Differences in the Shiga Toxin (Stx) 2a Phage Regulatory Switch Region Influence Stx2 Localization and Virulence of Stx-Producing Escherichia coli in Mice
Abstract
1. Introduction
2. Materials and Methods
3. Results
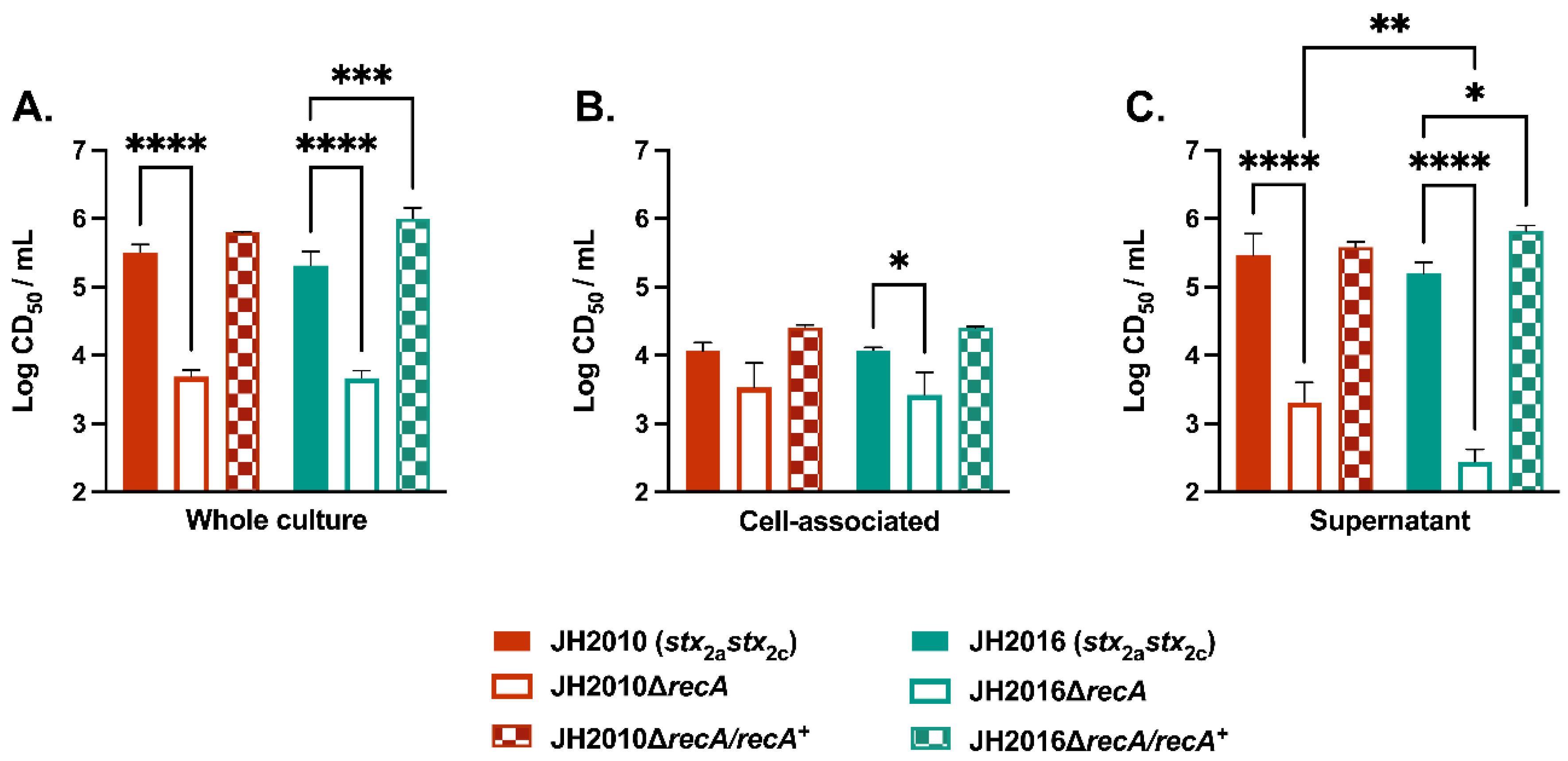
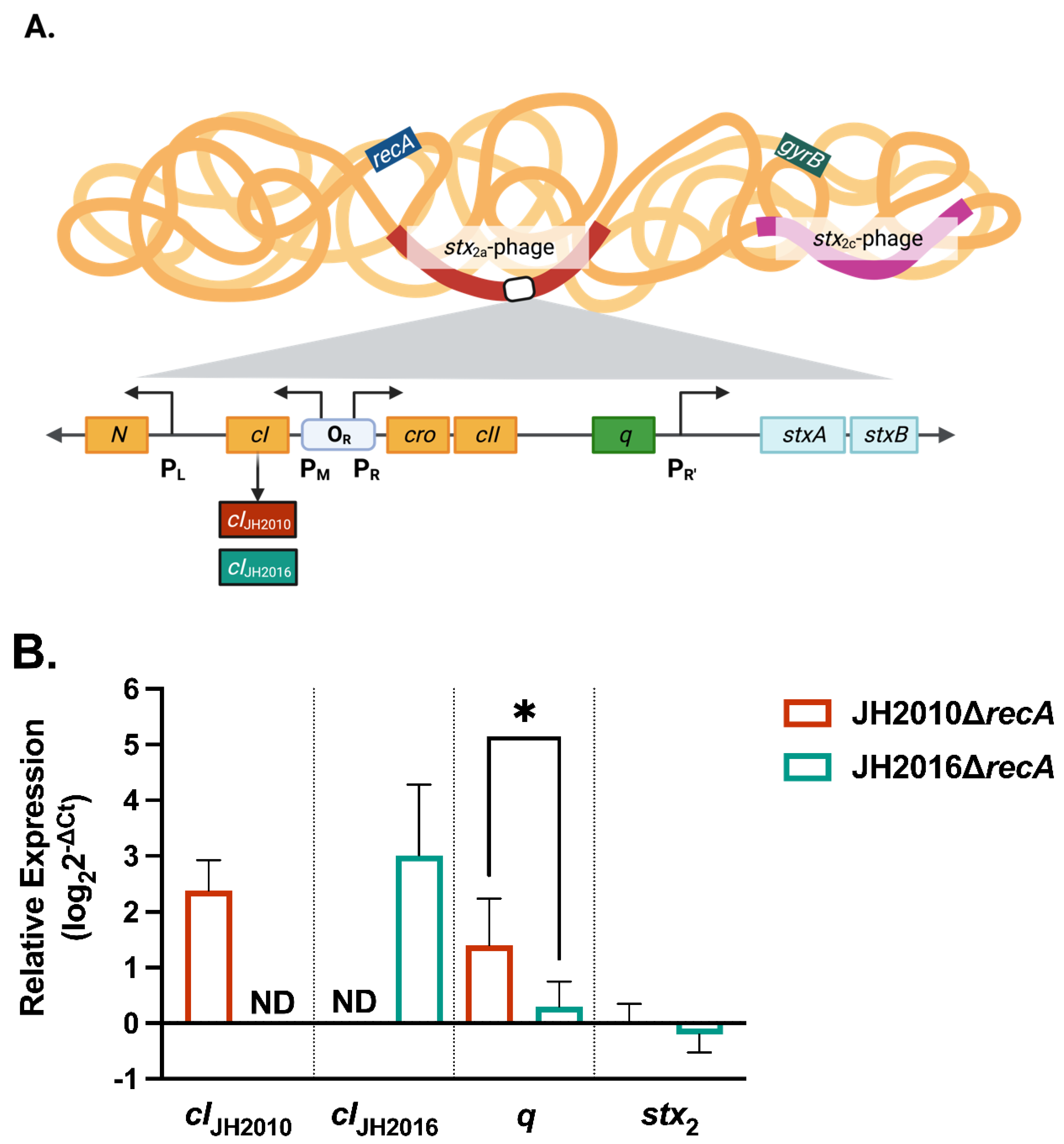
3.1. RecA-Independent Production of Stx2 from STEC Strains JH2010 and JH2016
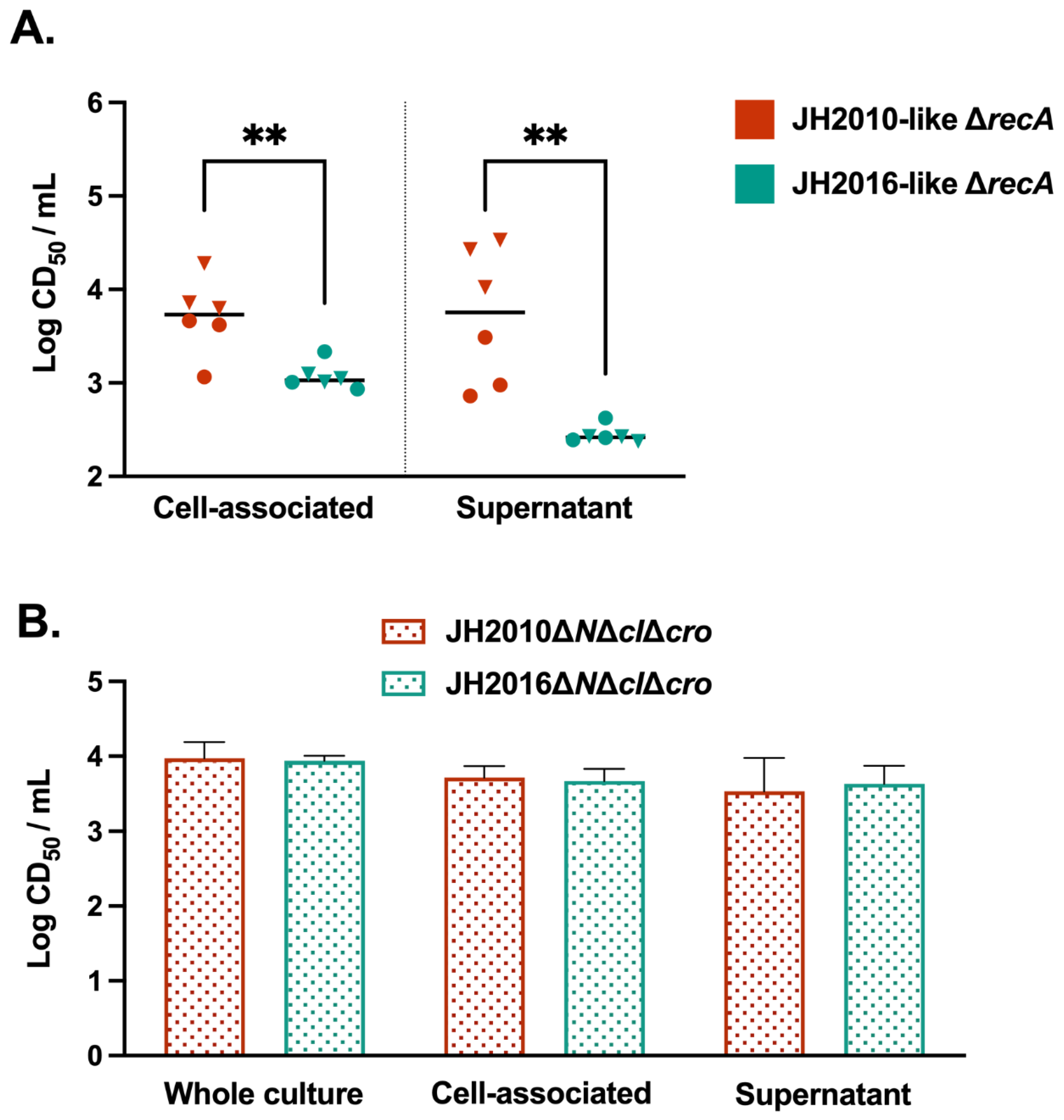
3.2. Cytotoxicity from Feces of Mice