Fecal Microbiota and Diet Composition of Buryatian Horses Grazing Warm- and Cold-Season Grass Pastures
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Ethical Considerations
2.3. Microhistological Fecal Analysis
2.4. DNA Extraction, Amplicon Library Construction, Sequencing, and Data Analysis
3. Results
3.1. Microhistology
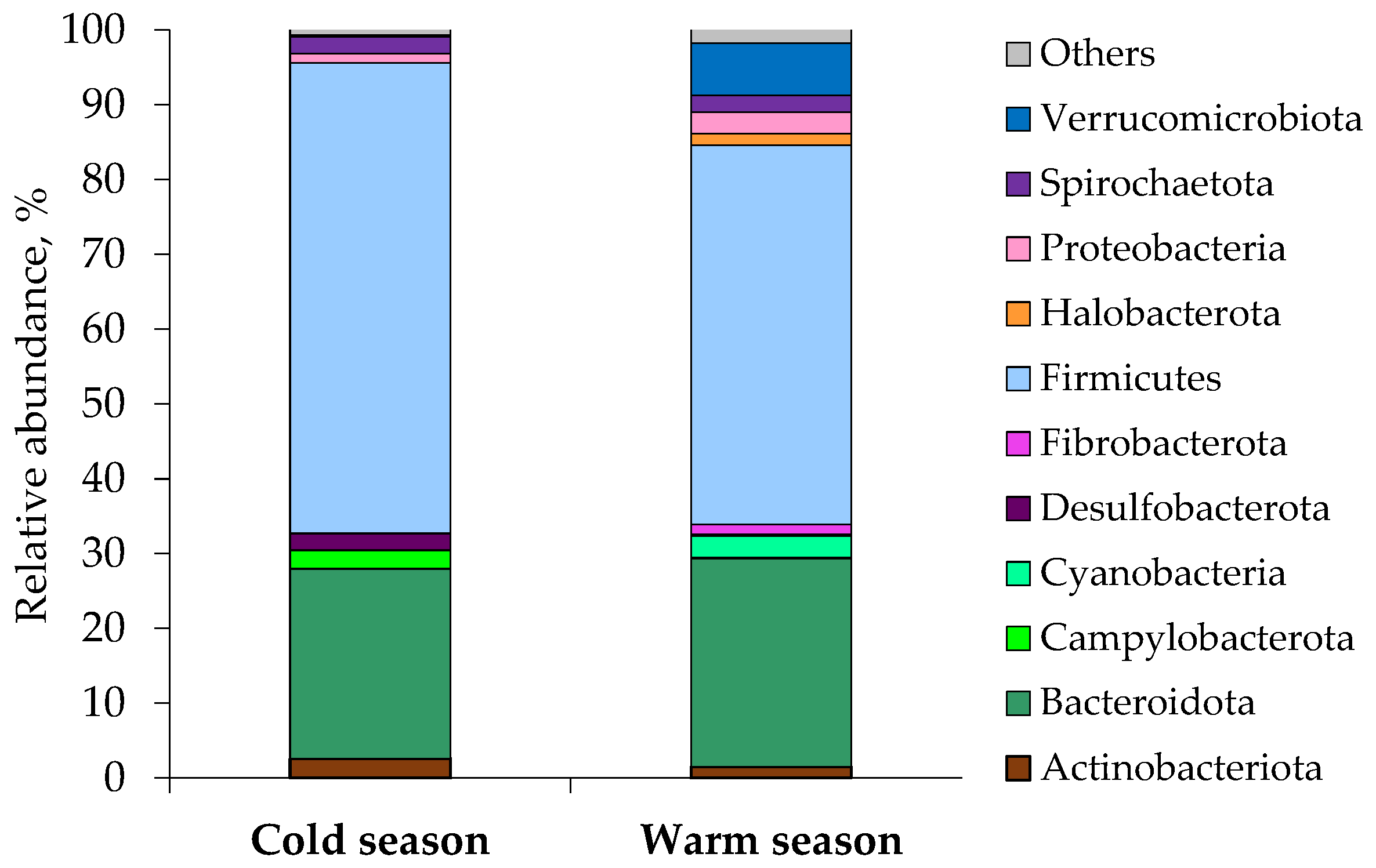
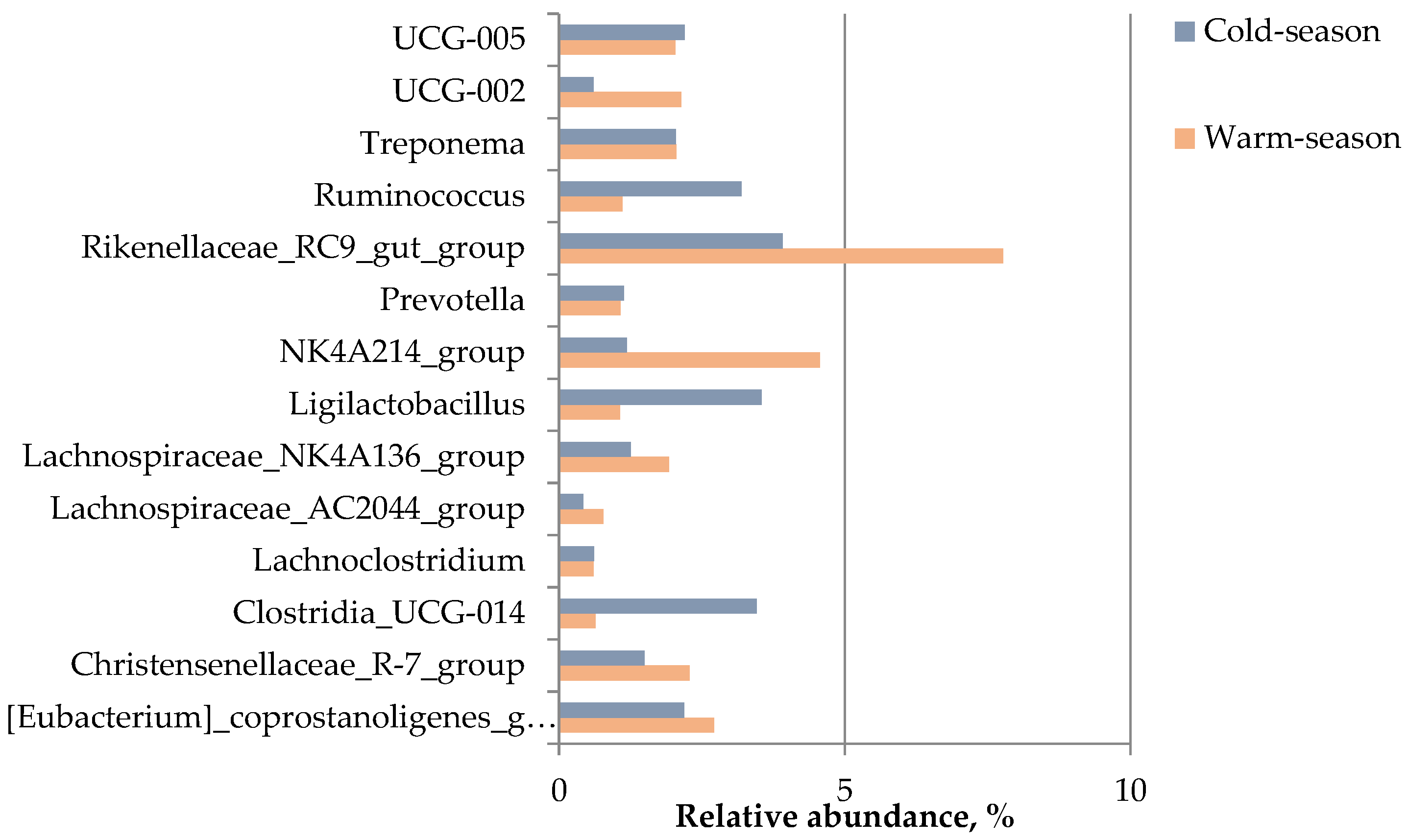
3.2. Analysis of the Fecal Microbiota (Microbial Communities) Using 16S rRNA Profiling
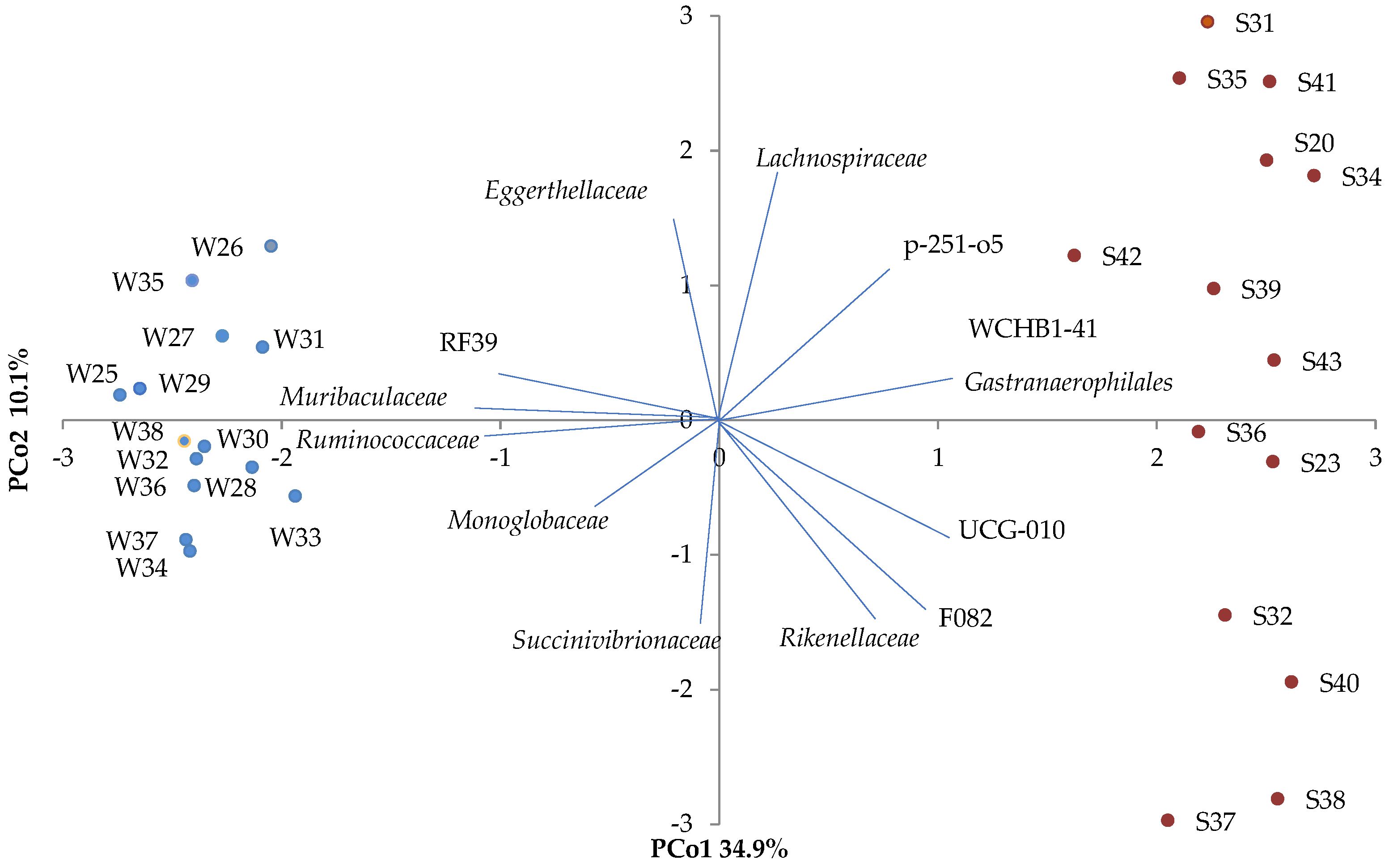
Seasonal-Specific Differences in Diversity of the Fecal Microbial Community
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ang, L.; Vinderola, G.; Endo, A.; Kantanen, J.; Jingfeng, C.; Binetti, A.; Burns, P.; Qingmiao, S.; Suying, D.; Zujiang, Y.; et al. Gut Microbiome Characteristics in feral and domesticated horses from different geographic locations. Commun. Biol. 2022, 5, 172. [Google Scholar] [CrossRef]
- Fernandes, K.A.; Gee, E.K.; Rogers, C.W.; Kittelmann, S.; Biggs, P.J.; Bermingham, E.N.; Bolwell, C.F.; Thomas, D.G. Seasonal Variation in the Faecal Microbiota of Mature Adult Horses Maintained on Pasture in New Zealand. Animals 2021, 11, 2300. [Google Scholar] [CrossRef]
- Park, T.; Cheong, H.; Yoon, J.; Kim, A.; Yun, Y.; Unno, T. Comparison of the Fecal Microbiota of Horses with Intestinal Disease and Their Healthy Counterparts. Vet. Sci. 2021, 8, 113. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, B.; Bai, D.; Huang, J.; Shiraigo, W.; Yang, L.; Zhao, Q.; Ren, X.; Wu, J.; Bao, W.; et al. Comparison of fecal microbiota of mongolian and thoroughbred horses by high-throughput sequencing of the V4 region of the 16s rRNA gene. Asian-Australas J. Anim. Sci. 2016, 29, 1345–1352. [Google Scholar] [CrossRef]
