Escherichia coli Nissle 1917 Antagonizes Candida albicans Growth and Protects Intestinal Cells from C. albicans-Mediated Damage
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
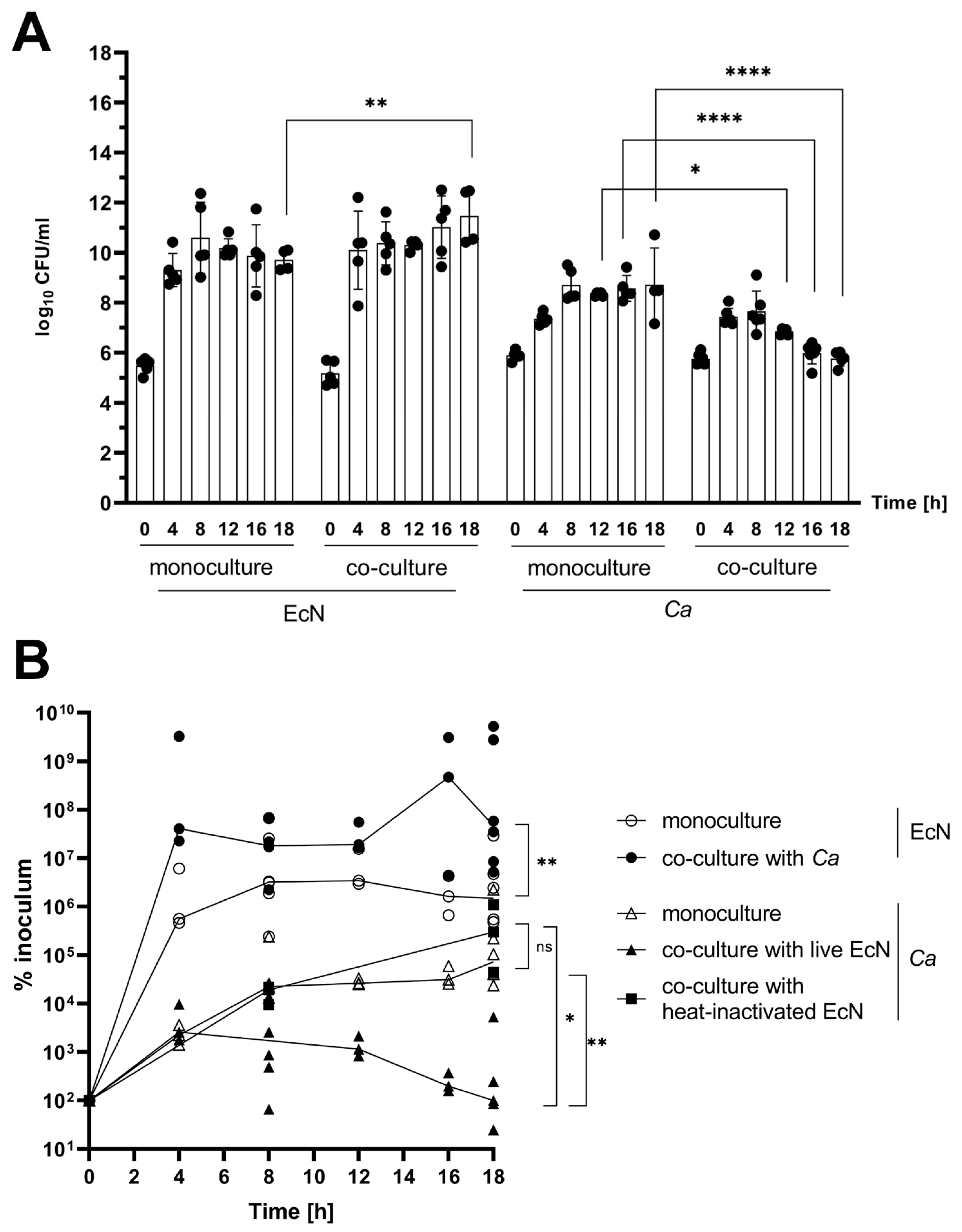
2.2. In Vitro Co-Culture Experiments
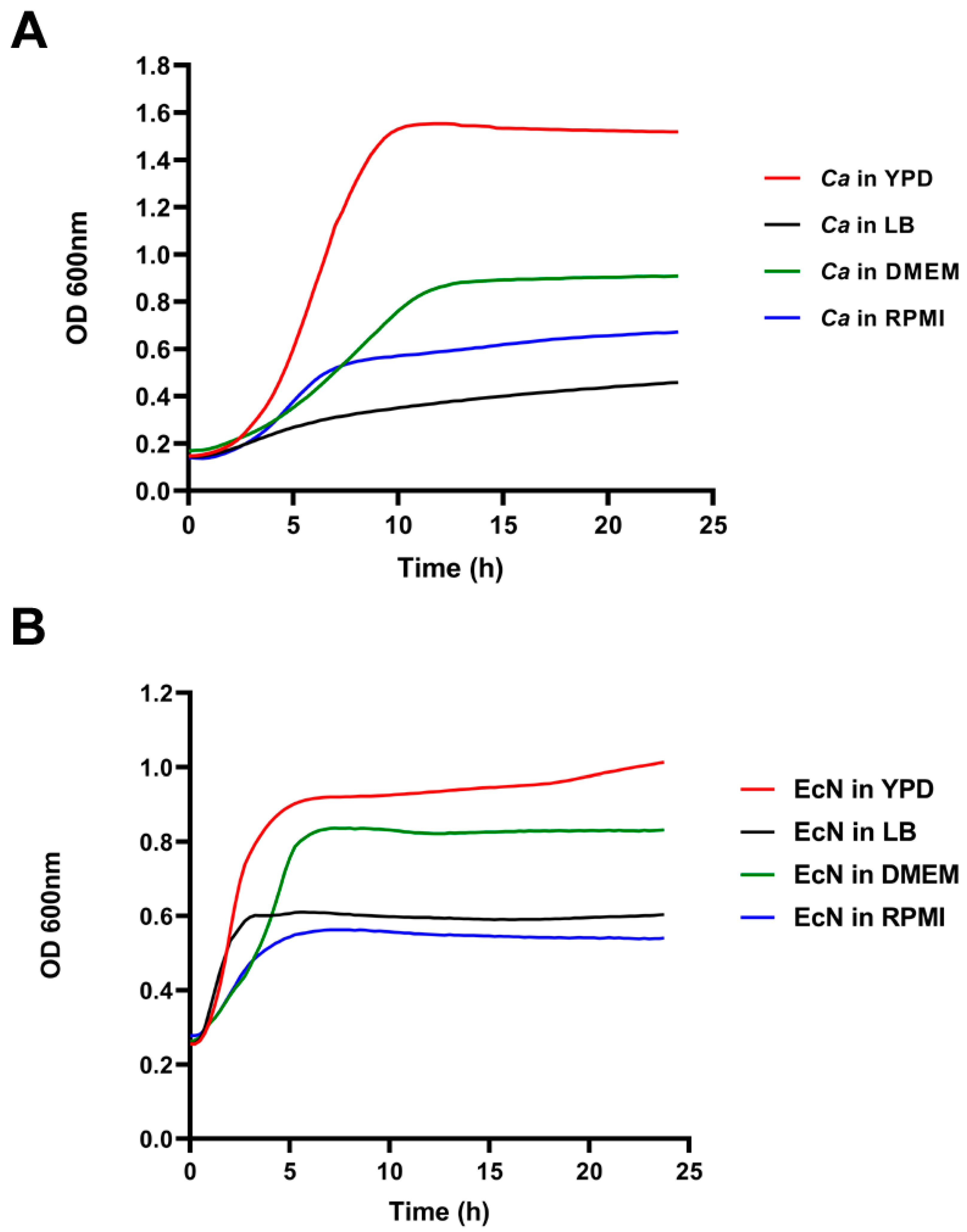
2.3. Growth Curve Assays
2.4. Collection of Cell-Free Supernatant (CFS)
2.5. Intestinal Epithelial Cell Model
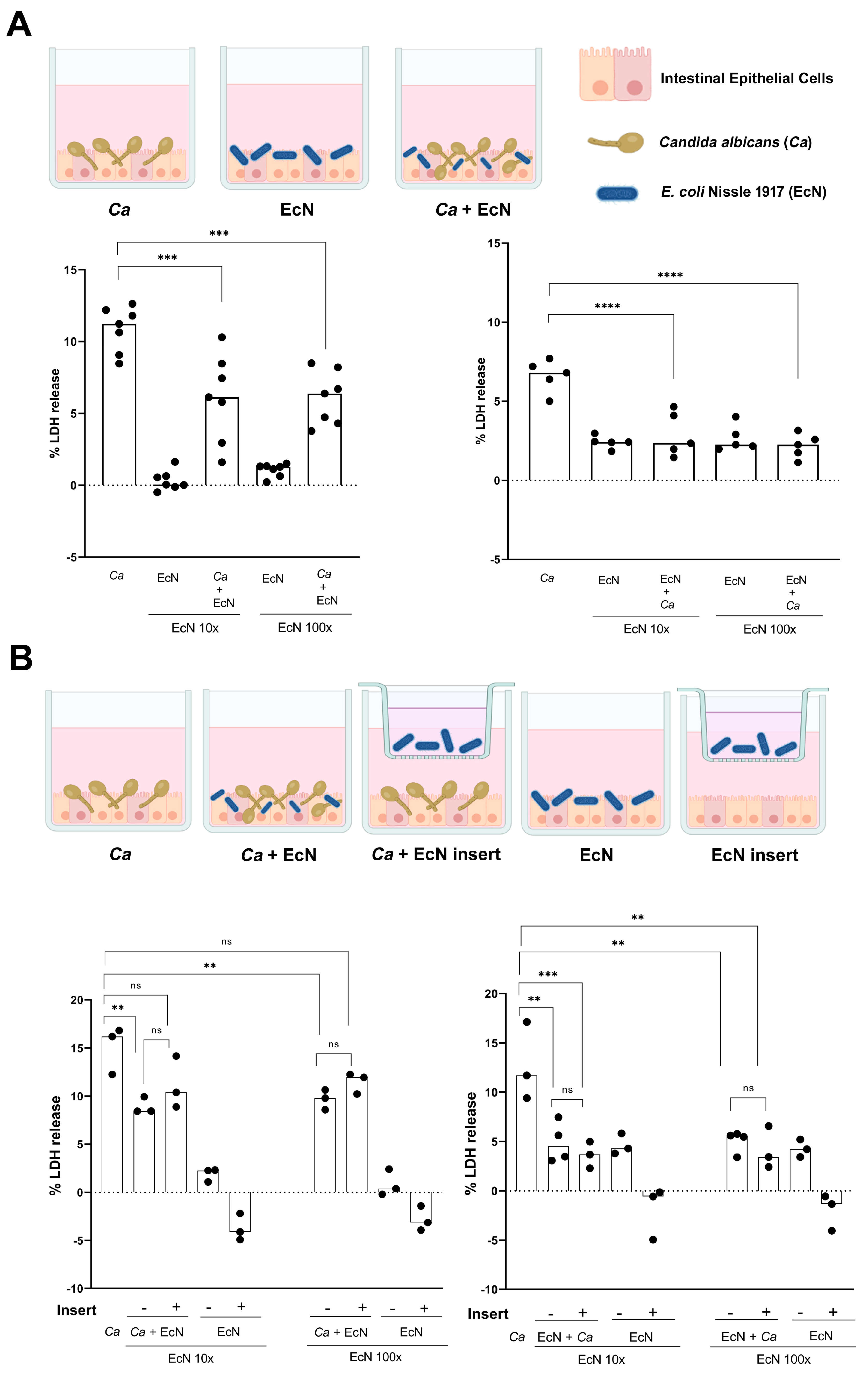
2.6. Intestinal Epithelial Cell Infection Model
2.7. Cytotoxicity Assay LDH
2.8. Cell Contact Assay
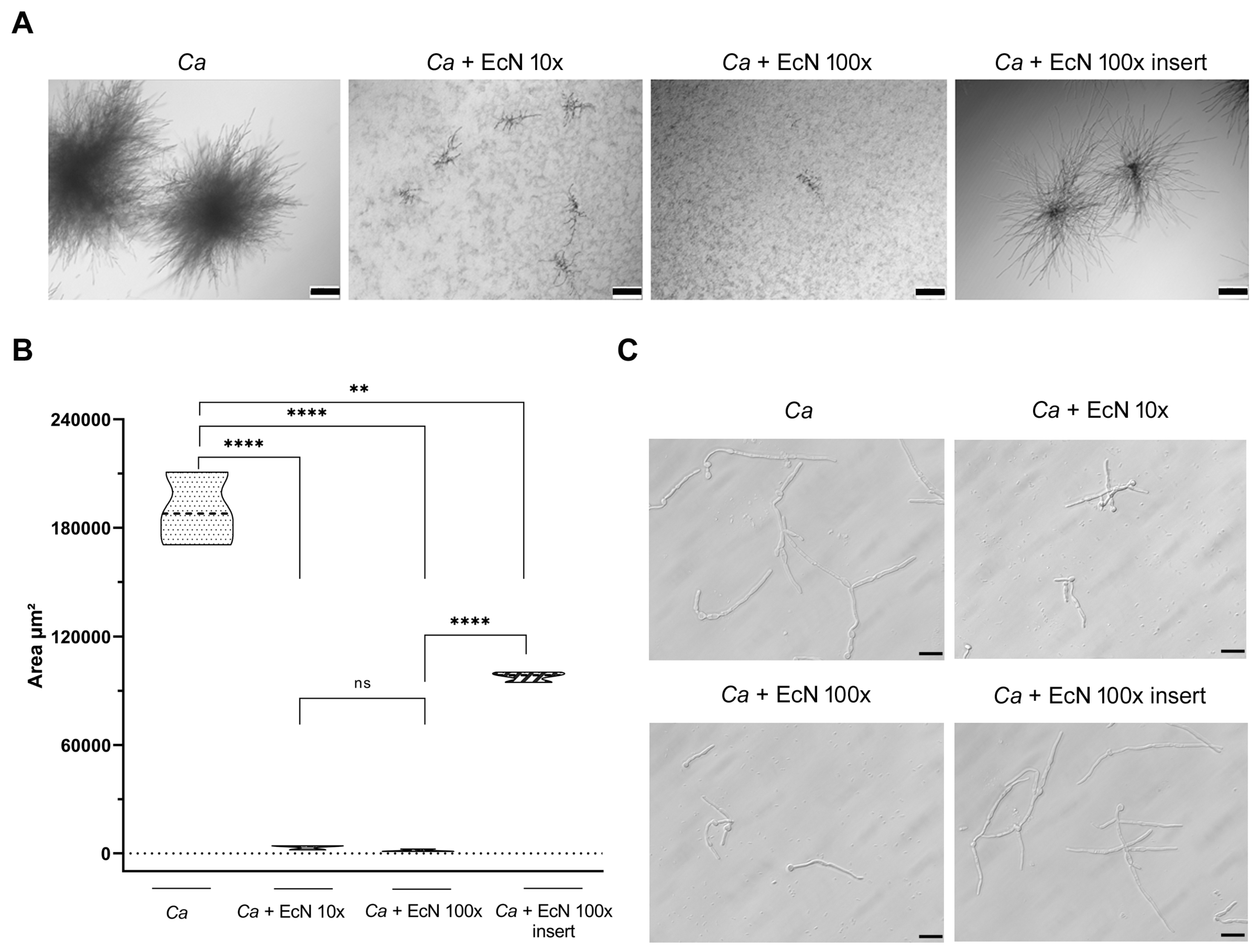
2.9. Microcolony Formation
2.10. Filamentation Assay
2.11. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- Francino, M.P. Antibiotics and the Human Gut Microbiome: Dysbioses and Accumulation of Resistances. Front. Microbiol. 2016, 6, 1543. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Sugimura, N.; Burgermeister, E.; Ebert, M.P.; Zuo, T.; Lan, P. The gut virome: A new microbiome component in health and disease. EBioMedicine 2022, 81, 104113. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Jawhara, S. How Gut Bacterial Dysbiosis Can Promote Candida albicans Overgrowth during Colonic Inflammation. Microorganisms 2022, 10, 1014. [Google Scholar] [CrossRef]
- Mishra, A.A.; Koh, A.Y. Adaptation of Candida albicans during gastrointestinal tract colonization. Curr. Clin. Microbiol. Rep. 2018, 5, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, C.A.; Gresnigt, M.S.; Hube, B. The gut, the bad and the harmless: Candida albicans as a commensal and opportunistic pathogen in the intestine. Curr. Opin. Microbiol. 2020, 56, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Swidergall, M.; LeibundGut-Landmann, S. Immunosurveillance of Candida albicans commensalism by the adaptive immune system. Mucosal. Immunol. 2022, 15, 829–836. [Google Scholar] [CrossRef]