Infected with JH2010ΔrecA or JH2016ΔrecA
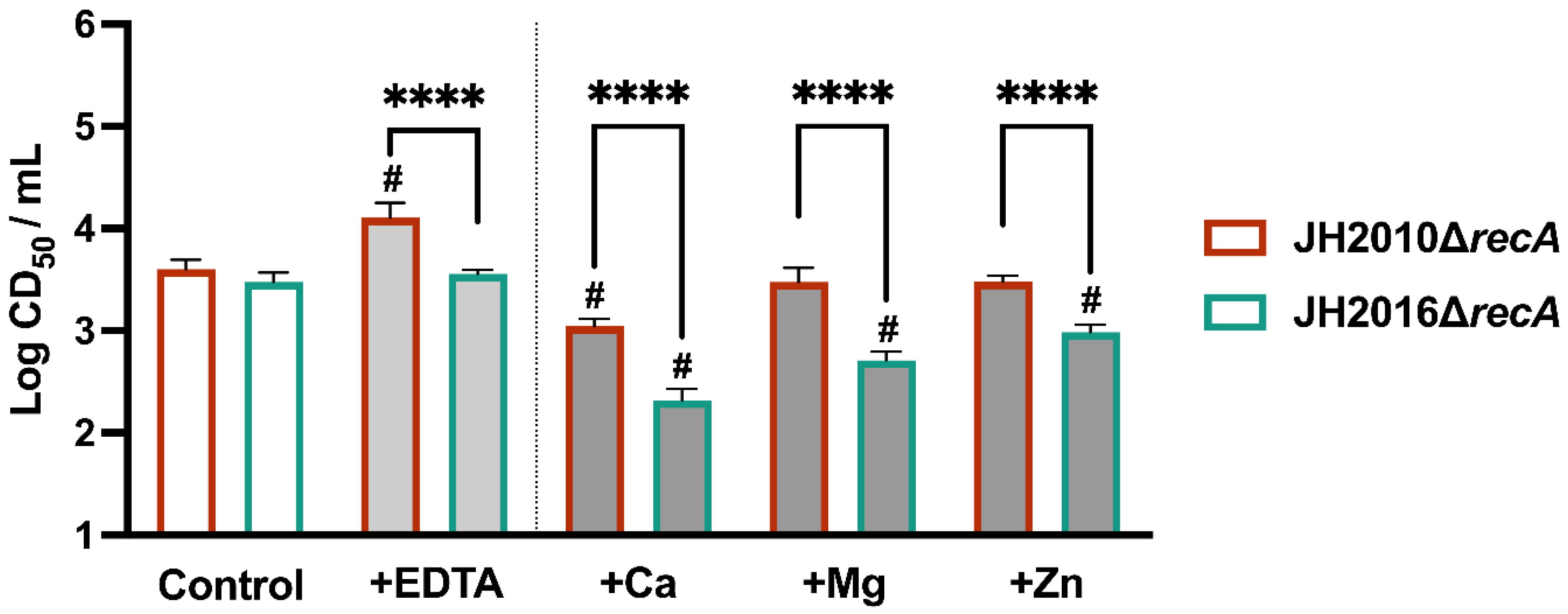
3.3. Test of Potential Inducers of RecA-Independent Cytotoxicity
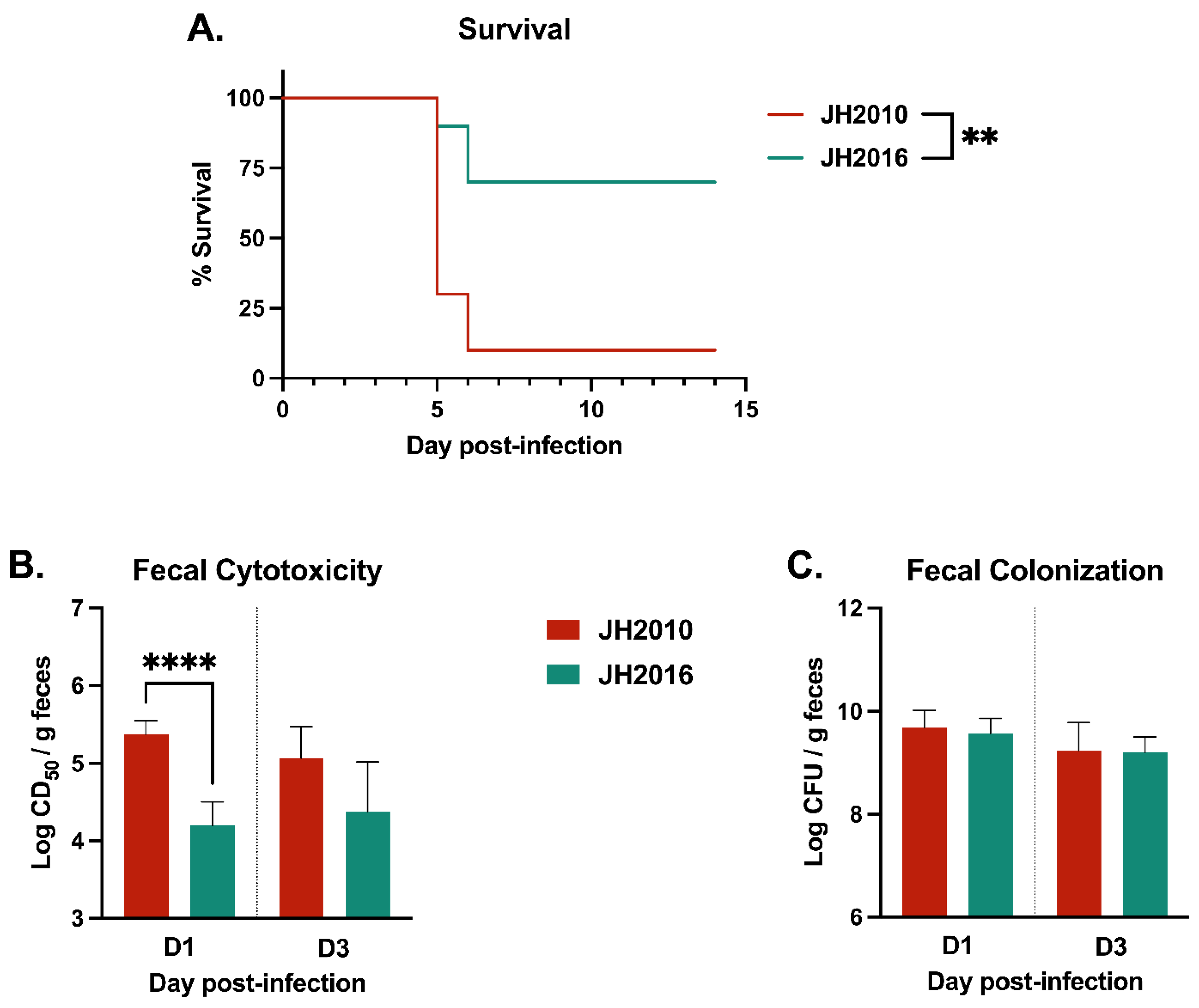
3.4. Infection of Mice with an Intermediate Dose of JH2010 or JH2016