- Wen, X.; Luo, S.; Lv, D.; Jia, C.; Zhou, X.; Zhai, Q.; Xi, L.; Yang, C. Variations in the fecal microbiota and their functions of Thoroughbred, Mongolian, and Hybrid horses. Front. Vet. Sci. 2022, 9, 920080. [Google Scholar] [CrossRef] [PubMed]
- Weinert-Nelson, J.R.; Biddle, A.S.; Sampath, H.; Williams, C.A. Fecal Microbiota, Forage Nutrients, and Metabolic Responses of Horses Grazing Warm- and Cool-Season Grass Pastures. Animals 2023, 13, 790. [Google Scholar] [CrossRef]
- Theelen, M.J.P.; Luiken, R.E.C.; Wagenaar, J.A.; Sloet van Oldruitenborgh-Oosterbaan, M.M.; Rossen, J.W.A.; Zomer, A.L. The Equine Faecal Microbiota of Healthy Horses and Ponies in The Netherlands: Impact of Host and Environmental Factors. Animals 2021, 11, 1762. [Google Scholar] [CrossRef]
- Metcalf, J.L.; Song, S.J.; Morton, J.T.; Weiss, S.; Seguin-Orlando, A.; Joly, F.; Feh, C.; Taberlet, P.; Coissac, E.; Amir, A.; et al. Evaluating the impact of domestication and captivity on the horse gut microbiome. Sci. Rep. 2017, 7, 15497. [Google Scholar] [CrossRef] [PubMed]
- Prišenk, J.; Filipič, N.; Rozman, Č.; Pažek, K.; Turk, J. Evaluation of Traditional and Indigenous Horse Breeds for Wider Intended Use: Case Study from Slovenia. Sustainability 2022, 14, 1971. [Google Scholar] [CrossRef]
- Mendelsohn, R. The challenge of conserving indigenous domesticated animals. Ecol. Econ. 2003, 45, 501–510. [Google Scholar] [CrossRef]
