Identifying the Microbiome of the Adenoid Surface of Children Suffering from Otitis Media with Effusion and Children without Middle Ear Effusion Using 16S rRNA Genetic Sequencing
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Patient Selection, Inclusion, and Exclusion Criteria
2.2.1. Inclusion Criteria for Samples Collected from Patients without Middle Ear Effusion
- Age of patients: 3 to 7 years old;
- A-type tympanometry bilaterally (no evidence of middle ear fluid accumulation);
- Indications for adenoid surgery based on history of frequent recurrent upper airway infections, obstructive sleep apnea episodes, and maxillofacial anomalies;
- Endoscopical findings—adenoid tissue hyperplasia 2/3/4 grade;
- Operation under general anesthesia;
- The patient and his/her representative agree to participate in the study.
2.2.2. Inclusion Criteria for Samples Collected from Patients with Middle Ear Effusion
- Age of patients: 3 to 7 years old;
- B-type tympanometry unilaterally or bilaterally (evidence of middle ear effusion);
- Indications for adenoid surgery based on history of frequent recurrent upper airway infections, obstructive sleep apnea episodes, maxillofacial anomalies, frequent middle ear infections, and prolonged hearing impairment due to conductive hearing loss;
- Endoscopic findings—adenoid tissue hyperplasia 2/3/4 grade;
- Operation under general anesthesia;
- The patient and his/her representative agree to participate in the study.
2.2.3. Exclusion Criteria for Both Groups
- Patients over the age of 7 and under the age of 3;
- Patients from other geographical locations traveling to Latvia specifically for the operation;
- Immunocompromised patients: HIV positive patients, patients with hepatitis A/B/C, patients undergoing chemotherapy due to oncological diseases, diabetes mellitus patients, patients with chronic autoimmune diseases (sarcoidosis, Wegner’s granulomatosis);
- Patients with cleft malformations;