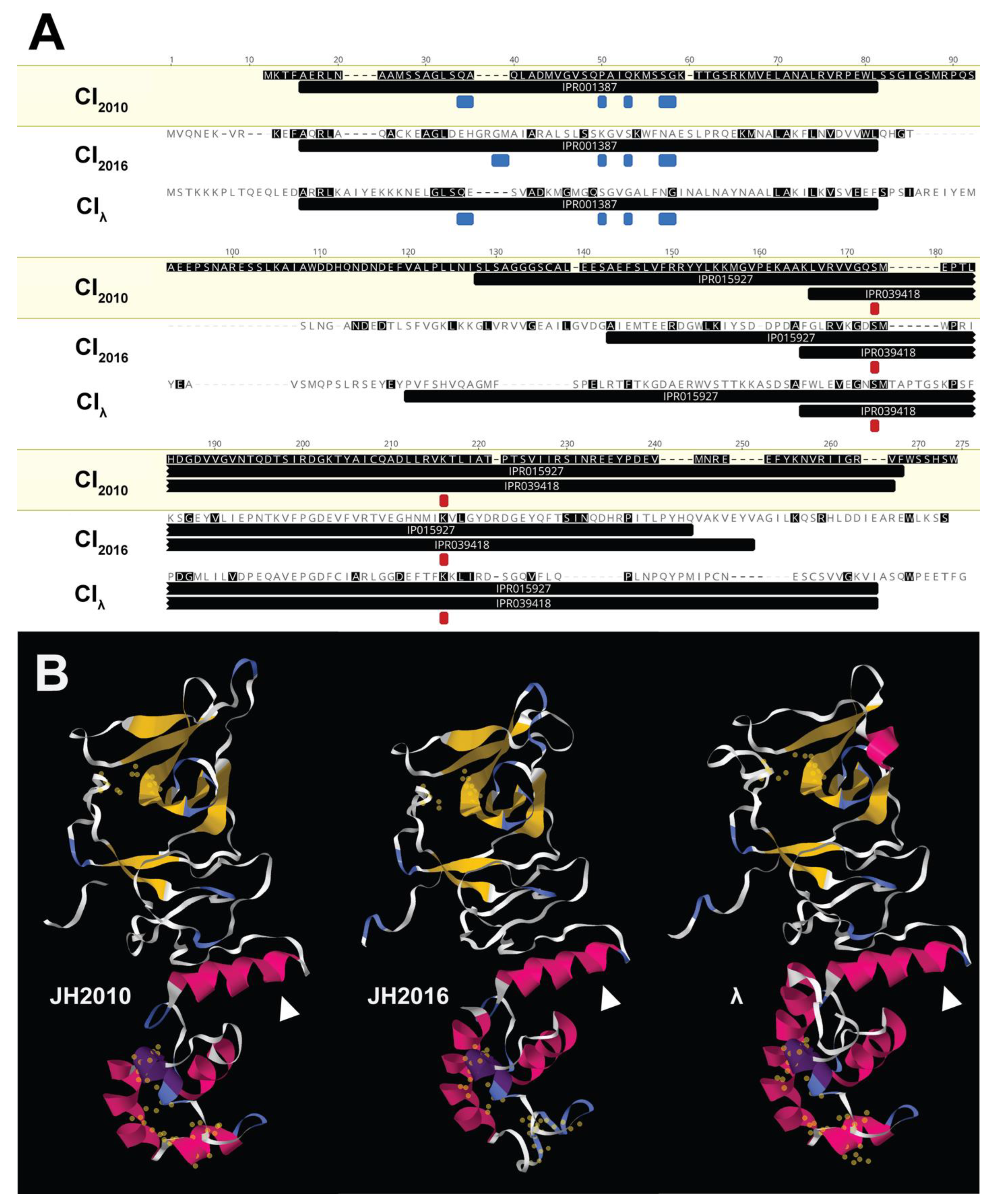
3.5. Comparison of the stx2a-Phage Sequences of JH2010 and JH2016
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Scallan, E.; Hoekstra, R.; Angulo, F.; Tauxe, R.; Widdowson, M.; Roy, S.; Jones, J.; Griffin, P. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. National Outbreak Reporting System Dashboard; U.S. Department of Health and Human Services, CDC: Atlanta, GA, USA, 2021.
