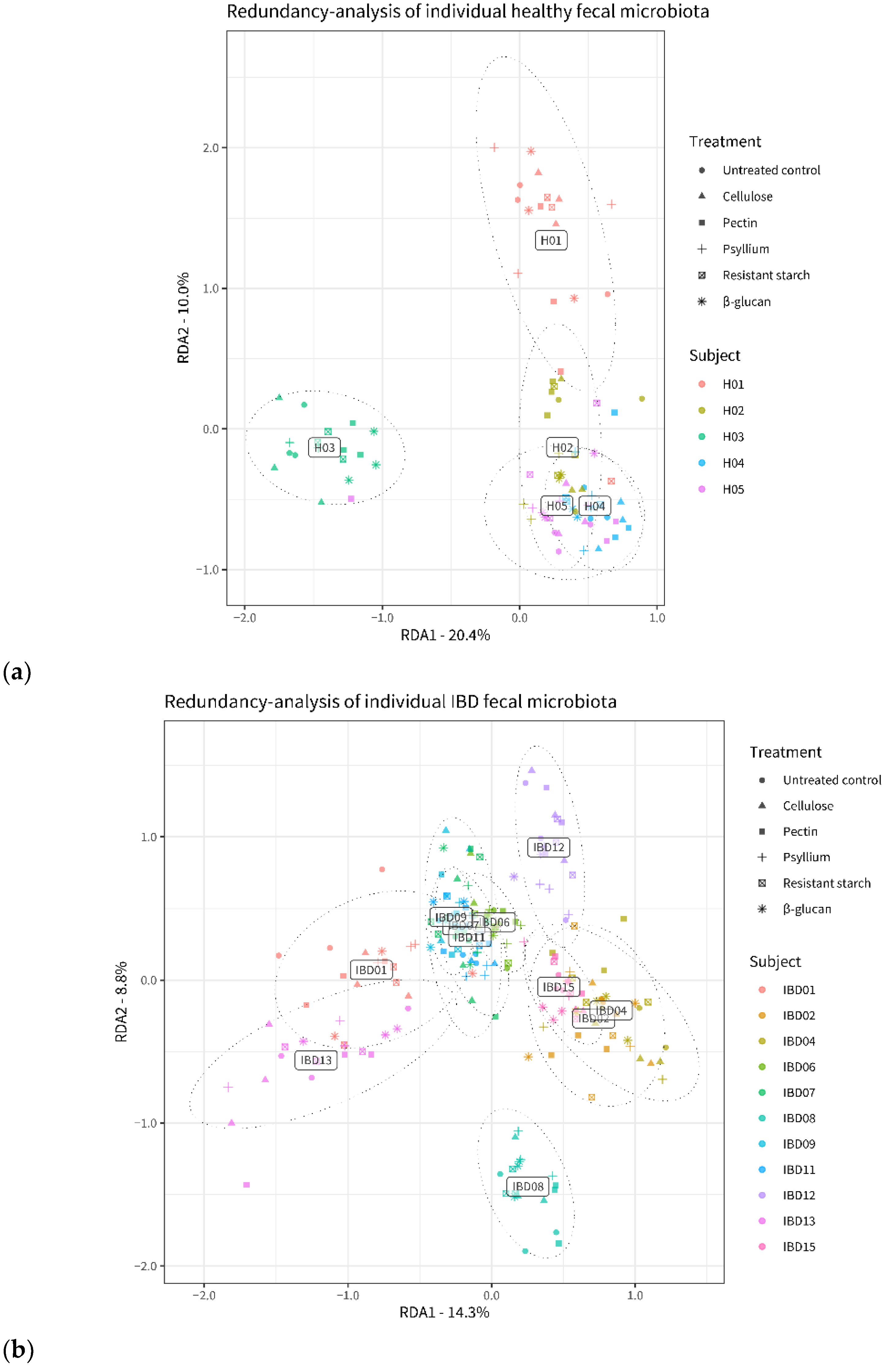
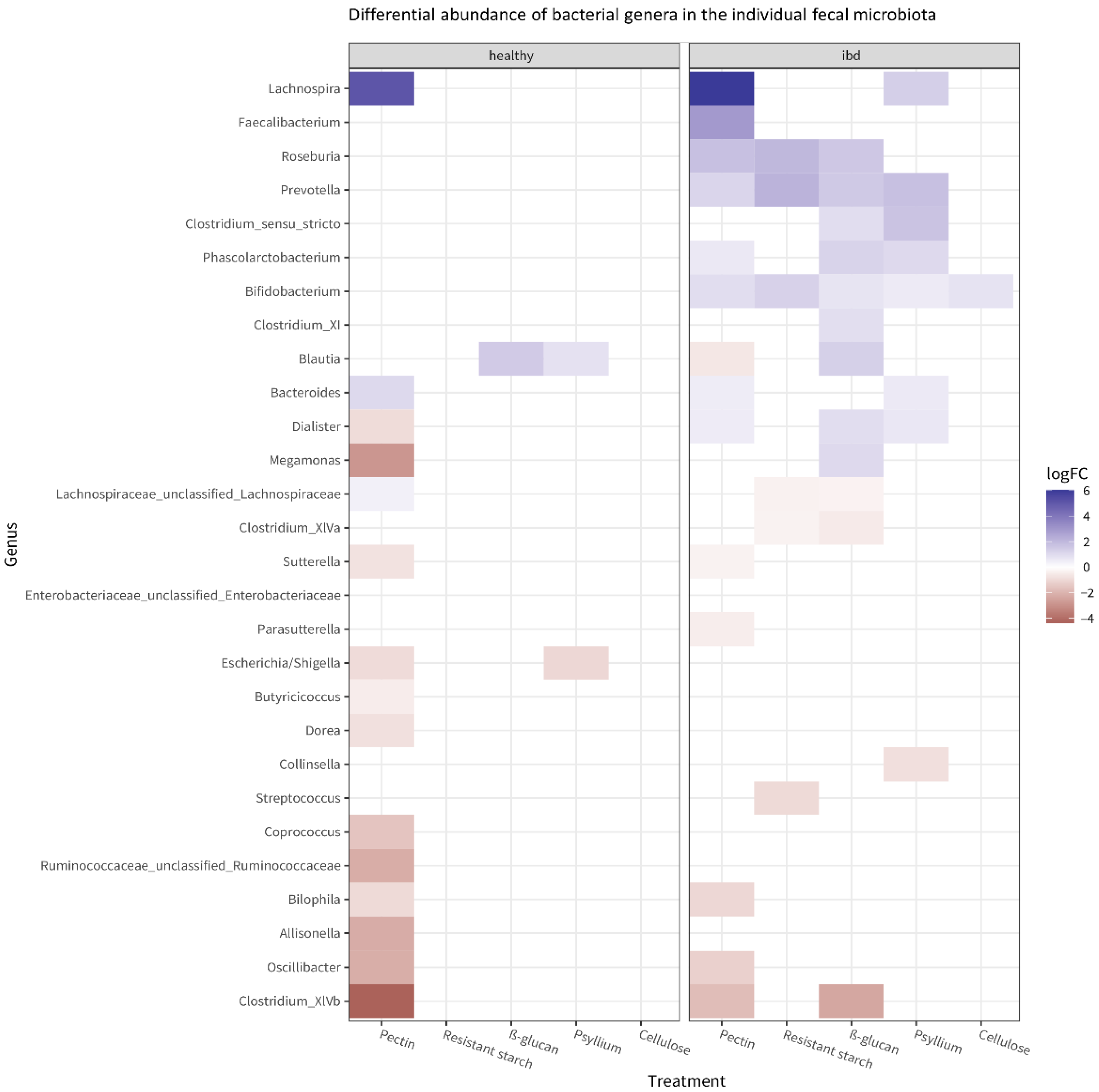
3.2. Fiber’s Effects on Microbiota Composition
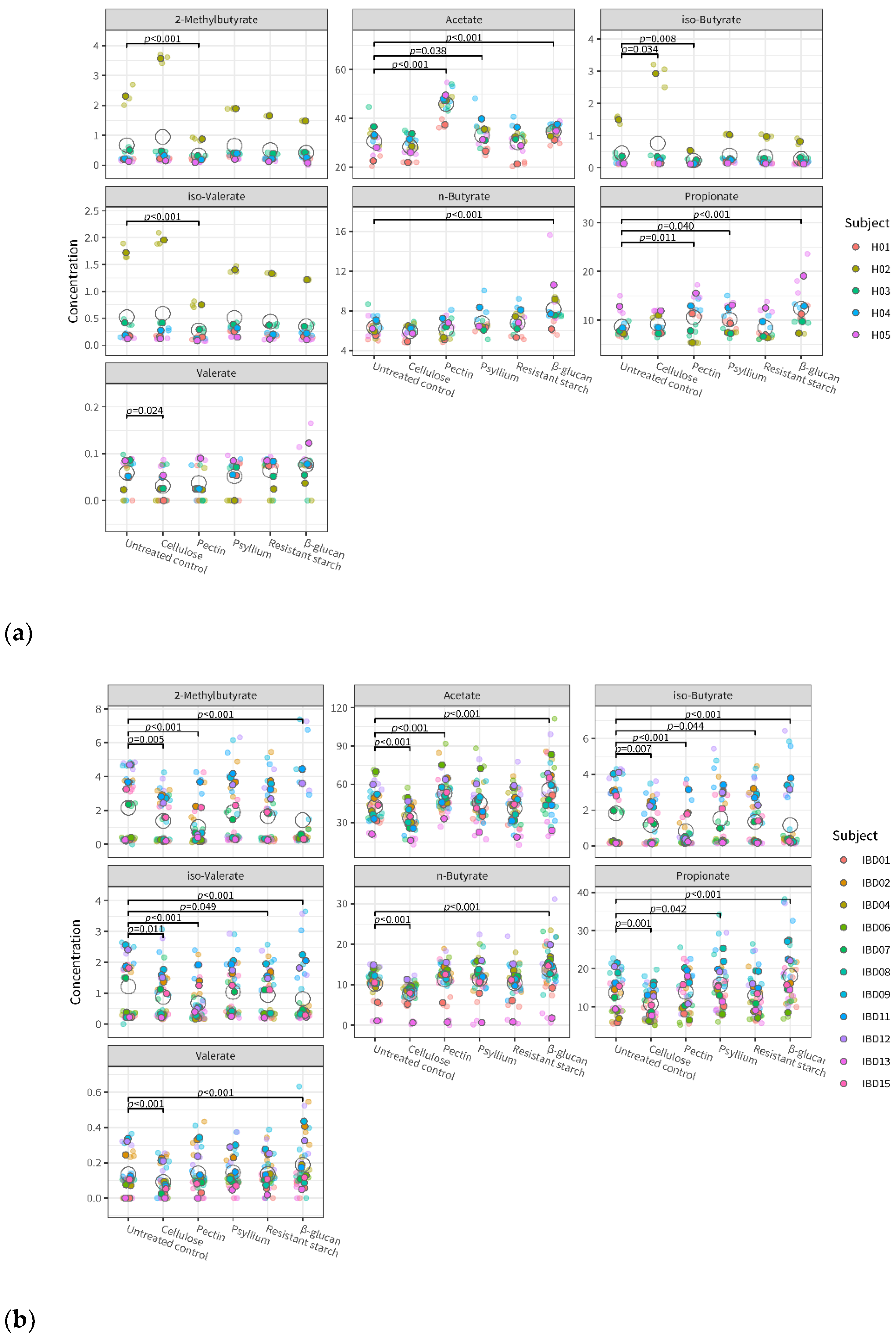
3.3. Fiber’s Effects on Metabolite Levels
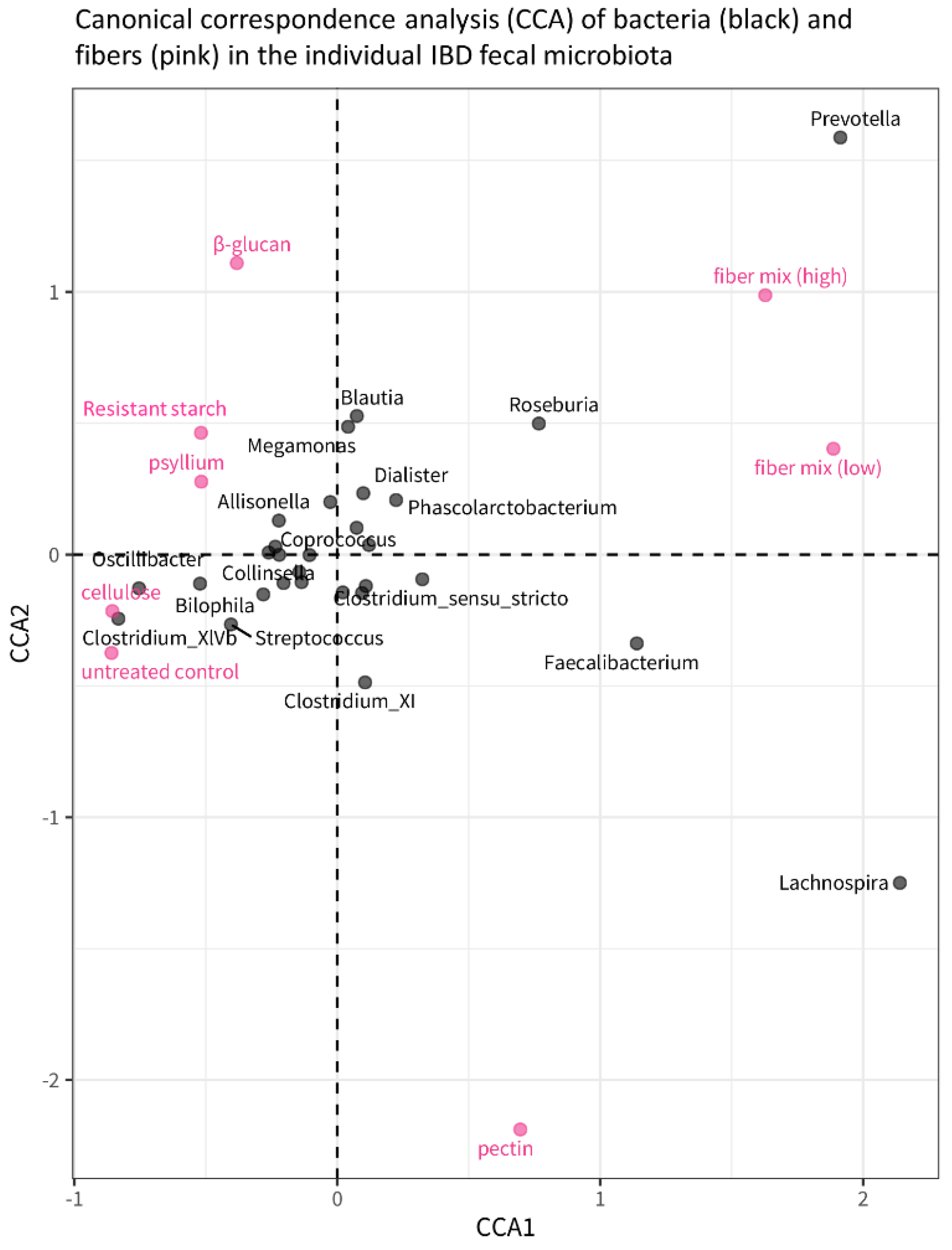
3.4. Effects of Single Fibers and Fiber Mix on Microbiota Composition and Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for the Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef] [Green Version]
- Kirwan, L.; Walsh, M.C.; Celis-Morales, C.; Marsaux, C.F.M.; Livingstone, K.M.; Navas-Carretero, S.; Fallaize, R.; O’Donovan, C.B.; Woolhead, C.; Forster, H.; et al. Phenotypic Factors Influencing the Variation in Response of Circulating Cholesterol Level to Personalised Dietary Advice in the Food4Me Study. Br. J. Nutr. 2016, 116, 2011–2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, S.H.; Anthony, J.C.; Carvajal, R.; Chae, L.; Khoo, C.S.H.; Latulippe, M.E.; Matusheski, N.V.; McClung, H.L.; Rozga, M.; Schmid, C.H.; et al. Perspective: Guiding Principles for