- Patients who received systemic antibiotic treatment for the last 2 weeks before the operation;
- Patients with signs of acute upper airway infections: fever >38 °C, purulent discharge from the nose and/or ear canals, productive cough, overall fatigue and shimmers;
- Patients who receive immunomudolators—microflora altering substances, for example, bifido-bacterium food supplements.
2.3. Material Collection
2.4. DNA Extraction and Sequencing
2.5. Data Analysis
3. Results
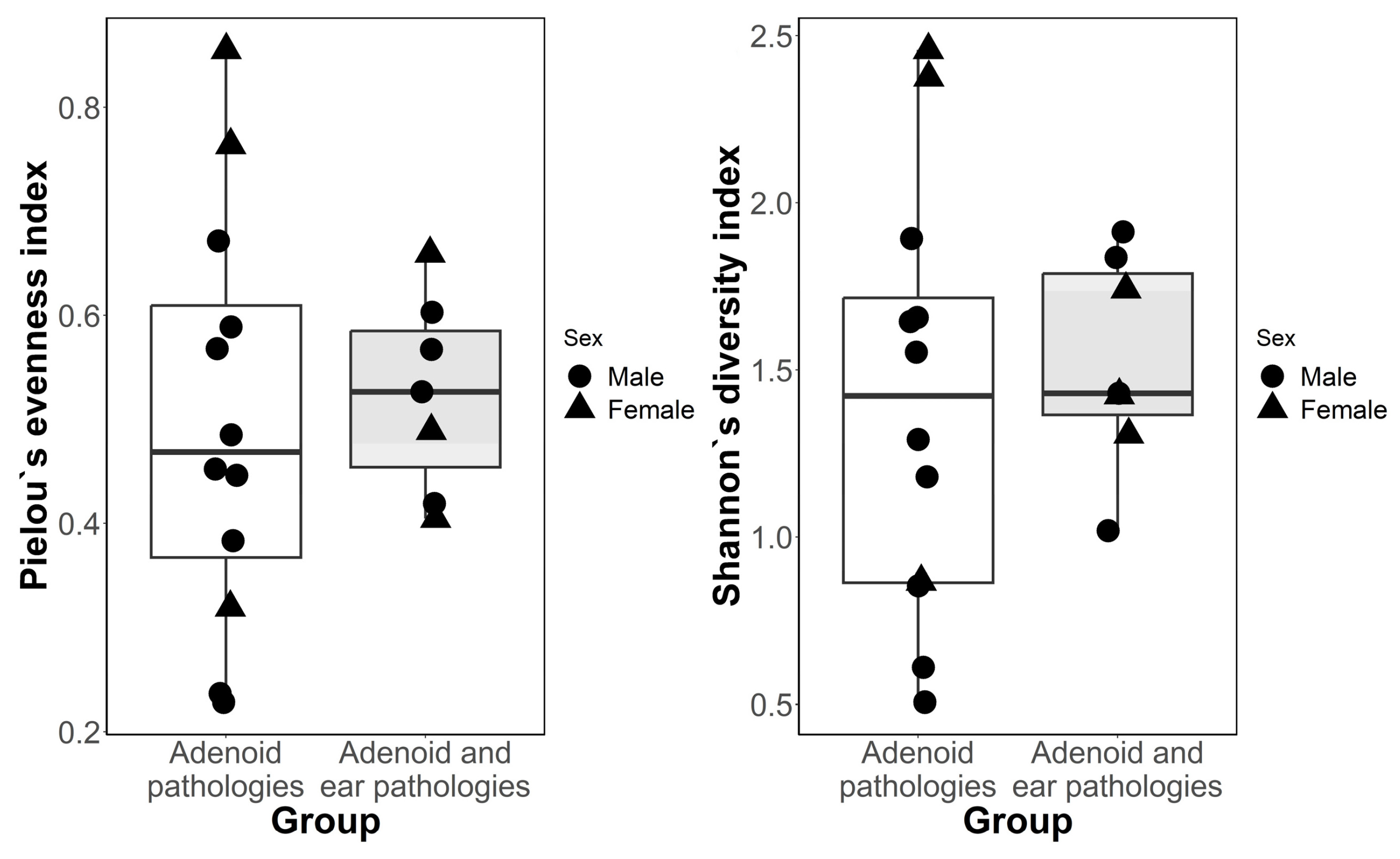
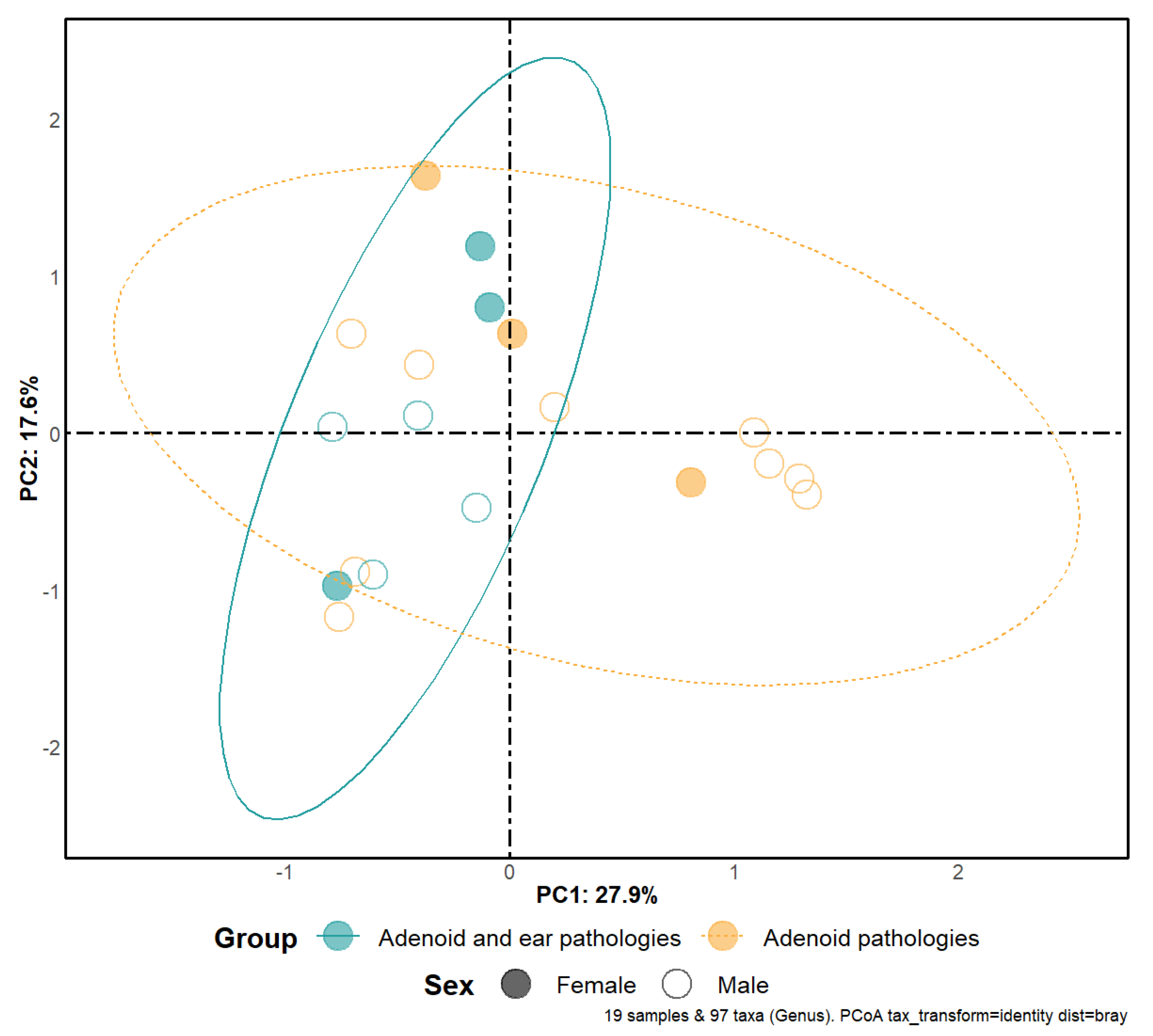
3.1. Alpha and Beta-Diversity
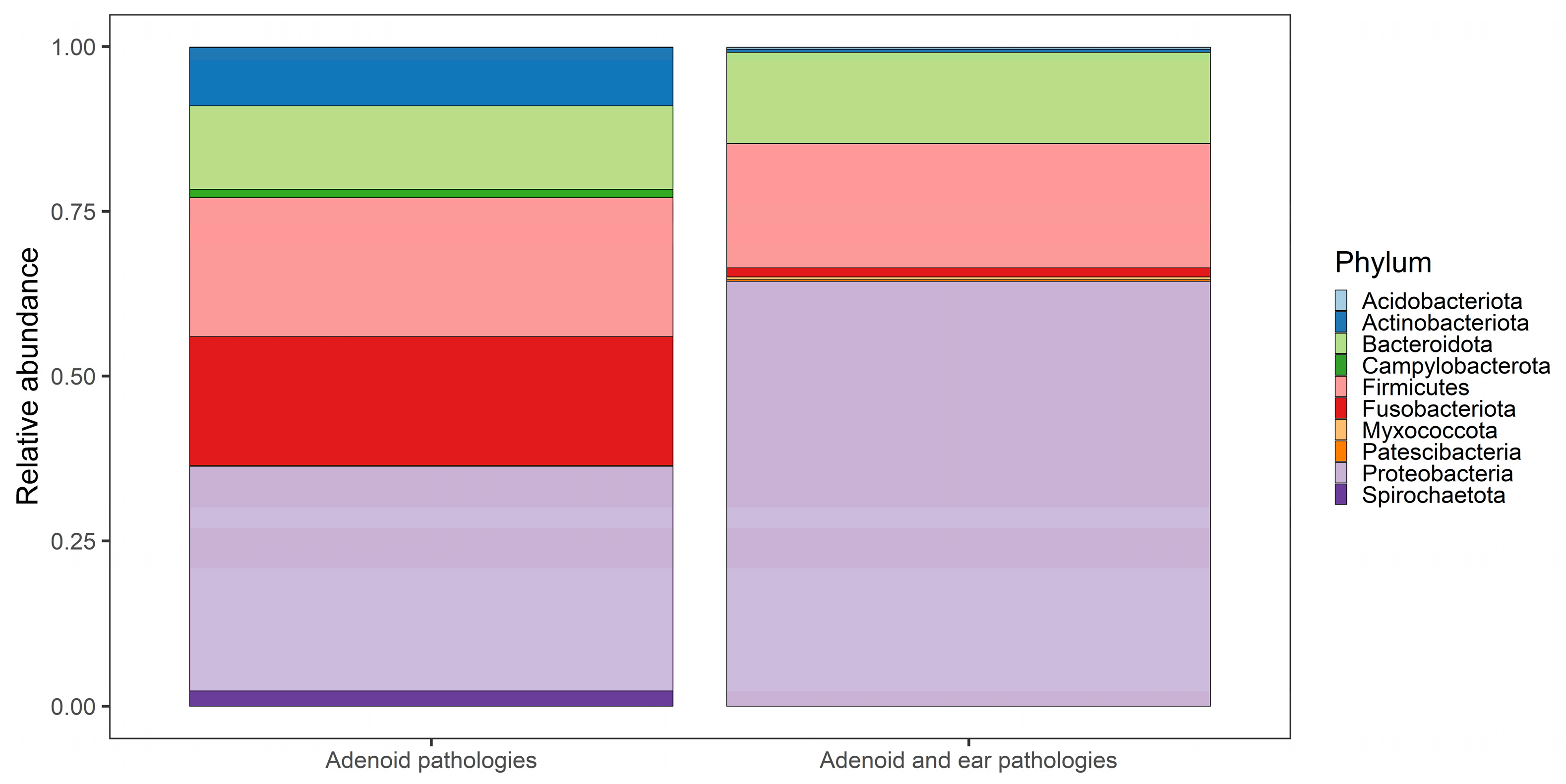
3.2. Taxonomy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hall-Stoodley, L.; Watts, G.; Crowther, J.E.; Balagopal, A.; Torrelles, J.B.; Robison-Cox, J.; Bargatze, R.F.; Harmsen, A.G.; Crouch, E.C.; Schlesinger, L.S. Mycobacterium tuberculosis binding to human surfactant proteins A and D, fibronectin, and small airway epithelial cells under shear conditions. Infect. Immun. 2006, 74, 3587–3596. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Blaser, M.J.; Falkow, S. What are the consequences of the disappearing human microbiota? Nat. Rev. Microbiol. 2009, 7, 887–894. [Google Scholar] [CrossRef]
- Requena, T.; Velasco, M. The human microbiome in sickness and in health. Rev. Clínica Española (Engl. Ed.) 2021, 221, 233–240. [Google Scholar]
- Proctor, D.M.; Relman, D.A. The Landscape Ecology and Microbiota of the Human Nose, Mouth, and Throat. Cell Host Microbe 2017, 21, 421–432. [Google Scholar]
- Kumpitsch, C.; Koskinen, K.; Schöpf, V.; Moissl-Eichinger, C. The microbiome of the upper respiratory tract in health and disease. BMC Biol. 2019, 17, 87. [Google Scholar] [CrossRef]
- Folino, F.; Ruggiero, L.; Capaccio, P.; Coro, I.; Aliberti, S.; Drago, L.; Marchisio, P.; Torretta, S. Upper Respiratory Tract Microbiome and Otitis Media Intertalk: Lessons from the Literature. J. Clin. Med. 2020, 9, 2845. [Google Scholar] [CrossRef]