- Centers for Disease Control and Prevention. National Enteric Disease Surveillance: Shiga Toxin-Producing E. coli (STEC) Annual Report, 2017; U.S. Department of Health and Human Services, CDC: Atlanta, GA, USA, 2021.
- Bryan, A.; Youngster, I.; McAdam, A.J. Shiga toxin producing Escherichia coli. Clin. Lab. Med. 2015, 35, 247–272. [Google Scholar] [CrossRef]
- Melton-Celsa, A.R. Shiga toxin (Stx) classification, structure, and function. Microbiol. Spectr. 2014, 2, EHEC-0024-2013. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.; Cointe, A.; Kurkdjian, P.; Rafat, C.; Hertig, A. Shiga toxin-associated hemolytic uremic syndrome: A narrative review. Toxins 2020, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rubio, L.; Haarmann, N.; Schwidder, M.; Muniesa, M.; Schmidt, H. Bacteriophages of Shiga toxin-producing Escherichia coli and their contribution to pathogenicity. Pathogens 2021, 10, 404. [Google Scholar] [CrossRef]
- Wong, C.S.; Jelacic, S.; Habeeb, R.L.; Watkins, S.L.; Tarr, P.I. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N. Engl. J. Med. 2000, 342, 1930–1936. [Google Scholar] [CrossRef]
- Rozanov, D.V.; D’Ari, R.; Sineoky, S.P. RecA-independent pathways of lambdoid prophage induction in Escherichia coli. J. Bacteriol. 1998, 180, 6306–6315. [Google Scholar] [CrossRef]
- Yamamoto, K.; Shinagawa, H.; Kondo, S. Induction of prophage lambda in Escherichia coli recA− strain by N-methyl-N′-nitro-N-nitrosoguanidine. Mutat. Res. 1983, 107, 33–40. [Google Scholar] [CrossRef]
- Fuchs, S.; Mühldorfer, I.; Donohue-Rolfe, A.; Kerényi, M.; Emödy, L.; Alexiev, R.; Nenkov, P.; Hacker, J. Influence of RecA on in vivo virulence and Shiga toxin 2 production in Escherichia coli pathogens. Microb. Pathog. 1999, 27, 12–23. [Google Scholar] [CrossRef]
- Imamovic, L.; Muniesa, M. Characterizing RecA-independent induction of Shiga toxin2-encoding phages by EDTA treatment. PLoS ONE 2012, 7, e32393. [Google Scholar] [CrossRef]
- Huerta-Uribe, A.; Marjenberg, Z.; Yamaguchi, N.; Fitzgerald, S.; Connolly, J.; Carpena, N.; Uvell, H.; Douce, G.; Elofsson, M.; Byron, O.; et al. Identification and characterization of novel compounds blocking Shiga toxin expression in Escherichia coli O157:H7. Front. Microbiol. 2016, 7, 1930. [Google Scholar] [CrossRef]
- Hauser, J.; Atitkar, R.; Petro, C.; Lindsey, R.L.; Strockbine, N.; O’Brien, A.D.; Melton-Celsa, A.R. The virulence of Escherichia coli O157:H7 isolates in mice depends on Shiga toxin type 2a (Stx2a)-induction and high levels of Stx2a in stool. Front. Cell. Infect. Microbiol. 2020, 10, 62. [Google Scholar] [CrossRef]
- Xiaoli, L.; Figler, H.; Banerjee, K.; Hayes, C.; Dudley, E. Non-pathogenic Escherichia coli enhance Stx2a production of E. coli O157:H7 through both bamA-dependent and independent mechanisms. Front. Microbiol. 2018, 9, 1325. [Google Scholar] [CrossRef]
- Kulasekara, B.; Jacobs, M.; Zhou, Y.; Wu, Z.; Sims, E.; Saenphimmachak, C.; Rohmer, L.; Ritchie, J.; Radey, M.; McKevitt, M.; et al. Analysis of the genome of the Escherichia coli O157:H7 2006 spinach-associated outbreak isolated indicates candidate genes that may enhance virulence. Infect. Immun. 2009, 77, 3713–3721. [Google Scholar] [CrossRef]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Research Council: Washington, DC, USA, 2011. [Google Scholar]
- Wadolkowski, E.A.; Burris, J.A.; O’Brien, A.D. Mouse model for colonization and disease caused by enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 1990, 58, 2438–2445. [Google Scholar] [CrossRef]