- Marsoner, T.; Vigl, L.E.; Manck, F.; Jaritz, G.; Tappeiner, U.; Tasser, E. Indigenous livestock breeds as indicators for cultural ecosystem services: A spatial analysis within the Alpine Space. Ecol. Indic. 2018, 94, 55–63. [Google Scholar] [CrossRef]
- Khrabrova, L.A. Preservation and use of the gene pool of native breeds of horses. Eff. Anim. Husb. 2016, 4, 33–35. (In Russian) [Google Scholar]
- Taishin, V.A.; Lkhasaranov, B.B.; James, R. Atlas of Nomadic Animals; Publishing House of the Siberian Branch of the Russian Academy of Sciences: Novosibirsk, Russia, 1999; 284p. [Google Scholar]
- Khrabrova, L.A.; Blohina, N.V.; Bazaron, B.Z.; Khamiruev, T.N. Variability of mitochondrial DNA D-loop sequences in Zabaikalskaya horse breed. Vavilov J. Genet. Breed. 2021, 25, 486–491. [Google Scholar] [CrossRef]
- Kalashnikov, I.A. Scientific and Practical Aspects of Conservation, Selection and Use of Horses of Local Aboriginal Breeds (on the Example of the Buryatian Horse). Thesis of Doctor’s Degree, Moscow Timiryazev Agricultural Academy, Moscow, Russia, 1997; 36p. [Google Scholar]
- Schoenecker, K.A.; King, S.R.B.; Nordquist, M.K.; Nandintsetseg, D.; Cao, Q. Habitat and diet of equids. In Wild Equids: Ecology, Management, and Conservation; Ransom, J.I., Kaczensky, P., Eds.; EdsJohns Hopkins University Press: Baltimore, MD, USA, 2016; pp. 41–57. [Google Scholar]
- Kobayashi, Y.; Koike, S.; Miyaji, M.; Hata, H.; Tanaka, K. Hindgut microbes, fermentation and their seasonal variations in Hokkaido native horses compared to light horses. Ecol. Res. 2006, 21, 285–291. [Google Scholar] [CrossRef]