- Marseglia, G.; Caimmi, D.; Pagella, F.; Matti, E.; Labò, E.; Licari, A.; Salpietro, A.; Pelizzo, G.; Castellazzi, A. Adenoids during Childhood: The Facts. Int. J. Immunopathol. Pharmacol. 2011, 24, 1–5. [Google Scholar] [CrossRef]
- Xu, J.; Dai, W.; Liang, Q.; Ren, D. The microbiomes of adenoid and middle ear in children with otitis media with effusion and hypertrophy from a tertiary hospital in China. Int. J. Pediatr. Otorhinolaryngol. 2020, 134, 110058. [Google Scholar] [CrossRef]
- Santee, C.A.; Nagalingam, N.A.; Faruqi, A.A.; DeMuri, G.P.; Gern, J.E.; Wald, E.R.; Lynch, S.V. Nasopharyngeal microbiota composition of children is related to the frequency of upper respiratory infection and acute sinusitis. Microbiome 2016, 4, 34. [Google Scholar] [CrossRef]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Pintucci, J.P.; Corno, S.; Garotta, M. Biofilms and infections of the upper respiratory tract. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 683–690. [Google Scholar]
- Sontakke, S.; Cadenas, M.B.; Maggi, R.G.; Diniz, P.P.V.P.; Breitschwerdt, E.B. Use of broad range16S rDNA PCR in clinical microbiology. J. Microbiol. Methods 2009, 76, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 3. [Google Scholar] [CrossRef]
- Masella, A.P.; Bartram, A.K.; Truszkowski, J.M.; Brown, D.G.; Neufeld, J.D. PANDAseq: Paired-end assembler for illumina sequences. BMC Bioinform. 2012, 13, 31. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Davis, N.M.; Proctor, D.M.; Holmes, S.P.; Relman, D.A.; Callahan, B.J. Simple statistical identification and removal of contaminant sequences in marker-gene and metagenomics data. Microbiome 2018, 6, 226. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar]
- Lin, H.; Peddada, S.D. Analysis of compositions of microbiomes with bias correction. Nat. Commun. 2020, 11, 3514. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Hong, S.J.; Pak, K.H.; Hong, S.M. Analysis of the Microbiome in the Adenoids of Korean Children with Otitis Media with Effusion. J. Int. Adv. Otol. 2019, 15, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Ari, O.; Karabudak, S.; Kalcioglu, M.T.; Gunduz, A.Y.; Durmaz, R. The bacteriome of otitis media with effusion: Does it originate from the adenoid? Int. J. Pediatr. Otorhinolaryngol. 2019, 126, 109624. [Google Scholar] [PubMed]