the Implementation of Personalized Nutrition Approaches That Benefit Health and Function. Adv. Nutr. 2020, 11, 25–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, K.S.; de Vos, W.M.; Martens, E.C.; Gilbert, J.A.; Menon, R.S.; Soto-Vaca, A.; Hautvast, J.; Meyer, P.D.; Borewicz, K.; Vaughan, E.E.; et al. Effect of Fructans, Prebiotics and Fibres on the Human Gut Microbiome Assessed by 16S RRNA-Based Approaches: A Review. Benef. Microbes 2020, 11, 101–129. [Google Scholar] [CrossRef] [PubMed]
- Stephen, A.M.; Champ, M.M.J.; Cloran, S.J.; Fleith, M.; Van Lieshout, L.; Mejborn, H.; Burley, V.J. Dietary Fibre in Europe: Current State of Knowledge on Definitions, Sources, Recommendations, Intakes and Relationships to Health; Cambridge University Press: Cambridge, UK, 2017; Volume 30, ISBN 0954422417000. [Google Scholar]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary Fibre in Gastrointestinal Health and Disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 18, 101–116. [Google Scholar] [CrossRef]
- Rodriguez, J.; Hiel, S.; Neyrinck, A.M.; Le Roy, T.; Pötgens, S.A.; Leyrolle, Q.; Pachikian, B.D.; Gianfrancesco, M.A.; Cani, P.D.; Paquot, N.; et al. Discovery of the Gut Microbial Signature Driving the Efficacy of Prebiotic Intervention in Obese Patients. Gut 2020, 69, 1975–1987. [Google Scholar] [CrossRef]
- Christensen, L.; Roager, H.M.; Astrup, A.; Hjorth, M.F. Microbial Enterotypes in Personalized Nutrition and Obesity Management. Am. J. Clin. Nutr. 2018, 108, 645–651. [Google Scholar] [CrossRef] [Green Version]
- Fu, T.; Pan, L.; Shang, Q.; Yu, G. Fermentation of Alginate and Its Derivatives by Different Enterotypes of Human Gut Microbiota: Towards Personalized Nutrition Using Enterotype-Specific Dietary Fibers. Int. J. Biol. Macromol. 2021, 183, 1649–1659. [Google Scholar] [CrossRef]
- Chen, T.; Long, W.; Zhang, C.; Liu, S.; Zhao, L.; Hamaker, B.R. Fiber-Utilizing Capacity Varies in Prevotella- versus Bacteroides-Dominated Gut Microbiota. Sci. Rep. 2017, 7, 2594. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, H.; Mander, I.; Zhang, Z.; Armstrong, D.; Wine, E. Not All Fibers Are Born Equal; Variable Response to Dietary Fiber Subtypes in IBD. Front. Pediatr. 2021, 8, 620189. [Google Scholar] [CrossRef]
- Schuren, F.; Agamennone, V.; Keijser, B.; Abeln, E.; van der Vossen, J.; Montijn, R. The I-Screen: A Versatile Preclinical Platform for Gut Microbiota Studies. J. Probiotics Health 2019, 07, 7–10. [Google Scholar] [CrossRef] [Green Version]
- Ladirat, S.E.; Schols, H.A.; Nauta, A.; Schoterman, M.H.C.; Keijser, B.J.F.; Montijn, R.C.; Gruppen, H.; Schuren, F.H.J. High-Throughput Analysis of the Impact of Antibiotics on the Human Intestinal Microbiota Composition. J. Microbiol. Methods 2013, 92, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a Dual-Index Sequencing Strategy and Curation Pipeline for Analyzing Amplicon Sequence Data on the Miseq Illumina Sequencing Platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global Patterns of 16S RRNA Diversity at a Depth of Millions of Sequences per Sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing Mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [Green Version]
- Jouany, J.P. Volatile Fatty Acid and Alcohol Determination in Digestive Contents, Silage Juices, Bacterial Cultures and Anaerobic Fermentor Contents. Sci. Aliment. 1982, 2, 131–144. [Google Scholar]
- Van Nuenen, M.H.M.C.; Meyer, P.D.; Venema, K. The Effect of Various Inulins and Clostridium Difficile on the Metabolic Activity of the Human Colonic Microbiota In Vitro. Microb. Ecol. Health Dis. 2003, 15, 137–144. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Hoffman, G.E.; Schadt, E.E. VariancePartition: Interpreting Drivers of Variation in Complex Gene Expression Studies. BMC Bioinform. 2016, 17, 483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, G.E.; Roussos, P. Dream: Powerful Differential Expression Analysis for Repeated Measures Designs. Bioinformatics 2021, 37, 192–201. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package 2017. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 12 October 2022).