- Zangari, T.; Melton-Celsa, A.R.; Panda, A.; Smith, M.A.; Tatarov, I.; De Tolla, L.; O’Brien, A.D. Enhanced virulence of the Escherichia coli O157:H7 spinach-associated outbreak strain in two animal models is associated with higher levels of Stx2 production after induction with ciprofloxacin. Infect. Immun. 2014, 82, 4968–4977. [Google Scholar] [CrossRef][Green Version]
- Poteete, A. Recombination phenotypes of Escherichia coli greA mutants. BMC Mol. Biol. 2011, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Steyert, S.R.; Sahl, J.W.; Fraser, C.M.; Teel, L.D.; Scheutz, F.; Rasko, D.A. Comparative genomics and stx phage characterization of LEE-negative Shiga toxin-producing Escherichia coli. Front. Cell. Infect. Microbiol. 2012, 2, 133. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.; Petty, N.; Beatson, S. Easyfig: A genome comparison visualizer. Bioinformatics 2011, 27, 1009–1010. [Google Scholar] [CrossRef] [PubMed]
- Shkilnyj, P.; Koudelka, G.B. Effect of salt shock on stability of λimm434 lysogens. J. Bacteriol. 2007, 189, 3115–3123. [Google Scholar] [CrossRef] [PubMed]
- Stayrook, S.; Jaru-Ampornpan, P.; Ni, J.; Hochschild, A.; Lewis, M. Crystal structure of the λ repressor and a model for pairwise cooperative operator binding. Nature 2008, 452, 1022–1025. [Google Scholar] [CrossRef]
- Vareille, M.; de Sablet, T.; Hindré, T.; Martin, C.; Gobert, A.P. Nitric oxide inhibits Shiga-toxin synthesis by enterohemorrhagic Escherichia coli. Proc. Natl. Acad. Sci. USA 2007, 104, 10199–10204. [Google Scholar] [CrossRef]
- Colon, M.; Chakraborty, D.; Pevzner, Y.; Koudelka, G.B. Mechanisms that determine the differential stability of Stx+ and Stx− lysogens. Toxins 2016, 8, 96. [Google Scholar] [CrossRef]
- Chakraborty, D.; Clark, E.; Mauro, S.A.; Koudelka, G.B. Molecular mechanisms governing “hair-trigger" induction of Shiga toxin-encoding prophages. Viruses 2018, 10, 228. [Google Scholar] [CrossRef]
- Fagerlund, A.; Aspholm, M.; Węgrzyn, G.; Lindbäck, T. High diversity in the regulatory region of Shiga toxin encoding bacteriophages. BMC Genom. 2022, 23, 230. [Google Scholar] [CrossRef]
- de Paepe, M.; Hutinet, G.; Son, O.; Amarir-Bouhram, J.; Schbath, S.; Petit, M.A. Temperate phages acquire DNA from defective prophages by relaxed homologous recombination: The role of Rad52-like recombinases. PLOS Genet. 2014, 10, e1004181. [Google Scholar] [CrossRef]
- Moura de Sousa, J.; Pfeifer, E.; Touchon, M.; Rocha, E. Causes and consequences of bacteriophage diversification via genetic exchanges across lifestyles and bacterial taxa. Microb. Evol. Genom. 2021, 38, 2497–2512. [Google Scholar]


| Strain Name | Description | NCBI Accession # (Parental Strs Strain, Unless Noted) | Reference |
|---|---|---|---|
| JH2010 | Strr isolate of CDC #06-3462 | NZ_CP034794.1, OP797663 * | [14] |
| JH2016 | Strr isolate of CDC #2009c-4687 | NZ_CP034799.1 | [14] |
| JH2010ΔrecA | recA replaced with cat cassette in JH2010 | N/A | [14] |
| JH2016ΔrecA | recA replaced with cat cassette in JH2016 | N/A | This study |
| pJH206 | pACYC177 vector with recA + 500 bp upstream | N/A | [14] |
| JH2010ΔrecA/recA+ | Complementation of JH2010ΔrecA with pJH206 | N/A | [14] |
| JH2016ΔrecA/recA+ | Complementation of JH2016ΔrecA with pJH206 | N/A | This study |
| JH2010ΔNΔcIΔcro | stx2a-phage region from N to cro replaced with cat cassette in JH2010 | N/A | This study |