- Dirain, C.O.; Silva, R.C.; Collins, W.O.; Antonelli, P.J. The Adenoid Microbiome in Recurrent Acute Otitis Media and Obstructive Sleep Apnea. J. Int. Adv. Otol. 2017, 13, 333–339. [Google Scholar] [CrossRef]
- Sokolovs-Karijs, O.; Brīvība, M.; Saksis, R.; Sumeraga, G.; Girotto, F.; Erts, R.; Osīte, J.; Krūmiņa, A. An Overview of Adenoid Microbiome Using 16S rRNA Gene Sequencing-Based Metagenomic Analysis. Medicina 2022, 58, 920. [Google Scholar] [CrossRef]
- Johnston, J.; Hoggard, M.; Biswas, K.; Astudillo-García, C.; Radcliff, F.J.; Mahadevan, M.; Douglas, R.G. Paired analysis of the microbiota between surface tissue swabs and biopsies from pediatric patients undergoing adenotonsillectomy. Int. J. Pediatr. Otorhinolaryngol. 2018, 113, 51–57. [Google Scholar] [CrossRef]
- Huang, C.C.; Chang, T.H.; Lee, C.Y.; Wu, P.W.; Chen, C.L.; Lee, T.J.; Liou, M.L.; Chiu, C.H. Tissue microbiota in nasopharyngeal adenoid and its association with pneumococcal carriage. Microb. Pathog. 2021, 157, 104999. [Google Scholar] [CrossRef]
- Lim, S.J.; Jithpratuck, W.; Wasylik, K.; Sriaroon, P.; Dishaw, L.J. Associations of Microbial Diversity with Age and Other Clinical Variables among Pediatric Chronic Rhinosinusitis (CRS) Patients. Microorganisms 2023, 11, 422. [Google Scholar]
- Marazzato, M.; Zicari, A.M.; Aleandri, M.; Conte, A.L.; Longhi, C.; Vitanza, L.; Bolognino, V.; Zagaglia, C.; De Castro, G.; Brindisi, G.; et al. 16S Metagenomics Reveals Dysbiosis of Nasal Core Microbiota in Children with Chronic Nasal Inflammation: Role of Adenoid Hypertrophy and Allergic Rhinitis. Front. Cell. Infect. Microbiol. 2020, 10, 458. [Google Scholar] [CrossRef]
- Robitzski, D. Newly Renamed Prokaryote Phyla Cause Uproar. The Scientist, 2022. [Google Scholar]