- Velikonja, A.; Lipoglavšek, L.; Zorec, M.; Orel, R.; Avguštin, G. Alterations in Gut Microbiota Composition and Metabolic Parameters after Dietary Intervention with Barley Beta Glucans in Patients with High Risk for Metabolic Syndrome Development. Anaerobe 2019, 55, 67–77. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut Bacteria Selectively Promoted by Dietary Fibers Alleviate Type 2 Diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, N.; De Souza, C.B.; Krych, L.; Cahú, T.B.; Wiese, M.; Kot, W.; Hansen, K.M.; Blennow, A.; Venema, K.; Jespersen, L. Potential of Pectins to Beneficially Modulate the Gut Microbiota Depends on Their Structural Properties. Front. Microbiol. 2019, 10, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantu-Jungles, T.M.; Hamaker, B.R. New View on Dietary Fiber Selection for Predictable Shifts in Gut Microbiota. MBio 2020, 11, e02179-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ze, X.; Duncan, S.H.; Louis, P.; Flint, H.J. Ruminococcus bromii Is a Keystone Species for the Degradation of Resistant Starch in the Human Colon. ISME J. 2012, 6, 1535–1543. [Google Scholar] [CrossRef] [Green Version]
- Davis, L.M.G.; Martínez, I.; Walter, J.; Goin, C.; Hutkins, R.W. Barcoded Pyrosequencing Reveals That Consumption of Galactooligosaccharides Results in a Highly Specific Bifidogenic Response in Humans. PLoS ONE 2011, 6, e25200. [Google Scholar] [CrossRef] [Green Version]
- Miquel, S.; Martin, R.; Bridonneau, C.; Robert, V.; Sokol, H.; Bermúdez-Humarán, L.G.; Thomas, M.; Langella, P. Ecology and Metabolism of the Beneficial Intestinal Commensal Bacterium Faecalibacterium prausnitzii. Gut Microbes 2014, 5, 146–151. [Google Scholar] [CrossRef] [Green Version]
- Baxter, N.T.; Schmidt, A.W.; Venkataraman, A.; Kim, K.S.; Waldron, C.; Schmidt, T.M. Dynamics of Human Gut Microbiota and Short-Chain Fatty Acids in Response to Dietary Interventions with Three Fermentable Fibers. MBio 2019, 10, e02566-18. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.K.; Deehan, E.C.; Zhang, Z.; Jin, M.; Baskota, N.; Perez-muñoz, M.E.; Cole, J.; Tuncil, Y.E.; Seethaler, B.; Wang, T.; et al. Gut Microbiota Modulation with Long-Chain Corn Bran Arabinoxylan in Adults with Overweight and Obesity Is Linked to an Individualized Temporal Increase in Fecal Propionate. Microbiome 2020, 8, 118. [Google Scholar] [CrossRef]
- Hughes, S.A.; Shewry, P.R.; Gibson, G.R.; McCleary, B.V.; Rastall, R.A. In Vitro Fermentation of Oat and Barley Derived β-Glucans by Human Faecal Microbiota. FEMS Microbiol. Ecol. 2008, 64, 482–493. [Google Scholar] [CrossRef] [Green Version]
- Daou, C.; Zhang, H. Oat Beta-Glucan: Its Role in Health Promotion and Prevention of Diseases. Compr. Rev. Food Sci. Food Saf. 2012, 11, 355–365. [Google Scholar] [CrossRef]