| JH2016ΔNΔcIΔcro | stx2a-phage region from N to cro replaced with cat cassette in JH2016 | N/A | This study |
| JH2011ΔrecA | recA replaced with cat cassette in JH2011 (Strr isolate of CDC #08-3914) | NZ_CP034808.1 | This study |
| JH2017ΔrecA | recA replaced with cat cassette in JH2017 (Strr isolate of CDC #2010c-3142) | NZ_CP034801.1 | This study |
| PA28-StrrΔrecA | recA replaced with cat cassette in RA10 (Strr isolate of strain PA28) | NC_041935.1 * | This study, parental strain [15] |
| TW14359-StrrΔrecA | recA replaced with cat cassette in RA13 (Strr isolate of strain TW14359) | NC_013008.1 | This study, parental strain [16] |
| Primer/Primer Pairs | Sequence | Reference |
|---|---|---|
| Primers for mutagenesis and confirmation of deletion strains | ||
| recA-KO_F | CTCATTTTATGGCTATCGACGAAAACAAACAGAAAGCGTTGGCGGCAGCACTGGGCCAGCACGTAAGAGGTTCCAACTTTCACCATAATG | [14] |
| recA-KO_R | ACGAACGATTAAAAATCTTCGTTAGTTTCTGCTACGCCTTCGCTGTCATCTACAGAGAAATTACGCCCCGCCCTGCCACTCATC | [14] |
| 2010_N-CI-Cro-KO_F | TTAACATAAATAGTCTTTTTCACCATAAGCATACTCAATAAGTCCATACGGCACGTAAGAGGTTCCAACTTTCACCATAATG | This study |
| 2010_N-CI-Cro-KO_R | TTATTCAGCCTGGTTCGGATGAGGAAACAGGTGCGGTAAGTCCGGGCGAATTACGCCCCGCCCTGCCACTCATC | This study |
| 2016_N-CI-Cro-KO_F | TCACCTCGCCGTCAGTTGTTTTGATTTCCGGTAGCCTGCCGCGTAAATGGGCACGTAAGAGGTTCCAACTTTCACCATAATG | This study |
| 2016_N-CI-Cro-KO_R | TTATGCAGCCAGAAGGTTCTTTTTGCTTATTTCAAGCATTTCGCTTGCTTTTACGCCCCGCCCTGCCACTCATC | This study |
| ScreenRecA_upF | TAACAACTGCCGAGTCTTGTACCGG | This study |
| ScreenN_upF | CTGTCGCCTGGAATCTCC | This study |
| ScreenCm_R | CACGACGATTTCCGGCAGTTTC | [14] |
| qPCR primers qPCR was conducted with the following thermocycler conditions: 95 °C for 15 min, followed by 40 cycles of: 94 °C for 15 s, 52 °C for 30 s, and 72 °C for 30 s. | ||
| gyrB_rtF/gyrB_rtR | F: GGTAGATAACGCTATCGACG R: GGGTGAATACCGGTCGG | This study |
| CI-2010_rtF/CI-2010_rtR * | F: GAGTCCGCAGAATTCTCTC R: CAACCCTGACCAACTTTGC | This study |
| CI-2016_rtF/CI-2016_rtR * | F: GCGCTTGCGAAATTTCTAAAC R: CCATCAACACCAAGAATTGC | This study |
| Q3_rtF/Q3_rtR | F: GACTGATCCCCGAAAAAGTA R: CAACCAGCAAGTCATGCAG | [21] |
| stx2_rtF/stx2_rtR | F: CGGCGGATTGTGCTAAAG R: GGTACTGGATTTGATTGTGACAG | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atitkar, R.R.; Melton-Celsa, A.R. Differences in the Shiga Toxin (Stx) 2a Phage Regulatory Switch Region Influence Stx2 Localization and Virulence of Stx-Producing Escherichia coli in Mice. Microorganisms 2023, 11, 1925. https://doi.org/10.3390/microorganisms11081925
Atitkar RR, Melton-Celsa AR. Differences in the Shiga Toxin (Stx) 2a Phage Regulatory Switch Region Influence Stx2 Localization and Virulence of Stx-Producing Escherichia coli in Mice. Microorganisms. 2023; 11(8):1925. https://doi.org/10.3390/microorganisms11081925
Chicago/Turabian StyleAtitkar, Rama R., and Angela R. Melton-Celsa. 2023. "Differences in the Shiga Toxin (Stx) 2a Phage Regulatory Switch Region Influence Stx2 Localization and Virulence of Stx-Producing Escherichia coli in Mice" Microorganisms 11, no. 8: 1925. https://doi.org/10.3390/microorganisms11081925
APA StyleAtitkar, R. R., & Melton-Celsa, A. R. (2023). Differences in the Shiga Toxin (Stx) 2a Phage Regulatory Switch Region Influence Stx2 Localization and Virulence of Stx-Producing Escherichia coli in Mice. Microorganisms, 11(8), 1925. https://doi.org/10.3390/microorganisms11081925