- Marzouk, H.; Aynehchi, B.; Thakkar, P.; Abramowitz, T.; Goldsmith, A. The utility of nasopharyngeal culture in the management of chronic adenoiditis. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 1413–1415. [Google Scholar] [PubMed]
- Marchisio, P.; Claut, L.; Rognoni, A.; Esposito, S.; Passali, D.; Bellussi, L.; Drago, L.; Pozzi, G.; Mannelli, S.; Schito, G.; et al. Differences in nasopharyngeal bacterial flora in children with nonsevere recurrent acute otitis media and chronic otitis media with effusion: Implications for management. Pediatr. Infect. Dis. J. 2003, 22, 262–268. [Google Scholar]
- Lenartova, M.; Tesinska, B.; Janatova, T.; Hrebicek, O.; Mysak, J.; Janata, J.; Najmanova, L. The Oral Microbiome in Periodontal Health. Front. Cell. Infect. Microbiol. 2021, 11, 629723. [Google Scholar] [PubMed]
- Arane, K.; Goldman, R.D. Fusobacterium infections in children. Can. Fam. Physician 2016, 62, 813–814. [Google Scholar] [PubMed]
- Pettigrew, M.M.; Alderson, M.R.; Bakaletz, L.O.; Barenkamp, S.J.; Hakansson, A.P.; Mason, K.M.; Nokso-Koivisto, J.; Patel, J.; Pelton, S.I.; Murphy, T.F. Panel 6: Vaccines. Otolaryngol. Head. Neck Surg. 2017, 156, S76–S87. [Google Scholar]
- Ngo, C.C.; Massa, H.M.; Thornton, R.B.; Cripps, A.W. Predominant Bacteria Detected from the Middle Ear Fluid of Children Experiencing Otitis Media: A Systematic Review. PLoS ONE 2016, 11, e0150949. [Google Scholar]
- Gladstone, R.A.; Jefferies, J.M.; Tocheva, A.S.; Beard, K.R.; Garley, D.; Chong, W.W.; Bentley, S.D.; Faust, S.N.; Clarke, S.C. Five winters of pneumococcal serotype replacement in UK carriage following PCV introduction. Vaccine 2015, 33, 2015–2021. [Google Scholar]
- Zhang, X.; Li, X.; Xu, H.; Fu, Z.; Wang, F.; Huang, W.; Wu, K.; Li, C.; Liu, Y.; Zou, J.; et al. Changes in the oral and nasal microbiota in pediatric obstructive sleep apnea. J. Oral. Microbiol. 2023, 15, 2182571. [Google Scholar]