- Salem, S.E.; Maddox, T.W.; Berg, A.; Antczak, P.; Ketley, J.M.; Williams, N.J.; Archer, D.C. Variation in faecal microbiota in a group of horses managed on pasture over a 12-month period. Sci. Rep. 2018, 8, 8510. [Google Scholar] [CrossRef] [PubMed]
- Korolyuk, A.Y. Syntaxonomy of steppe vegetation of the Republic of Buryatia. Veg. Russ. 2017, 31, 3–32. [Google Scholar] [CrossRef]
- King, S.R.B.; Schoenecker, K.A. Comparison of Methods to Examine Diet of Feral Horses from Noninvasively Collected Fecal Samples. Rangel. Ecol. Manag. 2019, 72, 661–666. [Google Scholar] [CrossRef]
- Scasta, J.D.; Beck, J.L.; Anqwin, C.J. Meta-Analysis of Diet Composition and Potential Conflict of Wild Horses with Livestock and Wild Ungulates on Western Rangelands of North America. Rangel. Ecol. Manag. 2016, 69, 309–318. [Google Scholar] [CrossRef]
- Holechek, J.L.; Vavra, M.; Pieper, R.D. Botanical composition determination of range herbivore diets: A review. J. Range Manag. 1982, 35, 309–315. [Google Scholar] [CrossRef]
- Toshchakov, S.V.; Izotova, A.O.; Vinogradova, E.N.; Kachmazov, G.S.; Tuaeva, A.Y.; Abaev, V.T.; Evteeva, M.A.; Gunitseva, N.M.; Korzhenkov, A.A.; Elcheninov, A.G.; et al. Culture-Independent Survey of Thermophilic Microbial Communities of the North Caucasus. Biology 2021, 10, 1352. [Google Scholar] [CrossRef]
- Karnachuk, O.V.; Panova, I.A.; Panov, V.L.; Ikkert, O.P.; Kadnikov, V.V.; Rusanov, I.I.; Avakyan, M.P.; Glukhova, L.B.; Rakitin, A.V.; Begmatov, S.; et al. Active Sulfate-Reducing Bacterial Community in the Camel Gut. Microorganisms 2023, 11, 401. [Google Scholar] [CrossRef]