- Allert, S.; Forster, T.M.; Svensson, C.M.; Richardson, J.P.; Pawlik, T.; Hebecker, B.; Rudolphi, S.; Juraschitz, M.; Schaller, M.; Blagojevic, M.; et al. Candida albicans-Induced Epithelial Damage Mediates Translocation through Intestinal Barriers. mBio 2018, 9, 10–1128. [Google Scholar] [CrossRef]
- Sprague, J.L.; Kasper, L.; Hube, B. From intestinal colonization to systemic infections: Candida albicans translocation and dissemination. Gut Microbes 2022, 14, 2154548. [Google Scholar] [CrossRef]
- McCall, A.D.; Kumar, R.; Edgerton, M. Candida albicans Sfl1/Sfl2 regulatory network drives the formation of pathogenic microcolonies. PLoS Pathog. 2018, 14, e1007316. [Google Scholar] [CrossRef]
- Zuo, T.; Wong, S.H.; Cheung, C.P.; Lam, K.; Lui, R.; Cheung, K.; Zhang, F.; Tang, W.; Ching, J.Y.L.; Wu, J.C.Y.; et al. Gut fungal dysbiosis correlates with reduced efficacy of fecal microbiota transplantation in Clostridium difficile infection. Nat. Commun. 2018, 9, 3663. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.L.; Limon, J.J.; Bar, A.S.; Leal, C.A.; Gargus, M.; Tang, J.; Brown, J.; Funari, V.A.; Wang, H.L.; Crother, T.R.; et al. Immunological Consequences of Intestinal Fungal Dysbiosis. Cell Host Microbe 2016, 19, 865–873. [Google Scholar] [CrossRef]
- Matsubara, V.H.; Bandara, H.M.; Mayer, M.P.; Samaranayake, L.P. Probiotics as Antifungals in Mucosal Candidiasis. Clin. Infect. Dis. 2016, 62, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Sonnenborn, U. Escherichia coli strain Nissle 1917-from bench to bedside and back: History of a special Escherichia coli strain with probiotic properties. FEMS Microbiol. Lett. 2016, 363, fnw212. [Google Scholar] [CrossRef]