- Ulanova, M.; Tsang, R.S.W. Haemophilus influenzae serotype a as a cause of serious invasive infections. Lancet Infect. Dis. 2014, 14, 70–82. [Google Scholar]
- Hufnagl, K.; Pali-Schöll, I.; Roth-Walter, F.; Jensen-Jarolim, E. Dysbiosis of the gut and lung microbiome has a role in asthma. Semin. Immunopathol. 2020, 42, 75–93. [Google Scholar]
- Wu, B.G.; Sulaiman, I.; Wang, J.; Shen, N.; Clemente, J.C.; Li, Y.; Laumbach, R.J.; Lu, S.E.; Udasin, I.; Le-Hoang, O.; et al. Severe Obstructive Sleep Apnea Is Associated with Alterations in the Nasal Microbiome and an Increase in Inflammation. Am. J. Respir. Crit. Care Med. 2019, 199, 99–109. [Google Scholar]
- Agirdir, B.V.; Bozova, S.; Derin, A.T.; Turhan, M. Chronic otitis media with effusion and Helicobacter pylori. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 829–834. [Google Scholar]
- Yilmaz, T.; Ceylan, M.; Akyön, Y.; Ozçakýr, O.; Gürsel, B. Helicobacter pylori: A possible association with otitis media with effusion. Otolaryngol. Head. Neck Surg. 2006, 134, 772–777. [Google Scholar] [PubMed]
- Fancy, T.; Mathers, P.H.; Ramadan, H.H. Otitis media with effusion: A possible role for Helicobacter pylori? Otolaryngol. Head. Neck Surg. 2009, 140, 256–258. [Google Scholar] [PubMed]
- Sudhoff, H.; Rajagopal, S.; Baguley, D.M.; Ebmeyer, J.; Schmelzer, A.; Schreiber, S.; Moffat, D.A. A critical evaluation of the evidence on a causal relationship between Helicobacter pylori and otitis media with effusion. J. Laryngol. Otol. 2008, 122, 905–911. [Google Scholar] [PubMed]

| Name | Sequence |
|---|---|
| ci5_16S_V3_Fw(341F) | TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGNNNNNNCCTACGGGNGGCWGCAG |
| ci7_16S_V4_Rs(805R) | GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGNNNNNNGACTACHVGGGTATCTAATCC |
| Descriptives | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Shapiro-Wilk | |||||||||
| N | Missing | Mean | Median | SD | Minimum | Maximum | W | p | |
| Age | 19 | 4.20 | 4.00 | 1.47 | 3 | 7 | 0.774 | <0.001 | |