- Frey, B.; Rime, T.; Phillips, M.; Stierli, B.; Hajdas, I.; Widmer, F.; Hartmann, M. Microbial diversity in European alpine permafrost and active layers. FEMS Microbiol. Ecol. 2016, 92, fiw018. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Gohl, D.; Gohl, D.M.; MacLean, A.; Hauge, A.; Becker, A.; Walek, D.; Beckman, K.B. An Optimized Protocol for High-Throughput Amplicon-Based Microbiome Profiling. Protoc. Exch. 2016, 1–15. [Google Scholar] [CrossRef]
- Hugerth, L.W.; Wefer, H.A.; Lundin, S.; Jakobsson, H.E.; Lindberg, M.; Rodin, S.; Engstrand, L.; Andersson, A.F. DegePrime, a Program for Degenerate Primer Design for Broad-Taxonomic-Range PCR in Microbial Ecology Studies. Appl. Environ. Microbiol. 2014, 80, 5116–5123. [Google Scholar] [CrossRef]
- Merkel, A.Y.; Tarnovetskii, I.Y.; Podosokorskaya, O.A.; Toshchakov, S.V. Analysis of 16S RRNA Primer Systems for Profiling of Thermophilic Microbial Communities. Microbiology 2019, 88, 671–680. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.M.; Proctor, D.M.; Holmes, S.P.; Relman, D.A.; Callahan, B.J. Simple Statistical Identification and Removal of Contaminant Sequences in Marker-Gene and Metagenomics Data. Microbiome 2018, 6, 226. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.C.; Ma, K.H.; Chao, A. INEXT: An R Package for Rarefaction and Extrapolation of Species Diversity (H Ill Numbers). Methods Eco. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Vegan: Community Ecology Package. Ordination Methods, Diversity Analysis and Other Functions for Community and Vegetation Ecologists. Available online: https://www.worldagroforestry.org/publication/vegan-community-ecology-package-ordination-methods-diversity-analysis-and-other (accessed on 19 November 2022).