- Patzer, S.I.; Baquero, M.R.; Bravo, D.; Moreno, F.; Hantke, K. The colicin G, H and X determinants encode microcins M and H47, which might utilize the catecholate siderophore receptors FepA, Cir, Fiu and IroN. Microbiology 2003, 149, 2557–2570. [Google Scholar] [CrossRef]
- Hare, P.J.; Englander, H.E.; Mok, W.W.K. Probiotic Escherichia coli Nissle 1917 inhibits bacterial persisters that survive fluoroquinolone treatment. J. Appl. Microbiol. 2022, 132, 4020–4032. [Google Scholar] [CrossRef] [PubMed]
- Hove, P.R.; Nealon, N.J.; Chan, S.H.J.; Boyer, S.M.; Haberecht, H.B.; Ryan, E.P. Integrated Profiling of Gram-Positive and Gram-Negative Probiotic Genomes, Proteomes and Metabolomes Revealed Small Molecules with Differential Growth Inhibition of Antimicrobial-Resistant Pathogens. J. Diet. Suppl. 2022, 1–23. [Google Scholar] [CrossRef]
- Sassone-Corsi, M.; Raffatellu, M. No vacancy: How beneficial microbes cooperate with immunity to provide colonization resistance to pathogens. J. Immunol. 2015, 194, 4081–4087. [Google Scholar] [CrossRef]
- Graham, C.E.; Cruz, M.R.; Garsin, D.A.; Lorenz, M.C. Enterococcus faecalis bacteriocin EntV inhibits hyphal morphogenesis, biofilm formation, and virulence of Candida albicans. Proc. Natl. Acad. Sci. USA 2017, 114, 4507–4512. [Google Scholar] [CrossRef]
- Cabral, D.J.; Penumutchu, S.; Norris, C.; Morones-Ramirez, J.R.; Belenky, P. Microbial competition between Escherichia coli and Candida albicans reveals a soluble fungicidal factor. Microb. Cell 2018, 5, 249–255. [Google Scholar] [CrossRef]