| Children with no Middle Ear Effusion | Children with Middle Ear Effusion | |
|---|---|---|
| Gender | Male—10 participants Female—2 participants | Male—4 participants Female—3 participants |
| Age | 3 y.o.—3 participants 4 y.o.—5 participants 5. y.o.—3 participants 6 y.o.—1 participant | 3 y.o.—1 participant 4 y.o.—3 participants 5. y.o.—2 participants 6 y.o.—1 participant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokolovs-Karijs, O.; Brīvība, M.; Saksis, R.; Rozenberga, M.; Girotto, F.; Osīte, J.; Reinis, A.; Sumeraga, G.; Krūmiņa, A. Identifying the Microbiome of the Adenoid Surface of Children Suffering from Otitis Media with Effusion and Children without Middle Ear Effusion Using 16S rRNA Genetic Sequencing. Microorganisms 2023, 11, 1955. https://doi.org/10.3390/microorganisms11081955
Sokolovs-Karijs O, Brīvība M, Saksis R, Rozenberga M, Girotto F, Osīte J, Reinis A, Sumeraga G, Krūmiņa A. Identifying the Microbiome of the Adenoid Surface of Children Suffering from Otitis Media with Effusion and Children without Middle Ear Effusion Using 16S rRNA Genetic Sequencing. Microorganisms. 2023; 11(8):1955. https://doi.org/10.3390/microorganisms11081955
Chicago/Turabian StyleSokolovs-Karijs, Oļegs, Monta Brīvība, Rihards Saksis, Maija Rozenberga, Francesca Girotto, Jana Osīte, Aigars Reinis, Gunta Sumeraga, and Angelika Krūmiņa. 2023. "Identifying the Microbiome of the Adenoid Surface of Children Suffering from Otitis Media with Effusion and Children without Middle Ear Effusion Using 16S rRNA Genetic Sequencing" Microorganisms 11, no. 8: 1955. https://doi.org/10.3390/microorganisms11081955
APA StyleSokolovs-Karijs, O., Brīvība, M., Saksis, R., Rozenberga, M., Girotto, F., Osīte, J., Reinis, A., Sumeraga, G., & Krūmiņa, A. (2023). Identifying the Microbiome of the Adenoid Surface of Children Suffering from Otitis Media with Effusion and Children without Middle Ear Effusion Using 16S rRNA Genetic Sequencing. Microorganisms, 11(8), 1955. https://doi.org/10.3390/microorganisms11081955