- Lahti, L.; Shetty, S.; Blake, T.; Salojarvi, J. Microbiome; Version 1.0.2; Bioconductor: 2017. Available online: https://bioconductor.statistik.tutortmund.de/packages/3.6/bioc/html/microbiome.html (accessed on 19 November 2022).
- Zuur, A.F.; Ieno, E.N.; Smith, G.M. Analyzing Ecological Data; Springer: New York, NY, USA, 2007; p. 672. [Google Scholar]
- Peshkova, G.A. Florogenetic Analysis of the Steppe Flora of the Mountains of Southern Siberia; Science: Novosibirsk, Russia, 2001; 192p. (In Russian) [Google Scholar]
- Amarjargal, A.; Bayarkhuu, Y.; Bayarkhuu, B. Distribution and some results of chemical studies in Schizonepeta multifida L. Vestn. Tuvan State Univ. 2022, 3, 17–29. [Google Scholar]
- Zhao, X.; Zhou, M. Review on Chemical Constituents of Schizonepeta tenuifolia Briq. and Their Pharmacological Effects. Molecules 2022, 27, 5249. [Google Scholar] [CrossRef]
- Mojtahed Zadeh Asl, R.; Niakousari, M.; Hashemi Gahruie, H.; Saharkhiz, M.J.; Mousavi Khaneghah, A. Study of two-stage ohmic hydro-extraction of essential oil from Artemisia aucheri Boiss.: Antioxidant and antimicrobial characteristics. Food Res. Int. 2018, 107, 462–469. [Google Scholar] [CrossRef]
- Kļaviņa, A.; Keidāne, D.; Šukele, R.; Bandere, D.; Kovaļčuka, L. Traditional Latvian herbal medicinal plants used to treat parasite infections of small ruminants: A review. Vet. World 2021, 14, 1548–1558. [Google Scholar] [CrossRef]
- Lara, F.; Castro, R.; Thomson, P. Changes in the gut microbiome and colic in horses: Are they causes or consequences? Open Vet. J. 2022, 12, 242–249. [Google Scholar] [CrossRef]
- Qiu, X.; Qin, X.; Chen, L.; Chen, Z.; Hao, R.; Zhang, S.; Yang, S.; Wang, L.; Cui, Y.; Li, Y.; et al. Serum Biochemical Parameters, Rumen Fermentation, and Rumen Bacterial Communities Are Partly Driven by the Breed and Sex of Cattle When Fed High-Grain Diet. Microorganisms 2022, 10, 323. [Google Scholar] [CrossRef]
- Pereira, F.C.; Wasmund, K.; Cobankovic, I.; Jehmlich, N.; Herbold, C.W.; Lee, K.S.; Sziranyi, B.; Vesely, C.; Decker, T.; Stocker, R.; et al. Rational design of a microbial consortium of mucosal sugar utilizers reduces Clostridiodes difficile colonization. Nat. Commun. 2020, 11, 5104. [Google Scholar] [CrossRef]
- Dougal, K.; Harris, P.A.; Edwards, A.; Pachebat, J.A.; Blackmore, T.M.; Worgan, H.J.; Newbold, C.J. A comparison of the microbiome and the metabolome of different regions of the equine hindgut. FEMS Microbiol. Ecol. 2012, 82, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Fliegerova, K.; Mura, E.; Mrázek, J.; Moniello, G. A comparison of microbial profiles of different regions of the equine hindgut. Livest. Sci. 2016, 190, 16–19. [Google Scholar] [CrossRef]
- Lwin, K.O.; Matsui, H. Comparative analysis of the methanogen diversity in horse and pony by using mcrA gene and archaeal 16s rRNA gene clone libraries. Archaea 2014, 2014, 483574. [Google Scholar] [CrossRef] [PubMed]
| Summer | Winter | ||