- Joyce, S.A.; Kamil, A.; Fleige, L.; Gahan, C.G.M. The Cholesterol-Lowering Effect of Oats and Oat Beta Glucan: Modes of Action and Potential Role of Bile Acids and the Microbiome. Front. Nutr. 2019, 6, 171. [Google Scholar] [CrossRef] [PubMed]
- Elshahed, M.S.; Miron, A.; Aprotosoaie, A.C.; Farag, M.A. Pectin in Diet: Interactions with the Human Microbiome, Role in Gut Homeostasis, and Nutrient-Drug Interactions. Carbohydr. Polym. 2021, 255, 117388. [Google Scholar] [CrossRef] [PubMed]
- Casals-Pascual, C.; González, A.; Vázquez-Baeza, Y.; Song, S.J.; Jiang, L.; Knight, R. Microbial Diversity in Clinical Microbiome Studies: Sample Size and Statistical Power Considerations. Gastroenterology 2020, 158, 1524–1528. [Google Scholar] [CrossRef]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S.; et al. Population-Based Metagenomics Analysis Reveals Markers for Gut Microbiome Composition and Diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Fan, B.; Liu, S.; Imam, K.M.S.U.; Xie, Y.; Wen, B.; Xin, F. The in Vitro Effect of Fibers with Different Degrees of Polymerization on Human Gut Bacteria. Front. Microbiol. 2020, 11, 819. [Google Scholar] [CrossRef]
- Canfora, E.E.; Hermes, G.D.A.; Müller, M.; Bastings, J.; Vaughan, E.E.; van Den Berg, M.A.; Holst, J.J.; Venema, K.; Zoetendal, E.G.; Blaak, E.E. Fiber Mixture-Specific Effect on Distal Colonic Fermentation and Metabolic Health in Lean but Not in Prediabetic Men. Gut Microbes 2022, 14, 2009297. [Google Scholar] [CrossRef]
- Tily, H.; Patridge, E.; Cai, Y.; Gopu, V.; Gline, S.; Genkin, M.; Lindau, H.; Sjue, A.; Slavov, I.; Perlina, A.; et al. Gut Microbiome Activity Contributes to Prediction of Individual Variation in Glycemic Response in Adults. Diabetes Ther. 2021, 13, 89–111. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agamennone, V.; van den Broek, T.J.; de Kat Angelino-Bart, A.; Hoevenaars, F.P.M.; van der Kamp, J.W.; Schuren, F.H.J. Individual and Group-Based Effects of In Vitro Fiber Interventions on the Fecal Microbiota. Microorganisms 2023, 11, 2001. https://doi.org/10.3390/microorganisms11082001
Agamennone V, van den Broek TJ, de Kat Angelino-Bart A, Hoevenaars FPM, van der Kamp JW, Schuren FHJ. Individual and Group-Based Effects of In Vitro Fiber Interventions on the Fecal Microbiota. Microorganisms. 2023; 11(8):2001. https://doi.org/10.3390/microorganisms11082001
Chicago/Turabian StyleAgamennone, Valeria, Tim J. van den Broek, Alie de Kat Angelino-Bart, Femke P. M. Hoevenaars, Jan Willem van der Kamp, and Frank H. J. Schuren. 2023. "Individual and Group-Based Effects of In Vitro Fiber Interventions on the Fecal Microbiota" Microorganisms 11, no. 8: 2001. https://doi.org/10.3390/microorganisms11082001
APA StyleAgamennone, V., van den Broek, T. J., de Kat Angelino-Bart, A., Hoevenaars, F. P. M., van der Kamp, J. W., & Schuren, F. H. J. (2023). Individual and Group-Based Effects of In Vitro Fiber Interventions on the Fecal Microbiota. Microorganisms, 11(8), 2001. https://doi.org/10.3390/microorganisms11082001