- Forster, T.M.; Mogavero, S.; Drager, A.; Graf, K.; Polke, M.; Jacobsen, I.D.; Hube, B. Enemies and brothers in arms: Candida albicans and gram-positive bacteria. Cell. Microbiol. 2016, 18, 1709–1715. [Google Scholar] [CrossRef]
- Alonso-Roman, R.; Last, A.; Mirhakkak, M.H.; Sprague, J.L.; Moller, L.; Grossmann, P.; Graf, K.; Gratz, R.; Mogavero, S.; Vylkova, S.; et al. Lactobacillus rhamnosus colonisation antagonizes Candida albicans by forcing metabolic adaptations that compromise pathogenicity. Nat. Commun. 2022, 13, 3192. [Google Scholar] [CrossRef] [PubMed]
- Niemiec, M.J.; Kapitan, M.; Himmel, M.; Doll, K.; Kruger, T.; Kollner, T.G.; Auge, I.; Kage, F.; Alteri, C.J.; Mobley, H.L.T.; et al. Augmented Enterocyte Damage During Candida albicans and Proteus mirabilis Coinfection. Front. Cell Infect. Microbiol. 2022, 12, 866416. [Google Scholar] [CrossRef]
- St Onge, R.P.; Mani, R.; Oh, J.; Proctor, M.; Fung, E.; Davis, R.W.; Nislow, C.; Roth, F.P.; Giaever, G. Systematic pathway analysis using high-resolution fitness profiling of combinatorial gene deletions. Nat. Genet. 2007, 39, 199–206. [Google Scholar] [CrossRef]
- Salvatori, O.; Kumar, R.; Metcalfe, S.; Vickerman, M.; Kay, J.G.; Edgerton, M. Bacteria Modify Candida albicans Hypha Formation, Microcolony Properties, and Survival within Macrophages. mSphere 2020, 5, 10–1128. [Google Scholar] [CrossRef]
- Ferraretto, A.; Bottani, M.; De Luca, P.; Cornaghi, L.; Arnaboldi, F.; Maggioni, M.; Fiorilli, A.; Donetti, E. Morphofunctional properties of a differentiated Caco2/HT-29 co-culture as an in vitro model of human intestinal epithelium. Biosci. Rep. 2018, 38, BSR20171497. [Google Scholar] [CrossRef] [PubMed]
- Kruger, W.; Vielreicher, S.; Kapitan, M.; Jacobsen, I.D.; Niemiec, M.J. Fungal-Bacterial Interactions in Health and Disease. Pathogens 2019, 8, 70. [Google Scholar] [CrossRef]