|---|---|---|---|
| Species | Mean% | Species | Mean% |
| Total graminoids: | 41.9 ± 11.7 | Total graminoids: | 7 ± 5.3 |
| Poa botryoides | 14.3 | Poa botryoides | 2.9 |
| Agropyron cristatum | 11.1 | Carex pediformis | 2.4 |
| Carex pediformis | 8.9 | Agropyron cristatum | 1.2 |
| Stipa capillata L. | 3.0 | Koeleria cristata | 0.3 |
| Achnatherum splendens | 1.7 | Achnatherum splendens | 0.2 |
| Koeleria cristata | 1.4 | ||
| Carex sp. | 1.1 | ||
| Carex duriuscula C. A. Meyer 1831 | 0.8 | ||
| Total forbs: | 57.7 ± 11.8 | Total forbs: | 92.6 ± 5.9 |
| Lappula myosotis | 8.9 | Schizonepeta multifida | 51.43 |
| Silene repens | 8.5 | Aster alpinus | 9.4 |
| Aster alpinus | 7.8 | Artemisia sieversiana | 8.7 |
| Unknown grass 6 | 4.3 | Heteropappus altaicus | 6.8 |
| Potentilla acaulis | 3.9 | Potentilla acaulis | 6.1 |
| Allium altaicum | 2.6 | Heteropappus biennis | 2.5 |
| Thalictrum etaloideum L. | 2.5 | Lappula myosotis | 2.4 |
| Unknown grass 2 | 2.3 | Cymbaria dahurica | 1.4 |
| Heteropappus biennis | 2.3 | ||
| Serratula centauroides L. s. str. | 1.7 | ||
| Achnatherum splendens | 1.7 | ||
| Pulsatilla turczaninovii | 1.5 | ||
| Artemisia dracunculus L. | 1.3 | ||
| Saussurea salicifolia L. | 1.1 | ||
| Phlomis tuberosa L. | 1.0 | ||
| Nonea rossica | 1.0 | ||
| Potentilla longifolia | 0.8 | ||
| Schizonepeta multifida | 0.8 | ||
| Artemisia frigida Wil | 0.7 | ||
| Axyris amaranthoides L. | 0.6 | ||
| Leontopodium leontopodoides | 0.4 | ||
| Unknown grass 9 | 0.4 | ||
| Hypecoum (Chiazospermum) erectum | 0.3 | ||
| Unknown grass 4 | 0.3 | ||
| Unknown grass 5 | 0.3 | ||
| Astragalus adsurgens Pallas | 0.3 | ||
| Artemisia gmelinii Webb ex Stechm. | 0.3 | ||
| Descurainia sophia (L.) Webb. et Berth. | 0.2 | ||
| Potentilla bifurca | 0.2 | ||
| Allium anisopodium Ledeb | 0.2 | ||
| Pulsatilla multifida | 0.2 | ||
| Cymbaria dahurica | 0.2 | ||
| Heteropappus altaicus | 0.1 | ||
| Artemisia sieversiana | 0.1 | ||
| Saposhnikovia divaricata (Turcz.) Hiroe | 0.1 | ||
| Thalictrum squarrosum Stephan ex Willd. | 0.1 | ||
| Galium verum L. | 0.1 | ||
| Leonurus sp. | 0.1 | ||
| Total shrubs: | 0.4 ± 1.3 | Total shrubs: | 0.43 ± 1.3 |
| Kochia prostrata Sschraber | 0.4 | Kochia prostrata Sschraber | 0.43 |
| Spiraea sp. | 0.1 |
| Season | ASVs/OTUs | Chao1 | Shannon Index | Simpson Index |
|---|---|---|---|---|
| Warm season | 887 | 1263.7 ± 203 | 5.74 ± 0.41 | 0.986 ± 0.024 |
| Cold season | 114 | 480.8 ± 35.7 | 5.51 ± 0.11 | 0.991 ± 0.003 |
| p-value * | <0.001 | <0.001 | <0.001 | 0.449 |
| Phylum | Class | Family | Cold Season Mean Relative Abundance, % | Warm Season Mean Relative Abundance, % |
|---|---|---|---|---|
| Firmicutes | 62.95 | 50.74 | ||
| Clostridia | 40.64 | 42.60 | ||
| Lachnospiraceae | 11.4 | 13.1 | ||
| Oscillospiraceae | 7.7 | 9.6 | ||
| Ruminococcaceae | 9.4 | 3.2 | ||
| Christensenellaceae | 1.5 | 2.3 | ||
| Order_Clostridia UCG-014 | 3.5 | |||
| Peptostreptococcaceae | 1.8 | |||