- Eshima, S.; Kurakado, S.; Matsumoto, Y.; Kudo, T.; Sugita, T. Candida albicans Promotes the Antimicrobial Tolerance of Escherichia coli in a Cross-Kingdom Dual-Species Biofilm. Microorganisms 2022, 10, 2179. [Google Scholar] [CrossRef]
- Fourie, R.; Pohl, C.H. Beyond Antagonism: The Interaction Between Candida Species and Pseudomonas aeruginosa. J. Fungi 2019, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Darwish, M.S.; Qiu, L.; Taher, M.A.; Zaki, A.A.; Abou-Zeid, N.A.; Dawood, D.H.; Shalabi, O.M.A.K.; Khojah, E.; Elawady, A.A. Health Benefits of Postbiotics Produced by E. coli Nissle 1917 in Functional Yogurt Enriched with Cape Gooseberry (Physalis peruviana L.). Fermentation 2022, 8, 128. [Google Scholar] [CrossRef]
- Graf, K.; Last, A.; Gratz, R.; Allert, S.; Linde, S.; Westermann, M.; Groger, M.; Mosig, A.S.; Gresnigt, M.S.; Hube, B. Keeping Candida commensal: How lactobacilli antagonize pathogenicity of Candida albicans in an in vitro gut model. Dis. Model. Mech. 2019, 12, dmm039719. [Google Scholar] [CrossRef]
- Vylkova, S.; Nayyar, N.; Li, W.; Edgerton, M. Human beta-defensins kill Candida albicans in an energy-dependent and salt-sensitive manner without causing membrane disruption. Antimicrob. Agents Chemother. 2007, 51, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Schlee, M.; Wehkamp, J.; Altenhoefer, A.; Oelschlaeger, T.A.; Stange, E.F.; Fellermann, K. Induction of human beta-defensin 2 by the probiotic Escherichia coli Nissle 1917 is mediated through flagellin. Infect. Immun. 2007, 75, 2399–2407. [Google Scholar] [CrossRef]
- Hockertz, S. Augmentation of host defence against bacterial and fungal infections of mice pretreated with the non-pathogenic Escherichia coli strain Nissle 1917. Arzneimittelforschung 1997, 47, 793–796. [Google Scholar]
- Schirbel, A.; Shouval, D.S.; Hebecker, B.; Hube, B.; Sturm, A.; Werner, L. Intestinal epithelial cells and TÂ cells differentially recognize and respond to Candida albicans yeast and hypha. Eur. J. Immunol. 2018, 48, 1826–1837. [Google Scholar] [CrossRef] [PubMed]
- Wehkamp, J.; Harder, J.; Wehkamp, K.; Wehkamp-von Meissner, B.; Schlee, M.; Enders, C.; Sonnenborn, U.; Nuding, S.; Bengmark, S.; Fellermann, K.; et al. NF-kappaB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: A novel effect of a probiotic bacterium. Infect. Immun. 2004, 72, 5750–5758. [Google Scholar] [CrossRef]
- Becker, H.M.; Apladas, A.; Scharl, M.; Fried, M.; Rogler, G. Probiotic Escherichia coli Nissle 1917 and commensal E. coli K12 differentially affect the inflammasome in intestinal epithelial cells. Digestion 2014, 89, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Huebner, C.; Ding, Y.; Petermann, I.; Knapp, C.; Ferguson, L.R. The probiotic Escherichia coli Nissle 1917 reduces pathogen invasion and modulates cytokine expression in Caco-2 cells infected with Crohn’s disease-associated E. coli LF82. Appl. Environ. Microbiol. 2011, 77, 2541–2544. [Google Scholar] [CrossRef][Green Version]
- Ukena, S.N.; Westendorf, A.M.; Hansen, W.; Rohde, M.; Geffers, R.; Coldewey, S.; Suerbaum, S.; Buer, J.; Gunzer, F. The host response to the probiotic Escherichia coli strain Nissle 1917: Specific up-regulation of the proinflammatory chemokine MCP-1. BMC Med. Genet. 2005, 6, 43. [Google Scholar] [CrossRef]