| UCG-010 | 3.9 | |||
| Alkalibacteraceae | 2.1 | |||
| Anaerovoracaceae | 1.8 | |||
| Hungateiclostridiaceae | 1.2 | |||
| Bacilli | 20.15 | 5.87 | ||
| Erysipelatoclostridiaceae | 3.2 | 1.2 | ||
| Lactobacillaceae | 11.5 | 1.9 | ||
| Planococcaceae | 1.1 | |||
| Negativicutes | 1.12 | 2.20 | ||
| Acidaminococcaceae | 1.9 | |||
| Bacteroidota | 25.33 | 27.90 | ||
| Bacteroidia | 26.20 | 27.90 | ||
| Prevotellaceae | 10.9 | 6.7 | ||
| Rikenellaceae | 4.5 | 9.1 | ||
| Muribaculaceae | 6.4 | |||
| Bacteroidaceae | 1.6 | |||
| p-251-o5 | 4.4 | |||
| F082 | 3.5 | |||
| Bacteroidales_UCG-001 | 1.8 | |||
| Bacteroidales_RF16_group | 1.3 | |||
| Actinobacteriota | 2.61 | 1.45 | ||
| Coriobacteriia | 2.18 | 1.15 | ||
| Eggerthellaceae | 1.1 | |||
| Campylobacterota | 2.50 | |||
| Campylobacteria | 2.55 | |||
| Helicobacteraceae | 2.3 | |||
| Cyanobacteria | 2.86 | |||
| Vampirivibrionia | 2.86 | |||
| Gastranaerophilales | 2.9 | |||
| Desulfobacterota | 2.23 | |||
| Desulfovibrionia | 2.25 | |||
| Desulfovibrionaceae | 2.2 | |||
| Fibrobacterota | 1.34 | |||
| Fibrobacteria | 1.34 | |||
| Fibrobacteraceae | 1.3 | |||
| Halobacterota | 1.56 | |||
| Methanomicrobia | 1.49 | |||
| Methanocorpusculaceae | 1.5 | |||
| Proteobacteria | 1.26 | 2.88 | ||
| Alphaproteobacteria | 1.98 | |||
| Caulobacteraceae | 1.1 | |||
| Spirochaetota | 2.23 | 2.20 | ||
| Spirochaetia | 2.16 | 2.12 | ||
| Spirochaetaceae | 2.2 | 2.1 | ||
| Verrucomicrobiota | 7.00 | |||
| Kiritimatiellae | 6.49 | |||
| WCHB1-41 | 6.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaitseva, S.; Dagurova, O.; Radnagurueva, A.; Kozlova, A.; Izotova, A.; Krylova, A.; Noskov, S.; Begmatov, S.; Patutina, E.; Barkhutova, D.D. Fecal Microbiota and Diet Composition of Buryatian Horses Grazing Warm- and Cold-Season Grass Pastures. Microorganisms 2023, 11, 1947. https://doi.org/10.3390/microorganisms11081947
Zaitseva S, Dagurova O, Radnagurueva A, Kozlova A, Izotova A, Krylova A, Noskov S, Begmatov S, Patutina E, Barkhutova DD. Fecal Microbiota and Diet Composition of Buryatian Horses Grazing Warm- and Cold-Season Grass Pastures. Microorganisms. 2023; 11(8):1947. https://doi.org/10.3390/microorganisms11081947
Chicago/Turabian StyleZaitseva, Svetlana, Olga Dagurova, Aryuna Radnagurueva, Aleksandra Kozlova, Anna Izotova, Anastasia Krylova, Sergey Noskov, Shahjahon Begmatov, Ekaterina Patutina, and Darima D. Barkhutova. 2023. "Fecal Microbiota and Diet Composition of Buryatian Horses Grazing Warm- and Cold-Season Grass Pastures" Microorganisms 11, no. 8: 1947. https://doi.org/10.3390/microorganisms11081947
APA StyleZaitseva, S., Dagurova, O., Radnagurueva, A., Kozlova, A., Izotova, A., Krylova, A., Noskov, S., Begmatov, S., Patutina, E., & Barkhutova, D. D. (2023). Fecal Microbiota and Diet Composition of Buryatian Horses Grazing Warm- and Cold-Season Grass Pastures. Microorganisms, 11(8), 1947. https://doi.org/10.3390/microorganisms11081947