- Wan, M.L.; Chen, Z.; Shah, N.P.; El-Nezami, H. Effects of Lactobacillus rhamnosus GG and Escherichia coli Nissle 1917 Cell-Free Supernatants on Modulation of Mucin and Cytokine Secretion on Human Intestinal Epithelial HT29-MTX Cells. J. Food Sci. 2018, 83, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Nougayrede, J.P.; Homburg, S.; Taieb, F.; Boury, M.; Brzuszkiewicz, E.; Gottschalk, G.; Buchrieser, C.; Hacker, J.; Dobrindt, U.; Oswald, E. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science 2006, 313, 848–851. [Google Scholar] [CrossRef]
- Igarashi, Y.; Futamata, K.; Fujita, T.; Sekine, A.; Senda, H.; Naoki, H.; Furumai, T. Yatakemycin, a novel antifungal antibiotic produced by Streptomyces sp. TP-A0356. J. Antibiot. 2003, 56, 107–113. [Google Scholar] [CrossRef]
- Schulz, M.; Gaitanoglou, V.; Mantel, O.; Hovelmann, Y.; Hubner, F.; Dobrindt, U.; Humpf, H.U. Metabolomics Study on Pathogenic and Non-pathogenic E. coli with Closely Related Genomes with a Focus on Yersiniabactin and Its Known and Novel Derivatives. Metabolites 2020, 10, 221. [Google Scholar] [CrossRef]
- van der Hooft, J.J.J.; Goldstone, R.J.; Harris, S.; Burgess, K.E.V.; Smith, D.G.E. Substantial Extracellular Metabolic Differences Found Between Phylogenetically Closely Related Probiotic and Pathogenic Strains of Escherichia coli. Front. Microbiol. 2019, 10, 252. [Google Scholar] [CrossRef]
- Hull, R.A.; Rudy, D.C.; Donovan, W.H.; Wieser, I.E.; Stewart, C.; Darouiche, R.O. Virulence properties of Escherichia coli 83972, a prototype strain associated with asymptomatic bacteriuria. Infect. Immun. 1999, 67, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Mobley, H.L.; Green, D.M.; Trifillis, A.L.; Johnson, D.E.; Chippendale, G.R.; Lockatell, C.V.; Jones, B.D.; Warren, J.W. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: Role of hemolysin in some strains. Infect. Immun. 1990, 58, 1281–1289. [Google Scholar] [CrossRef]
- Vejborg, R.M.; Friis, C.; Hancock, V.; Schembri, M.A.; Klemm, P. A virulent parent with probiotic progeny: Comparative genomics of Escherichia coli strains CFT073, Nissle 1917 and ABU 83972. Mol. Genet. Genom. 2010, 283, 469–484. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebai, Y.; Wagner, L.; Gnaien, M.; Hammer, M.L.; Kapitan, M.; Niemiec, M.J.; Mami, W.; Mosbah, A.; Messadi, E.; Mardassi, H.; et al. Escherichia coli Nissle 1917 Antagonizes Candida albicans Growth and Protects Intestinal Cells from C. albicans-Mediated Damage. Microorganisms 2023, 11, 1929. https://doi.org/10.3390/microorganisms11081929
Rebai Y, Wagner L, Gnaien M, Hammer ML, Kapitan M, Niemiec MJ, Mami W, Mosbah A, Messadi E, Mardassi H, et al. Escherichia coli Nissle 1917 Antagonizes Candida albicans Growth and Protects Intestinal Cells from C. albicans-Mediated Damage. Microorganisms. 2023; 11(8):1929. https://doi.org/10.3390/microorganisms11081929
Chicago/Turabian StyleRebai, Yasmine, Lysett Wagner, Mayssa Gnaien, Merle L. Hammer, Mario Kapitan, Maria Joanna Niemiec, Wael Mami, Amor Mosbah, Erij Messadi, Helmi Mardassi, and et al. 2023. "Escherichia coli Nissle 1917 Antagonizes Candida albicans Growth and Protects Intestinal Cells from C. albicans-Mediated Damage" Microorganisms 11, no. 8: 1929. https://doi.org/10.3390/microorganisms11081929
APA StyleRebai, Y., Wagner, L., Gnaien, M., Hammer, M. L., Kapitan, M., Niemiec, M. J., Mami, W., Mosbah, A., Messadi, E., Mardassi, H., Vylkova, S., Jacobsen, I. D., & Znaidi, S. (2023). Escherichia coli Nissle 1917 Antagonizes Candida albicans Growth and Protects Intestinal Cells from C. albicans-Mediated Damage. Microorganisms, 11(8), 1929. https://doi.org/10.3390/microorganisms11081929







